Published online Jan 21, 2014. doi: 10.3748/wjg.v20.i3.804
Revised: October 21, 2013
Accepted: November 13, 2013
Published online: January 21, 2014
Processing time: 228 Days and 21.2 Hours
AIM: To assess laparoscopic fundoplication (LF) in partial responders to proton pump inhibitors (PPIs) for gastroesophageal reflux disease (GERD).
METHODS: We systematically searched PubMed and Embase (1966-Dec 2011) for articles reporting data on LF efficacy in partial responders. Due to a lack of randomized controlled trials, observational studies were included. Of 558 articles screened, 17 were eligible for inclusion. Prevalence data for individual symptoms were collated across studies according to mutually compatible time points (before and/or after LF). Where suitable, prevalence data were presented as percentage of patients reporting symptoms of any frequency or severity.
RESULTS: Due to a lack of standardized reporting of symptoms, the proportion of patients experiencing symptoms was recorded across studies where possible. After LF, the proportion of partial responders with heartburn was reduced from 93.1% (5 studies) to 3.8% (5 studies), with similar results observed for regurgitation [from 78.4% (4 studies) to 1.9% (4 studies)]. However, 10 years after LF, 35.8% (2 studies) of partial responders reported heartburn and 29.1% (1 study) reported regurgitation. The proportion using acid-suppressive medication also increased, from 8.8% (4 studies) in the year after LF to 18.2% (2 studies) at 10 years. In the only study comparing partial responders to PPI therapy with complete responders, higher symptom scores and more frequent acid-suppressive medication use were seen in partial responders after LF.
CONCLUSION: GERD symptoms improve after LF, but subsequently recur, and acid-suppressive medication use increases. LF may be less effective in partial responders than in complete responders.
Core tip: There are no high-level evidence to support the use of laparoscopic fundoplication (LF) in partial responders to proton pump inhibitor (PPI) therapy. The evidence that does exist suggests LF improves symptom control in these patients, but symptoms recur over time. There are limited data to suggest that LF is not as efficacious in partial responders as in those with an adequate response to PPI therapy.
- Citation: Lundell L, Bell M, Ruth M. Systematic review: Laparoscopic fundoplication for gastroesophageal reflux disease in partial responders to proton pump inhibitors. World J Gastroenterol 2014; 20(3): 804-813
- URL: https://www.wjgnet.com/1007-9327/full/v20/i3/804.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i3.804
Gastroesophageal reflux disease (GERD) affects approximately 10%-20% of adults in Western countries, with cardinal symptoms of heartburn and regurgitation[1]. Current treatment options include pharmacological and surgical approaches. The most common pharmacological therapy is acid-suppression with proton pump inhibitors (PPIs) (or, less commonly, histamine-2 receptor antagonists)[2]. GERD symptoms can also be treated surgically by the fundoplication technique, which involves wrapping the gastric fundus partly or completely around the lower end of the esophagus. More recently, the introduction of laparoscopic techniques has reduced perioperative complications and facilitated postoperative recovery, without compromising the level of GERD control. These improvements could increase the likelihood of patients being referred for this procedure.
The clinical effectiveness of laparoscopic fundoplication (LF) has been extensively validated[3], but the critical question remains of how to select those patients with chronic GERD who will benefit most from such procedures. Most studies in the surgical literature have included only patients who respond adequately to PPIs (referred to as “complete responders” from this point onwards), but it is increasingly evident that a substantial proportion of patients experience only partial or no relief from reflux symptoms, even after optimized PPI treatment[4,5]. These patients are often referred to as “partial responders” or “non-responders”, depending on the degree of symptom alleviation[6].
Partial responders to PPI treatment are commonly referred for LF, even though evidence for the effectiveness of the procedure in these patients has not been systematically assessed. The aim of this study was therefore to systematically review data on the effectiveness of LF in partial responders to PPI treatment and thereby offer a scientific platform for clinical decision-making in patients with complex GERD.
Targeted literature searches were conducted in PubMed and Embase from 1966 until December 2011. Reviews, studies not conducted in adult humans and studies not published in English were excluded using search engine filters. The remaining studies were screened based on titles and abstracts, and full articles were reviewed when their relevance was unclear from the abstract.
