Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9476
Revised: March 24, 2014
Accepted: April 5, 2014
Published online: July 28, 2014
Processing time: 267 Days and 16.2 Hours
AIM: To establish an orthotopic mouse model of pancreatic cancer that mimics the pathological features of exocrine pancreatic adenocarcinoma.
METHODS: Pan02 cells were suspended in low-temperature Matrigel and injected into the parenchyma of pancreatic tails of C57BL/6 mice, with cells suspended in phosphate buffered saline (PBS) serving as a control. Primary and implanted tumors were confirmed pathologically. The rate of tumor formation and intraperitoneal implantation in the two groups were compared at different time points after injection. Leakage and intra-abdominal dispersion of Matrigel and PBS, both dyed with methylene blue, were compared after injection into the parenchyma of the pancreas. We observed adherence and proliferation in Pan02 cells suspended in Matrigel in vitro. We also compared the pathological manifestation of this orthotopic pancreatic cancer model in the head and tails of the pancreas. The characteristics of the origin of epithelial cells and exocrine markers of established orthotopic pancreatic tumors were confirmed using immunohistochemistry.
RESULTS: Diluted Matrigel could form a gel drip in the pancreatic parenchyma, effectively preventing leakage from the injection site and avoiding dispersion in the abdominal cavity. Pan02 cells were able to adhere to a dish, proliferate, and migrate in the gel drip. The tumor formation rate in the Matrigel group was 100% at both 2 and 3 wk after injection, whereas it was 25.0% and 37.5% in the PBS group at 2 and 3 wk, respectively (P < 0.05). The intraperitoneal tumor implantation rate was 75.0% in the PBS group after 3 wk of injection, while it was 12.5% in the Matrigel group (P < 0.05). Hepatoduodenal ligament and duodenal invasions with obstructive jaundice and upper digestive obstruction with mesenteric lymph node metastasis were observed in the pancreatic head group. In the pancreatic tail group, spleen and gastric invasion were dominant, leading to retroperitoneal lymph nodes metastasis. Positive immunohistochemical staining of cytokeratin and negative staining of vimentin and chromogranin A confirmed that the orthotopic pancreatic tumor injected with Pan02 cells suspended in Matrigel was of epithelial origin and expressed exocrine markers of cancer.
CONCLUSION: This method of low-temperature Matrigel suspension and injection is effective for establishing an orthotopic mouse model of pancreatic cancer.
Core tip: This article describes a simple and effective method to establish an orthotopic pancreatic cancer model in immunocompetent mice. The orthotopic pancreatic cancer models in the pancreatic head and tail both effectively mimic the relative pathological features of exocrine adenocarcinoma of the human pancreas. This model system will provide a platform for exploring new research outcomes for the effective treatment and management of pancreatic cancer.
- Citation: Jiang YJ, Lee CL, Wang Q, Zhou ZW, Yang F, Jin C, Fu DL. Establishment of an orthotopic pancreatic cancer mouse model: Cells suspended and injected in Matrigel. World J Gastroenterol 2014; 20(28): 9476-9485
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9476.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9476
Pancreatic cancer is one of the most malignant tumors of the gastrointestinal tract. Despite tremendous progress in the treatment of pancreatic cancer over the past two decades, treatment efficacy remains limited duo to late diagnosis, a low surgical resection rate, early development of recurrence and metastasis, and a lack of effective adjuvant options[1-4]. Thus, there is an urgent need for basic research in pancreatic cancer to understand the biological characteristics underlying the genesis of this disease.
Establishing animal models has proved to be an effective pathway for basic research in pancreatic cancer[5]. Among the available animal-based tumor models, the tumor xenograft model is the most widely used due to its susceptibility to forming stable, uniform tumors. The orthotopic transplantation strategy, in which a tumor is transplanted into the corresponding host organ as the primary tumor site, has recently gained popularity. An orthotopic xenograft tumor can simulate a similar microenvironment to that of the primary tumor via a variety of endogenous growth factors that promote tumor growth, invasion, metastasis, and other pathophysiological processes better than subcutaneous or intraperitoneal transplantation[6].
Due to the very thin structure of the mouse pancreas, local injection of a tumor cell suspension will cause high pressure in the local tissue and leakage from the injection site, resulting in intra-abdominal dissemination and a low tumor formation rate. Matrigel is a basement membrane matrix that exists as a liquid at low temperature and polymerizes to form a three-dimensional matrix at room temperature. Thus, Matrigel prevents leakage when injected at a low temperature, as it polymerized in response to the rise in body temperature in vivo. At the same time, cells can still adhere, proliferate, and migrate in Matrigel. In the present study, we established an orthotopic pancreatic carcinoma model in mice that mimics the pathological features of pancreatic cancer in both the head and tail region by injecting Pan02 cells suspended in low-temperature Matrigel.
The murine pancreatic adenocarcinoma cell line Pan02 was purchased from the Frederick National Laboratory for Cancer Research (New York, United States). Culture medium (RPMI 1640) and fetal bovine serum (FBS) were obtained from Gibco (California, United States). Matrigel was purchased from Sigma (Sannois, United States), and 6 to 8-week-old female C57BL/6 mice with a body weight of 18-22 g were obtained from Shanghai Slac Laboratory Animal Co. Ltd. (Shanghai, China).
Cell culture and laboratory animals: Pan02 cells were maintained in RPMI 1640 supplemented with 10% FBS and 2 mmol/L L-Glutamine. Cell cultures were maintained in a humidified incubator at 37 °C with 5% CO2. All laboratory mice were supplied with sterilized food and tap water ad libitum and maintained in a specific pathogen-free environment at a constant temperature and humidity, followed by 12 h light/dark cycles to acclimatize the animals to environmental variation. All of animal study protocols were duly approved by the Institutional Ethics Committee for Animal Care of Shanghai Laboratory Animal Center, Chinese Academy of Sciences.
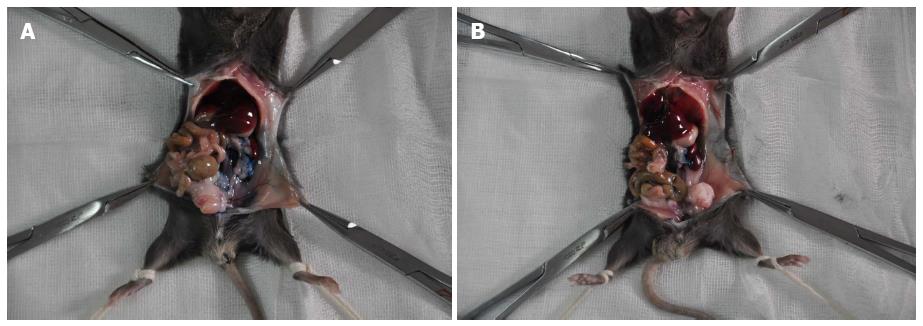
C57BL/6 mice were anesthetized by intraperitoneal injection of 1% pentobarbital (70 mg/kg). After local shaving and disinfection, the abdominal cavity was opened by a 1.5-cm longer longitudinal incision into the left upper quadrant. The tail of the pancreas was identified after the spleen was lifted. Twenty microlitre of ice-cold Matrigel containing methylene blue was then slowly injected into the pancreatic parenchyma using an ice-cold 27-gauge needle and an ice-cold calibrated syringe. To further prevent leakage, the needle was kept on the injection site for 30 s prior to removal. The control mouse was injected with the same amount of ice-cold phosphate buffered saline (PBS) containing methylene blue. Dye leakage from the injection site and contamination to the abdominal cavity was compared.
Subconfluent cultures of Pan02 cells were digested and harvested with 0.25% trypsin, re-suspended, and washed in pre-cooled PBS. The cells were counted and tested for viability using trypan blue exclusion, and cell viability was always > 95%. Thereafter, 1 × 106 cells were resuspended in ice-cold PBS and gently mixed with the same volume of ice-cold Matrigel. Sixty microlitre of the mixture was dripped into 5-cm culture dishes and incubated in a humidified incubator at 37 °C for 15 min. Then, 5 mL of pre-warmed complete medium was added to the cultures. Cell adherence, proliferation, and migration were monitored using an inverted phase-contrast microscope (Nikon).
Subconfluent cultures of Pan02 cells were collected, washed, counted, and tested for viability, as described previously. The cells were resuspended in ice-cold, serum free RPMI 1640 and divided into two groups, with the same volume of ice-cold Matrigel or ice-cold PBS added to one group, respectively. The mixtures were mixed gently to produce a homogeneous suspension.
General anesthesia, shaving, disinfection, abdomen cavity opening, and the identification of the pancreas tail of C57BL/6 mice were carried out as described previously. A total of 1 × 106 viable Pan02 cells were suspended in ice-cold Matrigel or ice-cold PBS in a 20 μL volume and injected to compare local tumor formation and extrapancreatic tumor implantation. The injection technique and highlights were described previously. To ensure that the same injection technique was used for all mice, injections were always carried out by the same person (Yong-Jian Jiang). The spleen and pancreas were placed back into the abdominal cavity, and the abdominal cavity was closed by a running two-layer silk suture. Postoperative status and wound healing were monitored every day for one week. To date, we have not observed any fatality or wound infection related to this surgery.
According to pilot studies in the Matrigel group, a visible tumor appeared 1 wk after injection and death occurred 4 wk after injection. Half of the mice in both groups were sacrificed by ether narcosis and cervical dislocation at 2 and 3 wk after tumor cell injection, respectively. Immediately after sacrifice, a cross incision was made to thoroughly assess the whole abdominal cavity. In the tail of pancreas, suspicious implantation lesions and possible metastasis foci were recorded, harvested, and routinely processed for histological microscopic examination.
The operation procedure was performed as mentioned above, except the incision made in the right upper quadrant. The head of the pancreas was identified after the duodenum was lifted. A total of 1 × 106 viable Pan02 cells suspended in ice-cold Matrigel were injected into the parenchyma of the pancreatic head. The mice were sacrificed, and the abdominal cavity was thoroughly inspected at 2 wk after tumor cell injection, when the mice displayed jaundice and some of them became moribund. Pancreatic head lesions and suspicion metastasis foci were recorded and harvested for pathological examination.