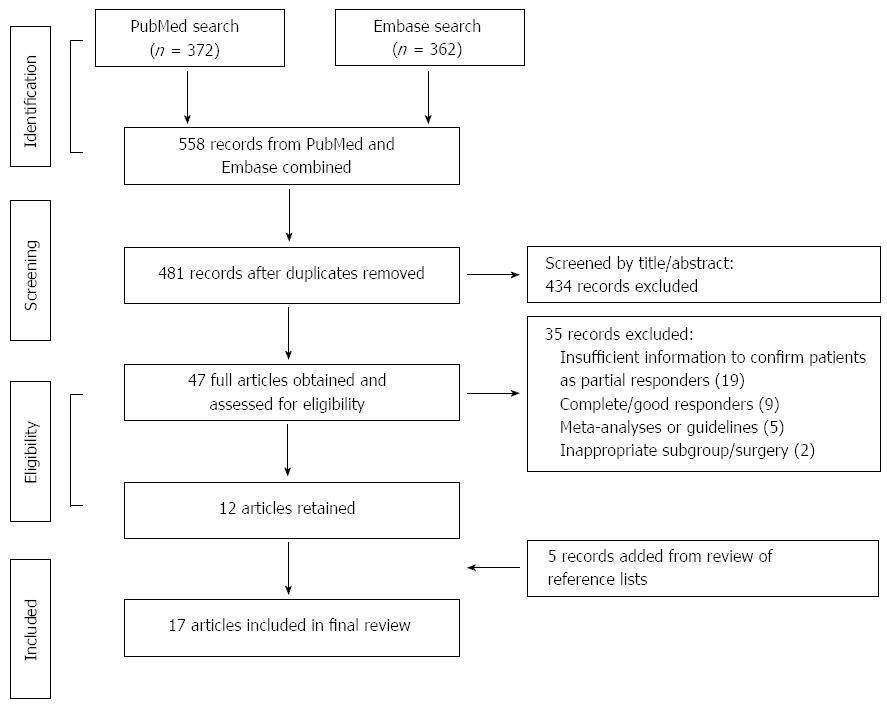
Study eligibility was assessed by all three authors. The primary targets were randomized controlled trials (RCTs) comparing LF with PPI maintenance therapy in partial responders. However, no such trials were identified and the selection criteria were therefore expanded to include any prospective studies assessing LF in partial responders, preferably including baseline data obtained before LF while patients were on PPI therapy. The search methodology used to identify relevant articles is summarized in Figure 1.
Data were abstracted by a single author (Bell M) and reviewed by the co-authors (Lundell L and Ruth M). Multiple methods of reporting symptoms obscure comparisons among studies. To minimize the impact of this variation, the authors focused (where possible) on collecting data on the prevalence of individual symptoms of any frequency and/or severity. These data were collated across studies according to mutually compatible time points (before and/or after LF). Where suitable, prevalence data were presented as percentage of patients reporting symptoms of any frequency or severity. Any other data providing insight into the effectiveness of LF, such as physiological or quality of life (QoL) measurements, were also collected. Nissen, Nissen-Rossetti, robot-assisted Nissen and Toupet, as well as other partial fundoplications, were all included under the broader definition of LF. Resting lower esophageal sphincter pressure (LESP) values reported in kPa were converted to mmHg, using standard conversion criteria stated by the International Bureau of Weights and Measures (1 mmHg = 0.133322 Pa).
Of 558 articles screened, 17 (reporting data from 13 trials) were deemed eligible for inclusion, with sample sizes of partial responders undergoing LF ranging from 10 to 1340; study characteristics are summarized in Table 1[7-23]. A PRISMA diagram outlining search strategy, study elimination and study selection is presented in Figure 1. Of the 17 articles, five (three trials) were conducted in the Netherlands, three (two trials) in the United Kingdom, two each in Austria and Italy, and one each in Canada, France, Belgium, Switzerland and Greece. The prevalence of individual symptoms (any frequency or severity) was reported by eleven studies (heartburn[7-10,16,17,19-21], regurgitation[7-10,16,17,20,21] and dysphagia[7-10,15-17,19-21,23]). Physiological reflux measures were presented by thirteen studies (LESP[7-10,16,18,20-23], esophageal acid exposure[7-9,11,12,18,19,21-23] and endoscopic evaluation of reflux esophagitis[7-10,18,21,22]). QoL measures were reported by nine studies [Gastrointestinal Quality of Life Index (GIQLI)[10,16,17], GERD Health-Related Quality of Life (GERD-HRQL)[16,21,22] or general QoL visual analogue scales(VASs)[7-9,18,22]]. Other measures indicative of surgical effectiveness were provided by eleven studies (patient satisfaction with the outcome of LF[7-10,15,18-20], postoperative use of acid-suppressive medication[7-9,11,15-20], intraoperative conversion to open fundoplication[10,15,17,18,20] and surgical reintervention rates after LF[7-10,15-20]). The definitions of partial response varied substantially across studies (Table 1).