Paraffin embedded samples of orthotopic pancreatic tumor, adjacent pancreas, and spleen were cut into 4-μm sections. Immunohistochemical staining was performed manually using the avidin-biotin complex horseradish-peroxidase method. Primary antibodies against the following antigens were applied according to the manufacturer’s guidelines: cytokeratin (Dako, AE1/AE3, 1:100), vimentin (Dako, V9, 1:200), chromogranin A (Dako, LK2H101, 1:100), and Ki67 (Dako, KiS5, 1:100). Heat-induced antigen-retrieval techniques were used for each antibody. Bound secondary antibodies were visualized with diaminobenzidine (Dako, Copenhagen, Denmark) and counterstained with Harris hematoxylin. Normal pancreatic tissues were used as a positive control, and the primary antibodies were omitted in the negative controls.
The tumor formation rate and implantation rate were expressed as frequencies (%). The Fisher’s exact probability test was performed for comparison, and P values < 0.05 were considered to be significant.
When 20 μL of PBS containing methylene blue dye was injected into the tail of the pancreatic parenchyma, leakage occurred after the needle was pulled out. As depicted in Figure 1A, the dye contaminated the surface of the pancreas, adjacent organs, and lateral abdominal wall, whereas the “Matrigel packet” was formed when the methylene blue stained Matrigel was injected in the same manner, and no leakage occurred after the needle was withdrawn (Figure 1B).
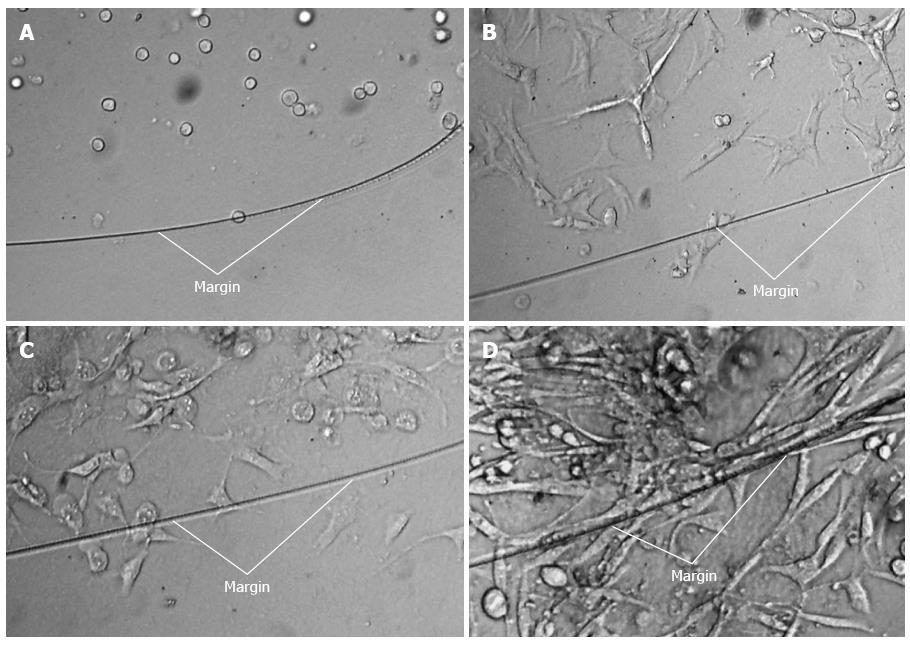
Another important question was whether Pan02 cells could survive, proliferate, and migrate in the Matrigel drip after the Matrigel turned from a watery consistency into a gel-like matrix in response to increased temperature. As shown in Figure 2, the cells adhered to dishes one day after the cells suspended in the Matrigel and cultured in complete medium. After 2 d, the cells proliferated and pierced out of the margin of the Matrigel, and cells proliferated out of the Matrigel within 2 additional days.
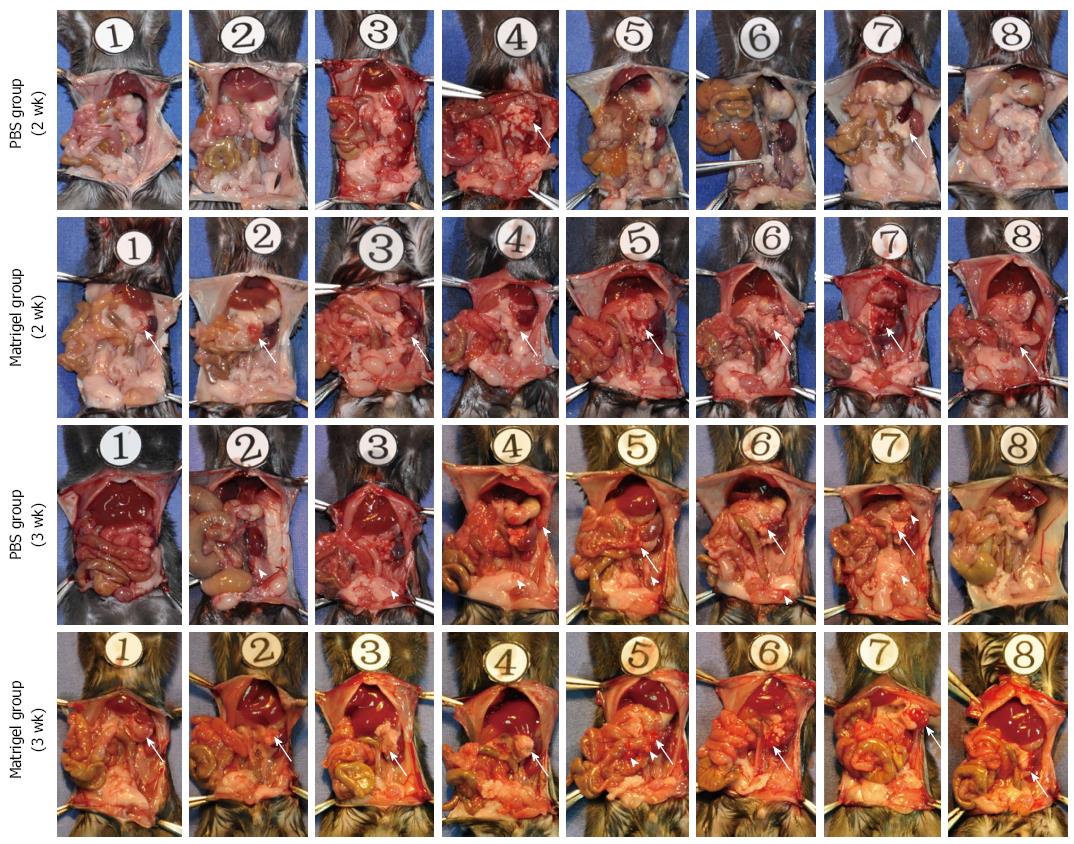
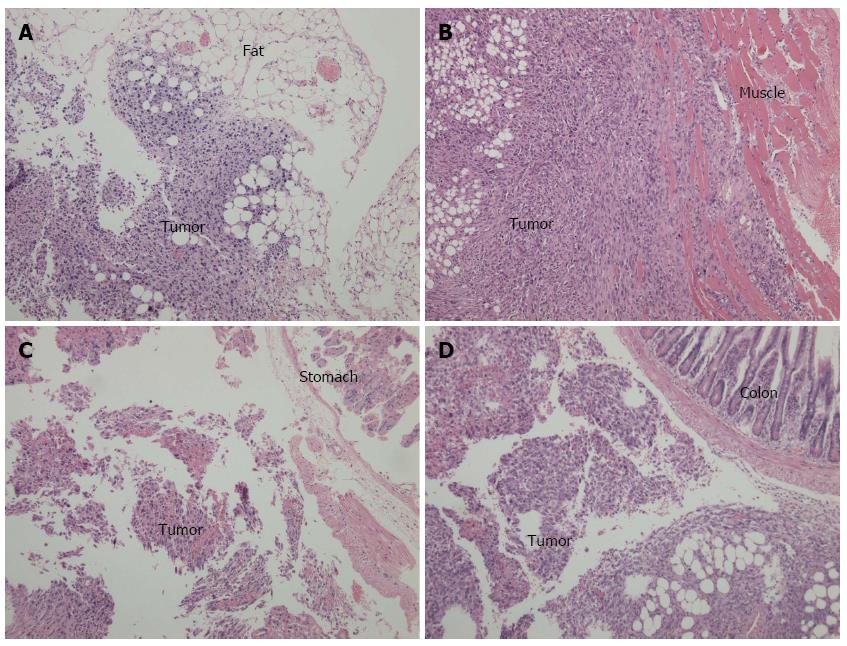
C57BL/6 mice injected in the pancreatic tail with Pan02 cells suspended in Matrigel or PBS were sacrificed at 2 or 3 wk, and tumor formation in the tail of the pancreas and intra-abdominal tumor implantation was assessed. As shown in Figure 3, tumors formed in the tail of the pancreas in all C57BL/6 mice in the Matrigel suspended group. The tumors were approximately 8 mm in diameter at 2 wk and 10 mm in diameter at 3 wk. Intra-abdominal cavity implantation was not observed in the Matrigel group, except in one C57BL/6 mouse at 3 wk after injection. In the PBS group, tumor formation only occurred in 25% of the C57BL/6 mice at 2 wk and 37.5% at 3 wk, which were both significantly less frequent than in the Matrigel group (Table 1). In the PBS group, intra-abdominal tumor implantation was observed at the lateral abdominal wall, stomach wall, colon serosa, and peritoneal omentum (Figure 3), and the incidence of tumor implantation was higher than that of the Matrigel group (Table 1).