| Ref. | Study design | Country | Definition of partial response | Age range (yr) | n |
| Bais et al[7] Draaisma et al[8] Broeders et al[9] | RCT 10-yr follow-up of LNF vs CNF | Netherlands | Bais et al: "…in patients with symptoms of GORD, insufficiently reacting to at least 40 mg of omeprazole daily, persisting oesophagitis, and pathological acid exposure, surgical treatment was proposed"… Broeders et al: "177 patients were included in a multi-center RCT to undergo Nissen fundoplication for PPI-refractory GERD" | 17-79 | 79 |
| Granderath et al[10] | Prospective 5-yr follow-up of LTF | Austria | "All patients had a long history of GERD symptoms (mean 7.1 years; range 6 mo to more than 10 yr) and had been receiving medical treatment with PPI for a mean period of 18.4 ± 6.8 mo (20-60 mg omeprazole daily)" | 29-74 | 155 |
| Anvari et al[11] | Prospective 5-yr follow-up of LF (one cohort of “poor responders”) | Canada | "Inadequate response (< 70% relief on a visual analogue scale, defined by the patient's subjective impression) to PPIs titrated to a dose of 120 mg/d in 445 patients" | Not specified | 445 |
| Jenkinson et al[12] | Prospective 6-8-wk follow-up after LF | United Kingdom | "All patients were on long term acid-suppression therapy (at least 4 mo) with PPIs and were either symptomatic or dissatisfied with the treatment" | 20-78 | 70 |
| Mahon et al[13] Mehta et al[14] | RCT 7-yr follow-up of LF vs PPI in GERD (PR subset) | United Kingdom | Mehta et al: "patients with symptoms of GERD for at least 6 mo" with "3 mo minimum of PPI maintenance therapy"..."After 12 mo, those who had been randomized to PPI were offered the opportunity to have surgery" | 26-69 | 54 |
| Pessaux et al[15] | Retrospective 5-yr follow-up of LF | France | "The indications for surgery were intractable or recurrent symptoms due to GERD after an adequate trial (minimum of 3 mo) of conservative treatment that consisted of proton pump inhibitors (n = 1234; 92.1%)" | 18-86 | 1340 |
| Ciovica et al[16] | Prospective 12-mo follow-up of LF | Austria | "Antireflux surgery was indicated and performed in patients with persistent or recurrent GERD symptoms and/or complications despite maximal conservative treatment, and in patients preferring surgery to medical treatment" | 16-81 | 351 |
| Dallemagne et al[17] | Prospective 5- and 10-yr follow-up of LF questionnaires | Belgium | "All patients were taking PPIs for acid suppression" … "For all patients, the primary symptoms indicating surgery were heartburn, associated with regurgitation in 54% and dysphagia in 8% of the patients" | 10-78 | 100 |
| Draaisma et al[18] | RCT 6-mo follow-up of robot-assisted vs standard laparoscopy LF | Netherlands | "Surgical treatment was proposed for patients with GORD insufficiently reacting to proton pump inhibitors..." | 20-74 | 50 |
| Zehetner et al[19] | Prospective 5-yr follow-up of LTF | Switzerland | All patients diagnosed with GERD according to SAGES criteria; "98% received a pre-operative medical treatment for > 3 mo with PPI/H2-blockers and/or prokinetic" | 27-81 | 100 |
| Antoniou et al[20] | Prospective 1-yr follow-up of LNF | Greece | “Continuous doses of PPIs for a minimum period of 6 mo incurred no or only partial relief of their symptoms. More specifically, refractory GERD symptoms to a standard dose of PPIs (omeprazole 20 mg/d) for at least 4 mo were followed by a therapeutic trial with a double dose of PPIs (omeprazole 40 mg/d) for 8-10 wk. Patients not responding to the high-dose PPI treatment were included in the study" | 17-65 | 32 |
| Brillantino et al[21] | Prospective 1-yr follow-up of LNF | Italy | "…patients referred for symptomatic gastroesophageal reflux poorly responsive to standard dose PPI therapy…" "...persisting heartburn or regurgitation during treatment for at least 3 mo with omeprazole at 40 mg or lansoprazole at 30 mg/d…" | 18-70 | 35 |
| Broeders et al[22] | Prospective 1-yr follow-up of LNF | Netherlands | "Thirty-one patients with PPI-refractory GORD with pathological acid exposure on pH monitoring…" | 26-67 | 31 |
| Frazzoni et al[23] | Prospective 3-mo follow-up of EsophyX vs LNF | Italy | "...adult patients referred to our centre because of troublesome heartburn/regurgitation persisting despite at least 4-wk high-dose PPI therapy" | “Adults” | 10 |
| Total: | 2852 |
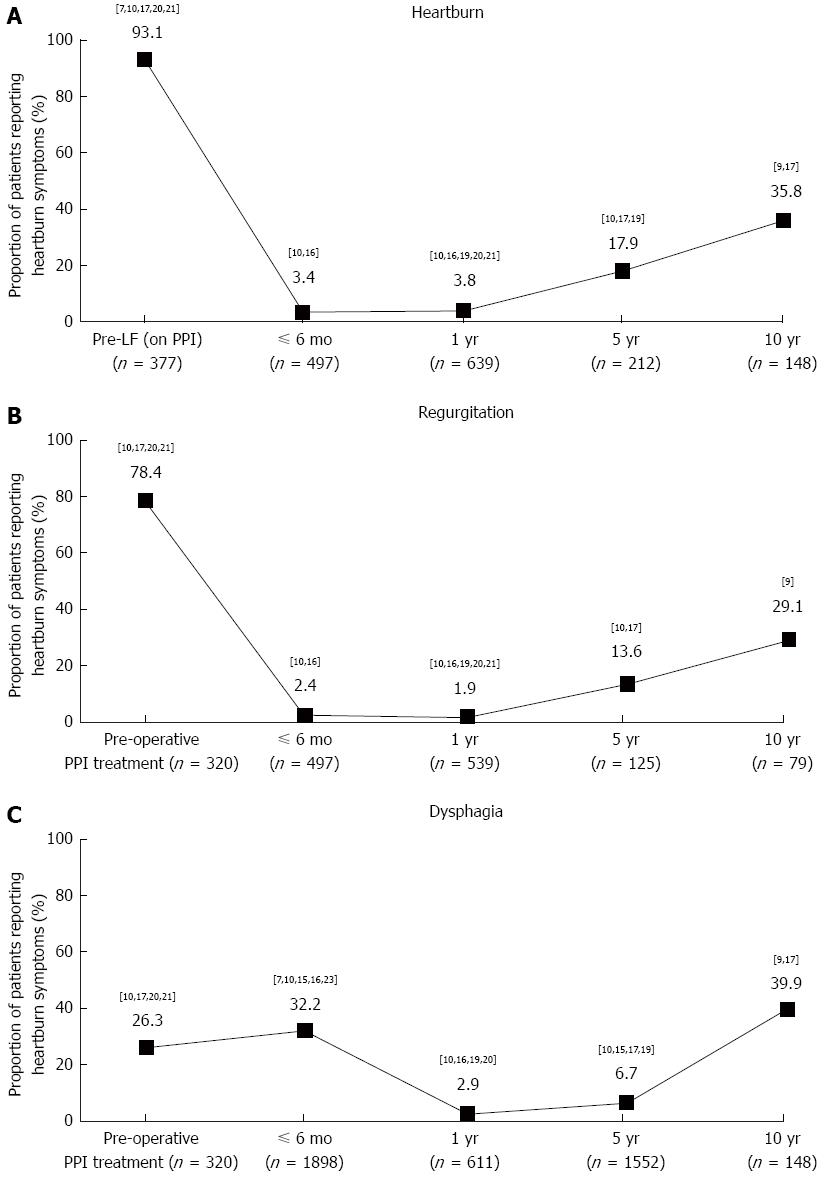
Heartburn: Data on the prevalence of heartburn (any frequency or severity) after LF were reported in seven trials[7-10,16,17,19-21]. Pooled data from these studies, some of which did not report baseline data (heartburn prevalence before LF in patients taking a PPI)[16,19], indicate that the proportion of patients experiencing heartburn decreased substantially in the year after LF but recurred over time, with 35.8% (53/148) of patients across two studies reporting heartburn after 10 years (Figure 2A)[9,17].