| Time after injection | Group | No. of mice | No. of tumor formed mice | Tumor formation rate | P value | No. of tumor implanted mice | Tumor implantation rate (100%) | P value |
| Two weeks | Matrigel group | 8 | 8 | 100.0% | 0.007 | |||
| PBS group | 8 | 2 | 25.0% | |||||
| Three weeks | Matrigel group | 8 | 8 | 100.0% | 0.026 | 1 | 12.5% | 0.041 |
| PBS group | 8 | 3 | 37.5% | 6 | 75.0% |
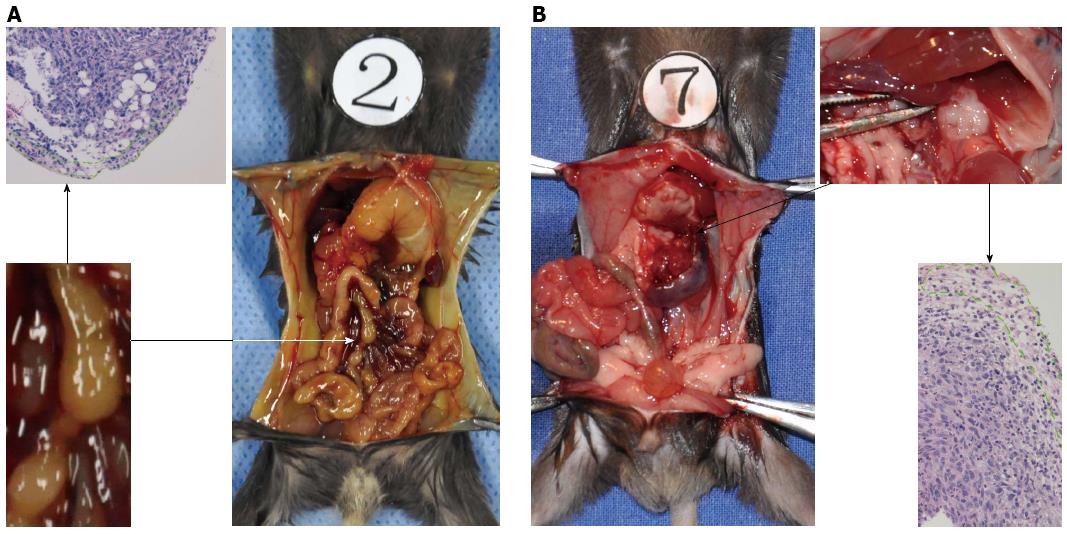
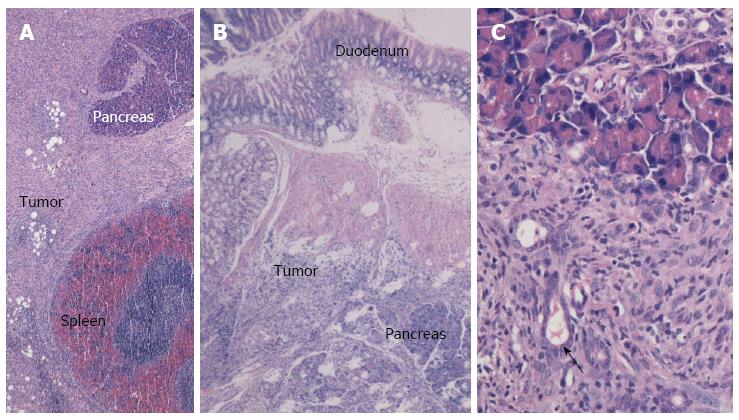
The paw gradually became yellow ten days after injection in the pancreatic head group. Two of the eight mice become moribund four days later. In total, eight mice in the pancreatic head group were sacrificed for exploratory examination. In this group, obstructive jaundice was frequently observed due to obvious parietal peritoneum, viscera yellow-staining, distended gall bladder, and dilated common bile duct (Figure 4). The tumor in the pancreatic head was 6-8 mm × 5-6 mm × 4-5 mm in size. Hepatoduodenal ligaments and duodenum invasion occurred in all eight mice. There was obvious stomach retention in three mice due to duodenal obstruction. No liver metastasis or other distant metastasis was observed, except an enlarged mesenteric lymph node, which was detected in three mice, with pathologically confirmed lymph node metastasis in two mice (Figure 4).
In the pancreatic tail injected group, none of the mice had jaundice or became moribund within 3 wk after injection. The size of the tumors in the pancreas tail was similar to those observed in the pancreas head. Invasion into adjacent organs, such as the spleen, stomach, and colon, was commonly observed. None of the mice with pancreatic tail cancer displayed liver or other distant metastasis. Lymph nodes metastases were pathologically confirmed in three of the eight mice in the 2- and 3- week groups after injection into the pancreatic tail. Pan02 cells tended to metastasize to retroperitoneal lymph nodes alongside the abdominal aorta (Figure 4).
The orthotopic pancreatic lesions and adjacent normal pancreas, duodenum and spleen, as well as suspicious intra-abdominal cavity implantation foci, were routinely collected, fixed in 10% formalin overnight, and sliced into 4-μm sections for hematoxylin-eosin staining. Figure 5A and B display images of the pancreatic tail and head lesions under light microscopy after injection with Pan02 cells suspended in Matrigel. Notably, we observed spindle or oval tumor cells located between the spleen and normal pancreatic tissue (Figure 5A) or tumor cells that invaded the duodenal mucosa (Figure 5B). Most of the tumor cells were arranged in a “weaved” pattern, while several formed duct-like structures (Figure 5C). Moreover, strong infiltration of leukocytes within the tumor area was observed. Tumors implanted in fat tissue, the abdominal wall, the gastric wall, and the colon serosa are shown in Figure 6A-D, respectively.
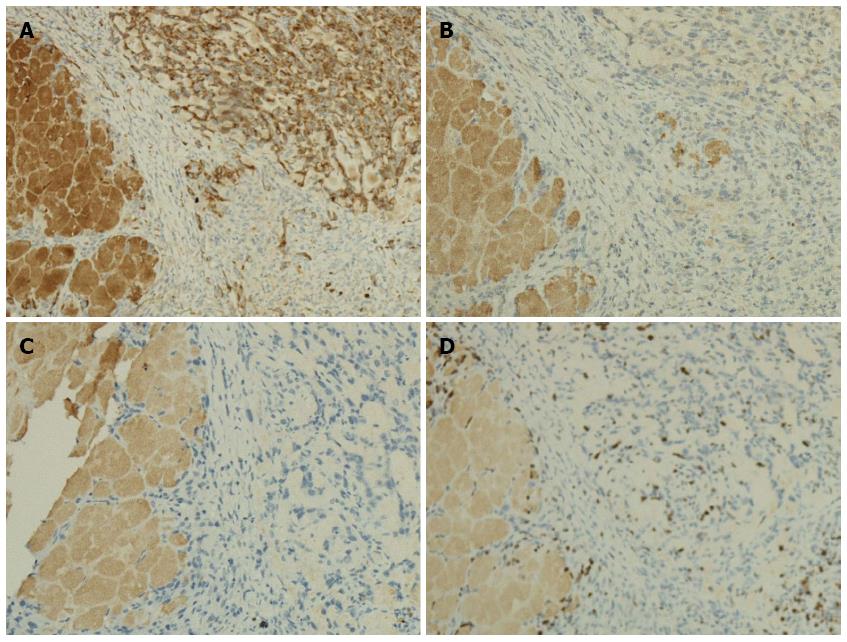
Immunohistochemistry revealed that the tumor cells were positive for cytokeratin and negative for vimentin and chromogranin A (Figure 7A-C). We also evaluated the tumor cell proliferation of the tumor using Ki67, and the percent of Ki67 positive cells in the tumor tissue was approximately 10%, demonstrating the high malignancy of Pan02 tumors (Figure 7D).
Current animal models of pancreatic cancer include xenograft models[7-9], carcinogen-induced models[10-13], and genetically engineered models[14-18]. In the carcinogen-induced model, when pancreatic cancer is induced, hepatocellular carcinoma and other tumors occur simultaneously due to poor specificity of the carcinogen; thereby, the accuracy of using such animal models is limited. A genetically engineered model was recently developed, and it is particularly useful for the study of certain genes in tumorigenesis and tumor progression. However, such models are not suitable for inducing tumors in animals at a large-scale due to their difficult technical requirements, long cycle time, and huge expense.
Xenograft models are extensively applicable for pancreatic cancer research due to their simple operation and short time for tumor formation[19]. There are two main types of xenograft model, subcutaneous transplantation, and orthotopic transplantation. The latter is more relevant, as it simulates local invasion and distant metastasis in a manner similar to human pancreatic cancer, although it is more difficult and complicated than subcutaneous xenograft models.
The use of orthotopic pancreatic cancer models in immunocompetent mice specifically benefits research on the interactions between the tumor and local microenvironment, especially in regard to immune aspects[20,21]. The Pan02 cell lines were established by Corbett et al[22] via injection of 3-methyl-cholanthrene into the pancreas of female C57BL/6 mice. It has been widely used for immunological studies of pancreatic cancer[23-25].
The direct injection method, which involves injection of pancreatic cancer cells directly into the mouse pancreas using surgical exposure and ultrasound guidance, is most commonly applied to establish orthotopic xenograft pancreatic carcinoma. However, the injection of a cell suspension causes high pressure within the local tissue and, because the mouse pancreatic tissue is very thin, the cell suspension can easily extravasate from the injection point (Figure 1A), resulting in reduced tumor formation and increased intra-abdominal tumor implantation. Matrigel is a biological matrix that remains in a liquid state at low temperatures and can form a gel when the temperature rises above 22 °C. Partecke et al[26] used Matrigel as a support to inject pancreatic cancer cell lines to establish orthotopic pancreatic cancer. In this study, we used Matrigel as three-dimensional matrix to contain mouse pancreatic cancer cells for direct injection to establish an orthotopic mouse pancreatic cancer model, prevent injection site leakage effectively, and improve the orthotopic tumor formation rate. In vitro experiments were performed to assess the feasibility of using of Matrigel as a three-dimensional support. In vivo experiments were used to compare the orthotopic tumor formation and intra-abdominal implantation rates. We also compared the difference between pathological characteristics in orthotopic pancreatic tail and head tumors.
In our study, when Matrigel containing methylene blue dye was injected into living tissues at low temperature, it changed from a liquid to a gel state as the local tissue temperature increased, and it effectively prevented leakage from the injection point (Figure 1B). Next, Pan02 cells were mixed with Matrigel at low temperature and then cultured in complete medium after the suspension was heated to form a gelatinous broth. As shown in Figure 2, cells adhered to the culture dish within one day. After 2 d, “pseudopodia” of some cells were observed protruding outside the edges of the Matrigel. After 4 d, the cells beyond the edge of the Matrigel were able to grow and formed a sheet. In vitro co-culture experiments demonstrated that Matrigel encapsulated cells within a short time after they mixed. Moreover, Pan02 cells could still adhere, grow, and migrate after being encapsulated within a Matrigel drip
Based on the two points delineated above, we hypothesized that an orthotopic xenograft tumor would be formed after Pan02 cells suspended in low-temperature Matrigel were injected into the pancreas of C57BL/6 mice. As shown in Figure 3, C57BL/6 mice injected with the suspension of Pan02 cells in low-temperature Matrigel formed tumor foci in the tail of the pancreas with a 100% success rate. The size of tumor foci was approximately 8 mm at 2 wk after implantation, and reached approximately 10 mm after 3 wk. The tumor formation rate of Pan02 cells suspended in PBS was only 25% at 2 wk after injection and 37.5% at 3 wk, significantly lower than those observed in the Matrigel group. Furthermore, in the PBS group, tumor implantation occurred in the abdominal wall, stomach, colon, and left lower quadrant abdominal adipose in some C57BL/6 mice at 3 wk after injection (Figures 3 and 6), and the incidence of intraperitoneal tumor implantation was 62.5%, which was significantly higher than that of the Matrigel group (12.5%). These results support the idea that Pan02 cells suspended in low-temperature Matrigel will effectively prevent leakage from the injection point, improve tumor formation rate, and reduce the risk of intraperitoneal tumor implantation.