Of the five trials reporting prevalence data before (during PPI treatment) and after LF, two reported complete remission of heartburn at 1 year after LF, from 60% (18/30) and 91.4% (32/35) before LF to 0% (0/30 and 0/28, respectively) after[20,21]. Another study reported that heartburn prevalence decreased from 95.5% (148/155) before LF to 0% (0/155) 1 year after and 2.6% (1/39) 5 years after[10]. In the two trials reporting the longest follow-up, heartburn prevalence was reduced from 93% (53/57) and 100% (100/100) before LF to 40.5% (32/79) and 30.4% (21/69) 10 years after, respectively[7,9,17].
Regurgitation: Data on the prevalence of regurgitation (any frequency or severity) after LF were reported in six trials[7-10,16,17,20,21]. Pooled data from these studies, some of which did not report baseline data (regurgitation prevalence before LF in patients taking a PPI)[7,16], indicate that the proportion of patients experiencing regurgitation decreased substantially in the year after LF but recurred over time, with 29.1% (23/79) of patients reporting regurgitation 10 years after LF in the only study reporting data over this period (Figure 2B)[9].
Of the four trials reporting prevalence data before (during PPI treatment) and after LF, two reported a decrease 1 year after LF, from 71.4% (25/35) and 93.3% (28/30) before LF to 3.6% (1/28) and 13.3% (4/30) after, respectively[20,21]. In the two trials reporting the longest follow-up, one reported that regurgitation prevalence was reduced from 54% (54/100) before LF to 17.4% (15/86) 5 years after, and the other reported that regurgitation prevalence was reduced from 92.9% (144/155) before LF to 0% (0/155) 1 year after and 5.1% (2/39) 5 years after[10,17].
Dysphagia: Data on the prevalence of dysphagia (any frequency or severity) after LF were reported in nine trials[7-10,15-17,19-21,23]. Pooled data from these studies, some of which did not report baseline data (dysphagia prevalence before LF in patients taking a PPI)[7,15,16,19,23], indicate that the proportion of patients experiencing dysphagia increased slightly in the 6 mo after LF, before decreasing substantially after 1 year (Figure 2C). The response appears to be biphasic, with the prevalence remaining low at 5 years[10,15,17,19], but increasing in the two trials with a 10-year follow-up period[9,17].
Of the three studies reporting prevalence data before (during PPI treatment) and after LF, two showed an improvement in symptoms 1 year after LF[10,20], from 43.9% (68/155) and 26.7% (8/30) before LF, to 2.6% (4/155) and 6.7% (2/30) after, respectively; in the former study, the decrease in dysphagia prevalence after LF was well maintained at 5-year follow-up with 5.1% (2/39) of patients reporting dysphagia[18]. However, the remaining study, with the longest follow-up period, showed an increase in the number of patients reporting dysphagia, from 8% (8/100) before LF to 37.2% (32/86) 5 years after and 23.2% (16/69) 10 years after[17].
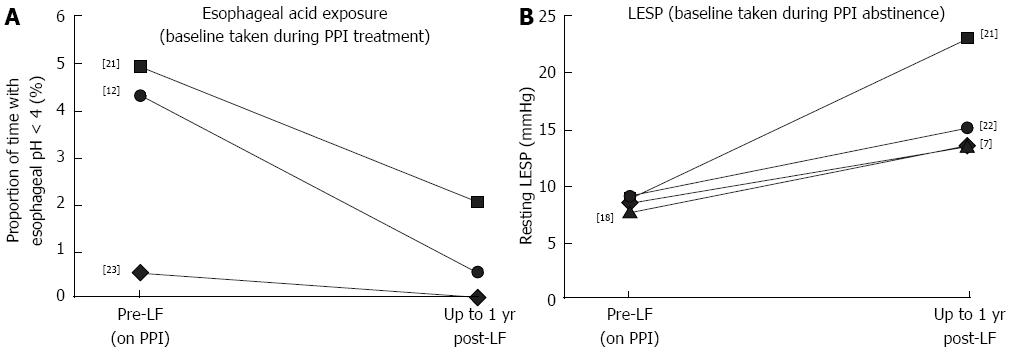
Ambulatory 24-h pH measurements: In five trials, esophageal acid exposure (proportion of time with esophageal pH < 4 during ambulatory 24-h pH measurements) was assessed before LF in patients who had not taken a PPI for at least 3 d, and again 0.25-1 years after LF[7-9,12,18,19,22]. Across these studies, esophageal acid exposure was reduced by 85.5%-97.0%, from 8.5%-17.8% (n = 753) before LF to 0.3%-1.8% (n = 242) after. In the only one of these trials reporting such data 5 years after LF[7,8], the 85.5% (n = 57) reduction in esophageal acid exposure observed 1 year after LF was largely maintained [82.3% (n = 48)].
In three studies, esophageal acid exposure was measured before LF in patients who were still taking a PPI, and repeated 0.25-1 year after LF (n = 115)[12,21,23]. Across these studies, esophageal acid exposure was reduced by 58.6%-100.0%, from 0.5%-4.9% (n = 115) before LF to 0.5%-2.0% (n = 108) after. Of these, one study reported reductions in esophageal acid exposure of 100%, but data were available for only 10 patients[23]. In the other two studies, esophageal acid exposure was reduced by 58.6% (n = 35)[21] and 88.4% (n = 70)[12] after LF. These changes are summarized in Figure 3A.
In the only study to compare esophageal acid exposure after LF with pre-LF measurements obtained on and off PPI therapy, esophageal acid exposure was lower in patients after LF (median: 0.5%) than in patients before LF, whether they were on or off PPIs [4.3% and 9.5%, respectively (n = 70)] [12].
Lower esophageal sphincter pressure: In four trials, resting LESP was assessed before LF in patients who had not taken a PPI for at least 3 d, and again 0.25-1 years after LF. Across these studies, resting LESP increased by 62.7%-164.4%, from 7.5-9.0 mmHg (n = 164) before LF to 13.5-23.0 mmHg (n = 157) after[7-9,18,21,22]. These changes are summarized in Figure 3B. In one of these trials, the follow-up period was extended to 5 years (n = 48)[7,8]. The 62.7% increase in LESP seen in this trial between baseline (8.3 mmHg) and 3 mo after LF (13.5 mmHg) was largely maintained after 5 years (12.8 mmHg, or a 54.2% increase relative to baseline). Only one study presented baseline data during PPI therapy compared with after LF; however, the values presented in this study (median value of 18 mmHg during PPI therapy before LF vs 21 mmHg after) are much higher than those reported by other studies, and furthermore, this study only included 10 patients[23].
Endoscopic evaluation of reflux esophagitis: Across the five trials presenting LA classification data (Table 2)[24], 74.6% (244/327) of participants were found to have some degree of reflux esophagitis before LF, although no study clarified whether pre-LF endoscopic assessments were taken during PPI treatment[7-10,18,21,22]. Grade C or D reflux esophagitis was found in 20.5% (67/327) of patients. Of the three studies reporting LA classification data both before and 6-12 mo after LF, all revealed substantial reductions in the proportion of patients with endoscopy-proven reflux esophagitis (range across studies at baseline: 48.4%-71.4%; range after LF: 2.9%-16.7%)[18,21,22]. Collectively, the proportion of patients presenting with reflux esophagitis was reduced from 63.6% (70/110) before LF to 8.1% (9/111) after; no patients were reported to have grade C or D reflux esophagitis after LF.
Only four trials compared QoL before LF while patients were taking a PPI with that after LF[7-9,16,21,22]. Of these, three found that GERD-HRQL scores improved (decreased) 1 year after LF [12-20.1 to 1-3.5 (n = 417)][16,21,22], two reported improvements (increases) in VAS scores[7-9], including a substantial increase at 1 year [50.2 to 71.5 (n = 31)][22] and at 10 years after LF [52.7 to 65.3 (n = 79)][7-9]. Only one study used the GIQLI scores and reported improved (increased) values at 1 year after LF [104 to 119 (n = 351)][16].
Patient satisfaction: The number of patients satisfied with the outcome of LF was reported by six trials[7-10,15,18-20]. In the year after LF, 88.8% (71/80) of patients expressed satisfaction with the procedure across two trials[18,20]; at follow-ups ranging from 3 to 5 years, 93.2% (1489/1598) of patients were satisfied across four trials[7,8,15,19]. In the only trial reporting patient satisfaction 10 years after LF, 78.5% (62/79) of patients were satisfied with the outcome of the operation[9].
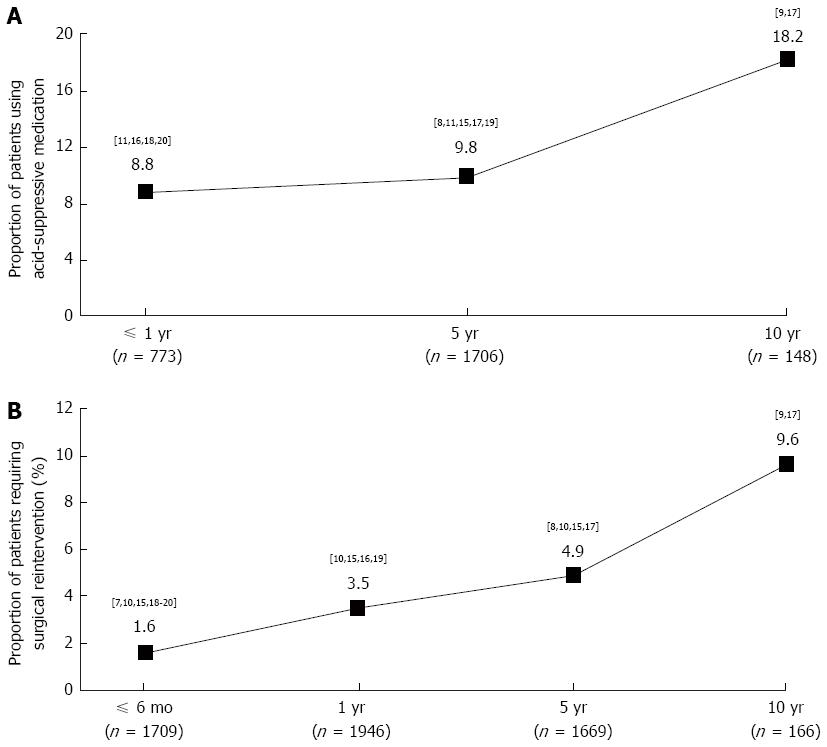
Postoperative acid-suppressive medication use: Use of acid-suppressive medication after LF was reported by eight trials for up to 10 years (Figure 4A)[7-9,11,15-20]. Across four trials reporting data for the year immediately after LF, 8.8% (68/773) of patients were using acid-suppressive medication[11,16,18,20]. Across five trials reporting 5-year outcomes of LF, 9.8% (167/1706) of patients were using acid-suppressive medication[7-9,11,15,17,19]. In two trials reporting results at 10 years, 18.2% (27/148) of patients were using acid-suppressive medication[7-9,17].
Intraoperative and postoperative complications: The proportion of patients scheduled for LF surgery who during the actual operation had to be converted to open fundoplication was 7.0% (115/1652)[10,15,17,18,20]. The number of patients who had undergone LF and required subsequent surgical reintervention (to repeat LF, to perform repetitive dilatation, or to repair an intrathoracic herniation), at some point during the follow-up period, was reported by eight trials (Figure 4B)[7-10,15-20]. Across the two studies with the longest follow-up, 9.6% (16/166) of patients required surgical reintervention during the decade following the initial LF[9,17].
Only one trial compared partial and complete responders (classified by patient-reported symptom relief on a VAS before and after LF[11]. Symptom scores were assessed by patients rating five GERD symptoms, with each symptom scored as a product of severity and frequency. Despite similar baseline symptom scores during PPI abstinence [complete responders: 33.7 (n = 274); partial responders: 34.3 (n = 445)], the partial responders experienced significantly less symptom relief during PPI treatment before LF than complete responders (13.2 and 22.6, respectively), and also 6 mo, 2 years and 5 years after LF (values not shown). This study also reported a higher rate of postoperative PPI use in partial responders than in complete responders (16.0% and 11.0%, respectively, at 5 years), although this difference was not statistically significant.
Across the selected studies, substantial reductions in the prevalence of heartburn and regurgitation in partial responders were observed immediately after LF compared with PPI therapy before LF. However, symptoms recurred in around 30%-35% of patients a decade after LF in those studies reporting long-term follow-up data. This coincided with an increase in the prevalence of acid-suppressive medication use from 9% to 18% over the same period. The impact of LF on the prevalence of dysphagia after LF is less clear. As expected, dysphagia may remain an issue for many patients during the first 6 mo after LF[25]. However, the prevalence of dysphagia also appeared to decrease 1 year and 5 years after LF but increased again after 10 years, suggesting a biphasic response. It seems possible that this late recrudescence of dysphagia coincides with the recurrence of heartburn and regurgitation symptoms observed over time after the operation.
Large reductions in esophageal acid exposure in partial responders were also seen after LF compared with PPI therapy alone before LF. A corresponding superiority of surgical repair in the reductions in esophageal acid exposure compared with PPI therapy alone has also been reported in complete responders 5 years after LF, in a recent study comparing long-term use of esomeprazole with surgery for treatment of chronic GERD (the LOTUS trial)[26]. The presented review suggests that surgery can elicit a further normalization of acid exposure in PPI partial responders, with a corresponding clinical improvement. These data would therefore suggest that in these patients it may be important to further minimize acid exposure in order to attain symptom control.
Additionally, increases in resting LESP of 60%-160% were observed 6 mo after LF. Furthermore, the durability of the hiatal repair appears robust in the years after LF. Some post-fundoplication problems may be a consequence of over-tightening of the sphincter by LF, but the optimal level of sphincter pressure following this procedure remains unclear. LF was found by several studies to also improve QoL according to various instruments.
Only one trial was identified that compared the effects of LF in partial responders with those in complete responders. This study clearly showed a greater benefit for LF in terms of reflux symptom scores at 5 years in the latter group[11]. This study also reported a higher rate of postoperative PPI use in partial responders than in complete responders. This albeit limited evidence lends support to the assertion that partial responders do not respond as favourably to LF as complete responders; therefore, appropriate patient selection for surgery is crucial.
Most studies reporting the efficacy of LF include only patients who respond well to PPI treatment[3]. However, patients for whom PPI treatment does not provide adequate symptom relief are also commonly referred for surgical therapy[27]. To our knowledge, this is the first systematic review to assess the short- and long-term effects of LF in patients with GERD whose symptoms respond poorly to PPI therapy, offering the most comprehensive assessment of this important clinical question to date.
Our systematic searches did not identify any “ideal” studies (i.e., RCTs comparing the results of LF in partial responders and complete responders with those of optimized PPI maintenance therapy). As such, we were forced to reduce the stringency of our study selection criteria. Another limitation of this review is the large variation in the design of the selected studies. For example, the definition of a partial response to PPI treatment in patients with GERD was often unclear and inconsistent across studies, although this is not surprising given that no consensus definition exists[6]. There was also substantial variation in the symptom definitions used and in the use of objective measures of reflux. Another weakness of the current review is that few of the studies reporting long-term follow-up data also presented baseline data. Data were therefore collated at each time point and this approach may mean that differences in trends among studies are missed. We cannot exclude the possibility that some degree of reporting bias contributes to the high prevalence of symptom recurrence after LF; patients with adequate symptom relief over time may be less likely to report for follow-up, meaning that recurrent symptoms may be relatively over-reported. Regardless, it is clear that a substantial proportion of partial responders exhibit GERD symptoms 10 years after LF. Despite the limitations of the included studies, useful inferences can be drawn about the effectiveness of LF in partial responders, and about recommendations for future studies in this area.
The data presented provide strong evidence that LF is superior to acid-suppressive medication at reducing reflux symptoms in partial responders to PPI therapy, but that these symptoms may recur and acid-suppressive medication use may increase in a substantial proportion of patients over time. Furthermore, although data are limited, LF appears to be less effective in patients with GERD whose symptoms only partially respond to PPI therapy than in complete responders to PPI therapy. Indeed, these results support the findings of a recent study that concluded that the response of symptoms to preoperative PPI treatment is an excellent predictor of the response of symptoms to fundoplication[28]. This may have implications for recent recommendations for LF as an alternative to the high costs of continuous acid-suppressive medication[29], because they are based mainly on data from complete responders and may thus overestimate the effectiveness of LF in partial responders. These factors must be carefully considered, especially in light of recent improvements in drug pharmacokinetics that may shift the balance towards pharmacological therapy, depending on the age and health status of the patients, and the pathology of GERD.
In a limited time horizon, LF offers a substantial and clinically relevant improvement in GERD symptoms, physiological measures of GERD and QoL parameters in partial responders beyond that provided by PPI treatment alone. However, in the long term a substantial proportion of these patients experience a recurrence of GERD symptoms. Limited data also suggest that LF is less effective at reducing symptoms in partial responders than in complete responders. This may affect cost arguments for using LF rather than acid-suppressive medications because data are based largely on complete responders.
When patients with gastroesophageal reflux disease (GERD) do not respond adequately to proton pump inhibitor (PPI) treatment, they are often referred for surgery (laparoscopic fundoplication). However, justification for this surgery is largely based on trials evaluating patients who respond well to PPIs.
This review aimed to systematically assess the available evidence for this procedure in patients responding only partially to PPI therapy (partial responders).
This is the first review to assess the available evidence for efficacy of laparoscopic fundoplication in this subset of patients who do not respond adequately to PPI treatment.
It is hoped referral for laparoscopic fundoplication can be made on an objective patient-by-patient basis, with pre-surgery response to PPI treatment taken into consideration. This review also outlines inconsistencies in patient definitions, and suggests preferred outcomes and methods of reporting.
Histamine-2 receptor antagonists: acid-suppressive medication often used to treat GERD. PPI: acid-suppressive medication often used to treat GERD. Laparoscopic fundoplication: a minimally invasive surgical procedure often used to treat GERD; the surgeon accesses via a small incision in the abdomen, and partially or completely wraps the patient’s fundus (the upper part of the stomach) around the lower esophageal sphincter, to minimize the degree of reflux.
The paper identifies that GERD symptoms improve after laparoscopic fundoplication, but subsequently recur, and acid-suppressive medication use increases. The research methodology is well written and well organized.
P- Reviewer: Maher MM S- Editor: Zhai HH L- Editor: A E- Editor: Wang CH
| 1. | Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1261] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 2. | van Pinxteren B, Sigterman KE, Bonis P, Lau J, Numans ME. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2010;CD002095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Wileman SM, McCann S, Grant AM, Krukowski ZH, Bruce J. Medical versus surgical management for gastro-oesophageal reflux disease (GORD) in adults. Cochrane Database Syst Rev. 2010;CD003243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Donnellan C, Sharma N, Preston C, Moayyedi P. Medical treatments for the maintenance therapy of reflux oesophagitis and endoscopic negative reflux disease. Cochrane Database Syst Rev. 2005;CD003245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | El-Serag H, Becher A, Jones R. Partial- and non-response of reflux symptoms to proton pump inhibitors: a systematic review of primary care and community-based studies. Gastroenterology. 2010;138:S648-649. |
| 6. | Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61:1340-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 248] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 7. | Bais JE, Bartelsman JF, Bonjer HJ, Cuesta MA, Go PM, Klinkenberg-Knol EC, van Lanschot JJ, Nadorp JH, Smout AJ, van der Graaf Y. Laparoscopic or conventional Nissen fundoplication for gastro-oesophageal reflux disease: randomised clinical trial. The Netherlands Antireflux Surgery Study Group. Lancet. 2000;355:170-174. [PubMed] |
| 8. | Draaisma WA, Rijnhart-de Jong HG, Broeders IA, Smout AJ, Furnee EJ, Gooszen HG. Five-year subjective and objective results of laparoscopic and conventional Nissen fundoplication: a randomized trial. Ann Surg. 2006;244:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Broeders JA, Rijnhart-de Jong HG, Draaisma WA, Bredenoord AJ, Smout AJ, Gooszen HG. Ten-year outcome of laparoscopic and conventional nissen fundoplication: randomized clinical trial. Ann Surg. 2009;250:698-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Granderath FA, Kamolz T, Schweiger UM, Pasiut M, Wykypiel H, Pointner R. Quality of life and symptomatic outcome three to five years after laparoscopic Toupet fundoplication in gastroesophageal reflux disease patients with impaired esophageal motility. Am J Surg. 2002;183:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Anvari M, Allen C. Surgical outcome in gastro-esophageal reflux disease patients with inadequate response to proton pump inhibitors. Surg Endosc. 2003;17:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Jenkinson AD, Kadirkamanathan SS, Scott SM, Yazaki E, Evans DF. Relationship between symptom response and oesophageal acid exposure after medical and surgical treatment for gastro-oesophageal reflux disease. Br J Surg. 2004;91:1460-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Mahon D, Rhodes M, Decadt B, Hindmarsh A, Lowndes R, Beckingham I, Koo B, Newcombe RG. Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton-pump inhibitors for treatment of chronic gastro-oesophageal reflux. Br J Surg. 2005;92:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Mehta S, Bennett J, Mahon D, Rhodes M. Prospective trial of laparoscopic nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: Seven-year follow-up. J Gastrointest Surg. 2006;10:1312-1316; discussion 1316-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Pessaux P, Arnaud JP, Delattre JF, Meyer C, Baulieux J, Monier H. Laparoscopic antireflux surgery: five-year results and beyond in 1340 patients. Arch Surg. 2005;140:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Ciovica R, Gadenstätter M, Klingler A, Lechner W, Riedl O, Schwab GP. Quality of life in GERD patients: medical treatment versus antireflux surgery. J Gastrointest Surg. 2006;10:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Dallemagne B, Weerts J, Markiewicz S, Dewandre JM, Wahlen C, Monami B, Jehaes C. Clinical results of laparoscopic fundoplication at ten years after surgery. Surg Endosc. 2006;20:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Draaisma WA, Ruurda JP, Scheffer RC, Simmermacher RK, Gooszen HG, Rijnhart-de Jong HG, Buskens E, Broeders IA. Randomized clinical trial of standard laparoscopic versus robot-assisted laparoscopic Nissen fundoplication for gastro-oesophageal reflux disease. Br J Surg. 2006;93:1351-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Zehetner J, Holzinger F, Breuhahn T, Geppert C, Klaiber C. Five-year results of laparoscopic Toupet fundoplication as the primary surgical repair in GERD patients: is it durable? Surg Endosc. 2006;20:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Antoniou SA, Delivorias P, Antoniou GA, Natsiopoulos I, Kalambakas A, Dalenbäck J, Makridis C. Symptom-focused results after laparoscopic fundoplication for refractory gastroesophageal reflux disease--a prospective study. Langenbecks Arch Surg. 2008;393:979-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Brillantino A, Schettino M, Torelli F, Marano L, Porfidia R, Reda G, Grassia M, Braccio B, Di Martino N. Laparoscopic Nissen-Rossetti fundoplication is a safe and effective treatment for both Acid and bile gastroesophageal reflux in patients poorly responsive to proton pump inhibitor. Surg Innov. 2011;18:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Broeders JA, Bredenoord AJ, Hazebroek EJ, Broeders IA, Gooszen HG, Smout AJ. Effects of anti-reflux surgery on weakly acidic reflux and belching. Gut. 2011;60:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Frazzoni M, Conigliaro R, Manta R, Melotti G. Reflux parameters as modified by EsophyX or laparoscopic fundoplication in refractory GERD. Aliment Pharmacol Ther. 2011;34:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1518] [Cited by in RCA: 1653] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 25. | Makris KI, Cassera MA, Kastenmeier AS, Dunst CM, Swanström LL. Postoperative dysphagia is not predictive of long-term failure after laparoscopic antireflux surgery. Surg Endosc. 2012;26:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Galmiche JP, Hatlebakk J, Attwood S, Ell C, Fiocca R, Eklund S, Långström G, Lind T, Lundell L. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA. 2011;305:1969-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 27. | Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:2647-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 28. | Morgenthal CB, Lin E, Shane MD, Hunter JG, Smith CD. Who will fail laparoscopic Nissen fundoplication? Preoperative prediction of long-term outcomes. Surg Endosc. 2007;21:1978-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, Macran S, Kilonzo M, Vale L, Francis J. The effectiveness and cost-effectiveness of minimal access surgery amongst people with gastro-oesophageal reflux disease - a UK collaborative study. The REFLUX trial. Health Technol Assess. 2008;12:1-181, iii-iv. [PubMed] |