The orthotopic cancer models established in the pancreatic head and tail both mimicked the pathological behavior of the human pancreatic carcinoma. For example, duodenal and hepatoduodenal ligaments invasion were prominent in the pancreatic head group, causing upper digestive tract obstruction and obstructive jaundice, respectively, which are commonly present in human pancreatic head cancer patients. Alternatively, spleen, stomach, and colon invasion often occurred in the pancreatic tail group, which is similar to human pancreatic tail cancer. In the pancreatic head group, lymph nodes metastasis often emerged in the mesentery, whereas it tended to undergo metastasis to retroperitoneal lymph nodes in the pancreatic tail group. Next, we further investigated the different lymphatic metastasis pathway of these two groups.
HE staining of tumor sections revealed that spindle or oval-shaped tumor cells were arranged in a weave pattern, indicative of a poorly-differentiated tumor. The positive-cytokeratin and negative-vimentin staining also suggested that the tumors were derived from epithelia, not mesenchyme. The fact that the tumor stained negative for chromogranin A excluded the possibility of a neuroendocrine origin. The few morphological abnormal ducts formed in the tumor tissue also suggest that the tumor was of exocrine origin. The tumor also displayed a high proliferation index (Ki67), suggesting high malignancy. Moreover, this tumor displayed the identical poor-differentiation characteristics and poor-survival observed in C57BL/6 mice after orthotopic implantation of Pan02 cells[26]. The rapid progress and poor survival of the orthotopic pancreatic cancer in C57BL/6 mice markedly simulate that of human pancreatic cancer.
In summary, the method of suspending and injecting tumor cells in low-temperature Matrigel proved to be a reliable method for establishing an orthotopic pancreatic cancer model in mice, demonstrating a high rate of tumor formation and less intraperitoneal tumor dissemination. The orthotopic pancreatic cancer established in both the pancreatic head and tail simulated the pathological manifestation of human pancreatic cancer. The difference in the lymph node metastatic pathway between the two groups requires further study.
Animal models represent promising tools for basic cancer research. In addition to carcinogen-induced models and genetically engineered models, xenograft models have a wide range of application due to their simple operation and short time to tumor formation. In particular, the orthotopic cancer model has gained great respect because it can simulate the local invasion and distant metastasis observed in human pancreatic cancer more effectively than other xenograft models. For pancreatic cancer, it is difficult to establish mouse orthotopic model via direct injection because the mouse pancreas is very thin, and local cells suspension injections cause high pressure within the local tissue, resulting in leakage from the injection site, reduced tumor formation, and intra-abdominal implantation.
Three-dimensional biocompatible materials are alternative choices to suspending or encapsulating cancer cells to overcome the shortcomings of injection site leakage, and directly increase the orthotopic pancreatic cancer formation rate. Matrigel is a basement membrane matrix that exists as a liquid at low temperature and polymerizes to form a three-dimensional matrix at room temperature. Matrigel has been reported to be a cancer cell supporting material for establishing orthotopic mice pancreatic cancer in mice.
In the present study, the authors verified the approach of establishing an orthotopic pancreatic cancer model in C57BL/6 mice by injecting Pan02 cells suspended in low-temperature Matrigel. We thoroughly compared the formation rate and intra-abdominal implantation rates of the Matrigel group and PBS groups. The authors’ data clarify the application of Matrigel for establishing an orthotopic pancreatic cancer model in mice. The poor differentiation and exocrine origin of the tumor were confirmed by HE staining and immunohistochemical methods. The orthotopic pancreatic cancer established in the pancreatic head and tail could mimic the pathological manifestations associated with human pancreatic cancer, including upper digestive tract obstruction, obstructive jaundice, spleen invasion, and stomach invasion. The different lymph node metastatic pathways involved in the pancreatic head and tail groups require further exploration.
This study provides an important foundation for the application of this method in establishing an orthotopic pancreatic cancer model in mice, thus providing a platform for basic research on the experimental treatment of pancreatic cancer.
Matrigel is a gelatinous protein mixture secreted by Engelbreth-Holm-Swarm mouse sarcoma cells. This mixture resembles the complex extracellular environment found in many tissues, and it is usually used as a substrate for culturing cells.
This article reports a simple and effective method for establishing an orthotopic pancreatic cancer model in mice. It provides a new methodology for investigating the effective treatment and management of pancreatic cancer. In the methodology of Matrigel and PBS injection, it is wise to use methylene blue, a dye, to ensure an adequate injection. This manuscript is also well written and easy to follow.
P- Reviewers: Caers J, Hori T, Jo J S- Editor: Wen LL L- Editor: Rutherford A E- Editor: Wang CH
| 1. | Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 628] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 2. | Costello E, Neoptolemos JP. Pancreatic cancer in 2010: new insights for early intervention and detection. Nat Rev Gastroenterol Hepatol. 2011;8:71-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1170] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 4. | Van Laethem JL, Verslype C, Iovanna JL, Michl P, Conroy T, Louvet C, Hammel P, Mitry E, Ducreux M, Maraculla T. New strategies and designs in pancreatic cancer research: consensus guidelines report from a European expert panel. Ann Oncol. 2012;23:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Pérez-Mancera PA, Guerra C, Barbacid M, Tuveson DA. What we have learned about pancreatic cancer from mouse models. Gastroenterology. 2012;142:1079-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Loi M, Di Paolo D, Becherini P, Zorzoli A, Perri P, Carosio R, Cilli M, Ribatti D, Brignole C, Pagnan G. The use of the orthotopic model to validate antivascular therapies for cancer. Int J Dev Biol. 2011;55:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Olive KP, Tuveson DA. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res. 2006;12:5277-5287. [PubMed] |
| 8. | Eibl G, Reber HA. A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer. Pancreas. 2005;31:258-262. [PubMed] |
| 9. | Jimeno A, Feldmann G, Suárez-Gauthier A, Rasheed Z, Solomon A, Zou GM, Rubio-Viqueira B, García-García E, López-Ríos F, Matsui W. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Jimenez RE, Z’graggen K, Hartwig W, Graeme-Cook F, Warshaw AL, Fernandez-del Castillo C. Immunohistochemical characterization of pancreatic tumors induced by dimethylbenzanthracene in rats. Am J Pathol. 1999;154:1223-1229. [PubMed] |
| 11. | Wendt LR, Osvaldt AB, Bersch VP, Schumacher Rde C, Edelweiss MI, Rohde L. Pancreatic intraepithelial neoplasia and ductal adenocarcinoma induced by DMBA in mice: effects of alcohol and caffeine. Acta Cir Bras. 2007;22:202-209. [PubMed] |
| 12. | Mathieu A, Clerc P, Portolan G, Bierkamp C, Lulka H, Pradayrol L, Seva C, Fourmy D, Dufresne M. Transgenic expression of CCK2 receptors sensitizes murine pancreatic acinar cells to carcinogen-induced preneoplastic lesions formation. Int J Cancer. 2005;115:46-54. [PubMed] |
| 13. | Cullingworth J, Hooper ML, Harrison DJ, Mason JO, Sirard C, Patek CE, Clarke AR. Carcinogen-induced pancreatic lesions in the mouse: effect of Smad4 and Apc genotypes. Oncogene. 2002;21:4696-4701. [PubMed] |
| 14. | Tuveson DA, Zhu L, Gopinathan A, Willis NA, Kachatrian L, Grochow R, Pin CL, Mitin NY, Taparowsky EJ, Gimotty PA. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res. 2006;66:242-247. [PubMed] |
| 15. | Kojima K, Vickers SM, Adsay NV, Jhala NC, Kim HG, Schoeb TR, Grizzle WE, Klug CA. Inactivation of Smad4 accelerates Kras(G12D)-mediated pancreatic neoplasia. Cancer Res. 2007;67:8121-8130. [PubMed] |
| 16. | Fendrich V, Schneider R, Maitra A, Jacobsen ID, Opfermann T, Bartsch DK. Detection of precursor lesions of pancreatic adenocarcinoma in PET-CT in a genetically engineered mouse model of pancreatic cancer. Neoplasia. 2011;13:180-186. [PubMed] |
| 17. | Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, Thompson JD, Cheng JH, Bou Reslan H, Ho CC. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol. 2010;28:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Singh M, Couto SS, Forrest WF, Lima A, Cheng JH, Molina R, Long JE, Hamilton P, McNutt A, Kasman I. Anti-VEGF antibody therapy does not promote metastasis in genetically engineered mouse tumour models. J Pathol. 2012;227:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, Shi C, Danenberg K, Danenberg PV, Kuramochi H. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652-4661. [PubMed] |
| 20. | Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 1027] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 21. | Kim HS, Choo YS, Koo T, Bang S, Oh TY, Wen J, Song SY. Enhancement of antitumor immunity of dendritic cells pulsed with heat-treated tumor lysate in murine pancreatic cancer. Immunol Lett. 2006;103:142-148. [PubMed] |
| 22. | Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP, Schabel FM. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717-726. [PubMed] |
| 23. | Jacobs C, Duewell P, Heckelsmiller K, Wei J, Bauernfeind F, Ellermeier J, Kisser U, Bauer CA, Dauer M, Eigler A. An ISCOM vaccine combined with a TLR9 agonist breaks immune evasion mediated by regulatory T cells in an orthotopic model of pancreatic carcinoma. Int J Cancer. 2011;128:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Okudaira K, Hokari R, Tsuzuki Y, Okada Y, Komoto S, Watanabe C, Kurihara C, Kawaguchi A, Nagao S, Azuma M. Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35:741-749. [PubMed] |
| 25. | Bauer C, Bauernfeind F, Sterzik A, Orban M, Schnurr M, Lehr HA, Endres S, Eigler A, Dauer M. Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut. 2007;56:1275-1282. [PubMed] |
| 26. | Partecke LI, Sendler M, Kaeding A, Weiss FU, Mayerle J, Dummer A, Nguyen TD, Albers N, Speerforck S, Lerch MM. A syngeneic orthotopic murine model of pancreatic adenocarcinoma in the C57/BL6 mouse using the Panc02 and 6606PDA cell lines. Eur Surg Res. 2011;47:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |