Published online Jul 7, 2014. doi: 10.3748/wjg.v20.i25.8201
Revised: February 8, 2014
Accepted: April 1, 2014
Published online: July 7, 2014
Processing time: 171 Days and 5.1 Hours
AIM: To explore the alteration of DNA methyltransferase expression in gastric cancer and to assess its prognostic value.
METHODS: From April 2000 to December 2010, 227 men and 73 women with gastric cancer were enrolled in the study. The expression of DNA methyltransferases (DNMTs), including DNMT1, DNMT3a and DNMT3b, in the 300 cases of gastric carcinoma, of which 85 had paired adjacent normal gastric mucus samples, was evaluated by immunohistochemistry using a tissue microarray. Serum anti-Helicobacter pylori (H. pylori) IgG was detected by enzyme-linked immunosorbent assay (ELISA). The relationships between the above results and the clinicopathological characteristics were analyzed. Their prognostic value was evaluated using the Cox proportional hazards model.
RESULTS: In gastric cancer, expression of DNMTs was mainly seen in the nucleus. Weak staining was also observed in the cytoplasm. Expression of DNMT1, DNMT3a and DNMT3b in gastric cancer was significantly higher compared to that in the paired control samples (60.0% vs 37.6%, 61.2% vs 4.7%, and 94.1% vs 71.8%, P < 0.01). The overall survival rate was significantly higher in the DNMT3a negative group than in the DNMT3a positive group in gastric cancer patients (Log-rank test, P = 0.032). No significant correlation was observed between DNMT1 and DNMT3b expression and the overall survival time (Log-rank test, P = 0.289, P = 0.347). Multivariate regression analysis indicated that DNMT3a expression (P = 0.025) and TNM stage (P < 0.001), but not DNMT1 (P = 0.54) or DNMT3b (P = 0.62), were independent prognostic factors in gastric cancer. H. pylori infection did not induce protein expression of DNMTs.
CONCLUSION: The results suggest that expression of DNMT3a is an independent poor prognostic indicator in gastric cancer. DNMT3a might play an important role in gastric carcinogenesis.
Core tip: Up to now, few studies investigated the expression of DNA methyltransferases (DNMTs) and the relationship between their expression and histopathology in gastric cancer. In this article, DNMT1, DNMT3a and DNMT3b expression has been investigated in 307 gastric cancer patients. The results suggest that expression of DNMT3a is an independent poor prognostic indicator in gastric cancer. DNA methylation plays essential roles in the development of gastric cancer.
- Citation: Cao XY, Ma HX, Shang YH, Jin MS, Kong F, Jia ZF, Cao DH, Wang YP, Suo J, Jiang J. DNA methyltransferase3a expression is an independent poor prognostic indicator in gastric cancer. World J Gastroenterol 2014; 20(25): 8201-8208
- URL: https://www.wjgnet.com/1007-9327/full/v20/i25/8201.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i25.8201
Gastric cancer is one of the most common malignancies in Asian countries, and remains the second leading cause of cancer-related death worldwide. Over 70% of new cases and deaths occur in developing countries[1,2]. DNA methylation plays an essential role in normal development and maintenance of tissue-specific gene expression patterns. In human cancer cells, increased CpG island methylation, which mediates tumor suppressor gene silencing, and genomic DNA hypomethylation can lead to genomic instability. Gastric cancer progression involves genes and numerous steps, such as the over-expression of oncogenes and inactivation of tumor suppressor genes[3,4]. In gastric cancer, DNA methylation change is a key contributor to human oncogenesis. Aberrant DNA methylation in the promoter regions of genes, which leads to inactivation of tumor suppressor and other cancer-related genes in cancer cells, is the most well-defined epigenetic hallmark in gastric cancer[5,6]. Several studies have indicated that DNA methylation occurs in the cancerous and para-cancerous areas.
The methylation process is catalyzed by DNA methyltransferases (DNMTs), including DNMT1, DNMT3a, and DNMT3b. With the assistance of DNMTs, methyl groups are transferred to the C5 position of the cytosine and guanine dinucleotides by S-adenosylmethionine[7]. The genetic expression of these regions is inhibited by methylation. DNMTs catalyze the transfer of methyl groups to cytosine and also participate in or maintain methylation. Several studies suggest that DNMT genes are over-expressed in human cancer and during cellular transformation[8-10]. Recently, Kim et al[11] reported that DNMT3A mutations are an independent adverse prognostic factor in younger AML patients with normal cytogenetics. Up to now, few studies have investigated the expression of DNMTs and the relationship between their expression and histopathology in gastric cancer. Ding et al[12] and Yang et al[13] reported the clinical significance of the expression of DNMT proteins. Our previous studies have shown that SNPs of DNMT influenced Helicobacter pylori (H. pylori) infection and gastric atrophy in humans[14]. However, it is still lacking the clinical evidence about the association between expression of DNMT proteins and prognosis of gastric cancer.
It was considered that change of expression of DNMTs accompanies genome-wide hypomethylation and oncogenic hypomethylation or genetic hypermethylation, may leading to tumor suppression. Therefore, the purpose of this study was to evaluate the expression of DNMT1, DNMT3a, and DNMT3b in gastric cancer and their possible predictive relevance in future clinical practice.
From April 2000 to December 2010, 307 patients, including 233 men and 74 women with gastric cancer who underwent surgery at the First Hospital of Jilin University were collected. Finally, 227 men and 73 women patients were enrolled in the study. None of the patients received adjuvant chemotherapy or radiotherapy before the surgical treatment. All specimens obtained after surgery were collected. The pathological diagnosis of gastric cancer was made on the basis of morphologic and immunohistochemical findings by senior pathologists. Adjacent normal gastric epithelial samples were collected from 85 patients as comparison controls. Patients ranged in age from 32 to 87 years, with a median age of 63 years. The study protocol was approved by the Ethics Committee of the First Hospital of Jilin University. Written informed consent was obtained from all of the patients.
The 4 μm thickness sections from the tissue block were chosen for immunohistochemical staining. The sections were deparaffinized and stained using a streptavidin-biotin immunoperoxidase technique. Briefly, the tissue sections were incubated overnight at 4 °C with primary anti-human DNMT1 polyclonal antibody (1:200 diluted, sc-20701, Santa Cruz, United States), DNMT3a polyclonal antibody (1:200 diluted, sc-20703, Santa Cruz), and DNMT3b polyclonal antibody (1:200 diluted, sc-20704, Santa Cruz), respectively. Signals were visualized with 3,3-Diaminobenzidine (DAB) and the slides were counterstained with hematoxylin. As negative controls, the slides were treated with the isotype IgGs to replace primary antibodies, respectively. The stained slides were evaluated by two independent pathologists (MSJ and YPW), who were blinded from clinical data and outcome. The widely accepted HSCORE system was used to assess staining intensity and percentages of cells stained with a specific magnitude of intensity. Briefly, the HSCORE was calculated by the following equation: HSCORE = ∑Pi(i) ( i = 0, 1, 2, 3, Pi = 0%-100%), where i means the intensity of staining, i.e. no staining = 0, weak staining = 1, moderate staining = 2, and strong staining = 3; Pi represents percentages of stained cells with intensities varying from 0 to 100%. The HSCORE ranges from 0 to 300. Furthermore, according to the HSCORE, the expression levels of DNMTs were described as follows: negative: no staining, low: HSCORE ≤ 100, moderate: HSCORE ≤ 200, high: HSCORE > 200.
Blood samples were collected from 101 patients for the examination of H. pylori infection before the surgery, among the total of 300 gastric cancer patients. Anti-H. pylori IgG was detected with an ELISA kit (Biohit, Helsink, Finland)[15]. The antibody titers were defined by optical density values according to the protocol, and titers higher than the cut off value of 30EIU were considered as positive for H. pylori infection.
Statistical software SPSS software package 18.0 (SPSS Inc. United States) was used for all statistical analyses. The expression of DNMTs was presented as median (inter quartile). The Mann-Whitney U test or Kruskal-Wallis H test was performed to comparing independent groups. The Wilcoxon signed rank test was used to compare paired groups. The overall survival rate was estimated by Kaplan-Meier method, and survival differences were analyzed by the log-rank test. The Cox proportional hazards model was used to calculate the hazard ratio (HR) and corresponding 95%CI. For all tests, P < 0.05 was considered statistically significant.
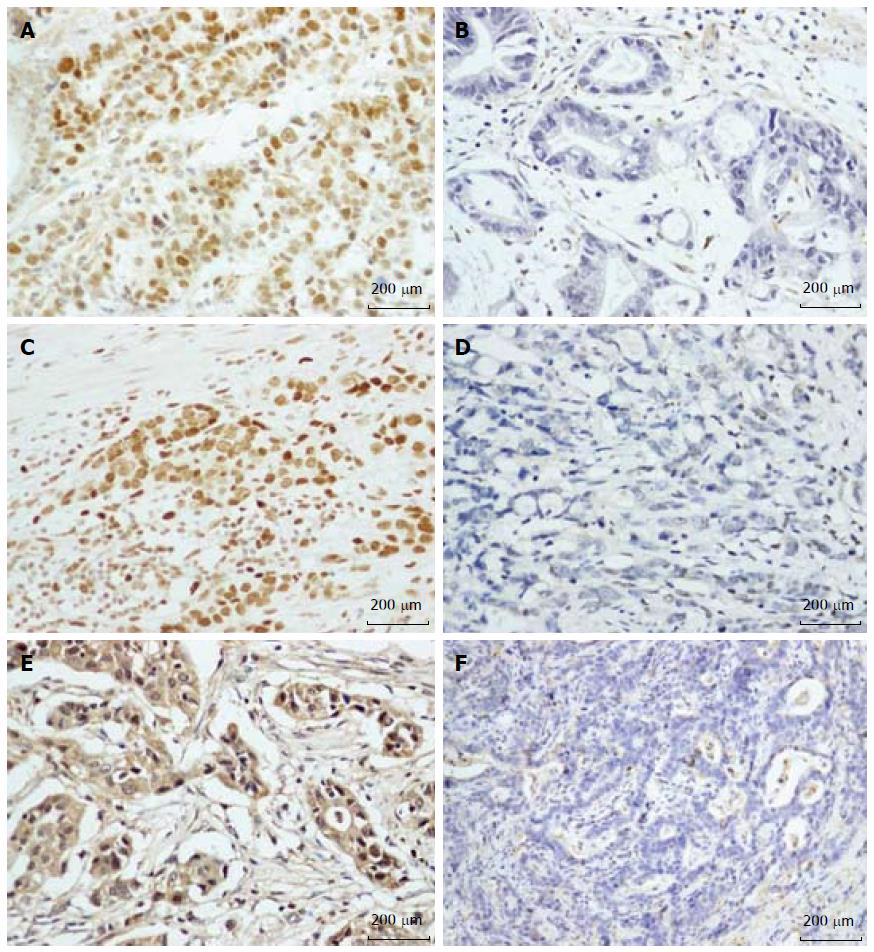
In gastric cancer, expression of DNMTs was mainly seen in the nucleus. Weak staining was also observed in the cytoplasm (Figure 1). All negative controls demonstrated negligible background staining. Among the 85 paired samples, DNMT1, DNMT3a and DNMT3b positive staining were found in 51/85, 52/85, and 80/85 in gastric cancer samples, respectively. They were significantly higher compared to those in the paired control samples (60.0% vs 37.6%, 61.2% vs 4.7%, and 94.1% vs 71.8%, P < 0.001) (Table 1).
| Gastric cancer (n = 85) | Control (n = 85) | P value | H. pylori (+) cancer (n = 67) | H. pylori (-) cancer (n = 34) | P value | |
| DNMT1 | 10 (0-40) | 0 (0-5) | < 0.001 | 20 (0-120) | 50 (5-110) | 0.302 |
| DNMT3a | 80 (5-150) | 0 (0-0) | < 0.001 | 180 (40-210) | 155 (58-240) | 0.859 |
| DNMT3b | 180 (140-240) | 90 (0-240) | < 0.001 | 180 (120-240) | 160 (98-210) | 0.179 |
H. pylori infection was tested in the 101 gastric cancer patients, and the positive rate of H. pylori infection was 66.3% (67/101). However, the analysis results showed no correlations between H. pylori infection and the expression levels of DNMT1, DNMT3a, and DNMT3b (P = 0.302, 0.859, and 0.179, respectively) (Table 1).
Expression of DNMT1 was significantly associated with lymph node metastasis of gastric cancer (P = 0.001). Meanwhile, there were significant higher HSCOREs of DNMT3a staining in patients with lymph-vascular invasion than those without infiltration (P = 0.02). There were significant higher HSCOREs of DNMT3b in patients with poor differentiation compared to those with well and moderate differentiation (P < 0.001). DNMT expression according to age, sex, and tumor differentiation, depth of invasion, lymph node metastasis, and distant metastasis and TNM stage were also analyzed and summarized in Table 2.
| DNMT1 Median (quantile range) | P value | DNMT3a Median (quantile range) | P value | DNMT3b Median (quantile range) | P value | |
| Gender | ||||||
| Male (n = 227) | 10 (0-60) | 0.283 | 90 (0-180) | 0.629 | 210 (120-240) | 0.011 |
| Female (n = 73) | 20 (0-90) | 80 (3-180) | 150 (95-240) | |||
| Age (yr) | ||||||
| ≤ 60 (n = 128) | 10 (0-80) | 0.839 | 60 (1-180) | 0.101 | 160 (90-240) | 0.003 |
| > 60 (n = 172) | 10 (0-60) | 100 (1-210) | 210 (140-240) | |||
| Smoking | ||||||
| Yes (n = 107) | 10 (0-70) | 0.502 | 120 (5-210) | 0.065 | 180 (120-240) | 0.722 |
| No (n = 193) | 10 (0-80) | 60 (0-180) | 180 (120-240) | |||
| Drinking | ||||||
| Yes (n = 72) | 10 (0-80) | 0.570 | 120 (40-233) | 0.003 | 195 (125-240) | 0.777 |
| No (n = 228) | 10 (0-60) | 60 (0-180) | 180 (120-240) | |||
| TNM stage | ||||||
| I (n = 22) | 45 (5-93) | 0.106 | 80 (0-180) | 0.905 | 135 (75-240) | 0.149 |
| II (n = 51) | 20 (0-80) | 60 (5-180) | 160 (90-240) | |||
| III (n = 195) | 10 (0-60) | 90 (5-180) | 210 (140-240) | |||
| IV (n = 32) | 10 (0-35) | 90 (0-180) | 170(90-240) | |||
| Differentiation | ||||||
| Well + moderate (n = 119) | 5 (0-60) | 0.051 | 100 (5-210) | 0.158 | 160 (100-240) | < 0.001 |
| Poor (n = 181) | 20 (0-80) | 80 (0-180) | 240 (150-270) | |||
| Lymph-vascular invasion | ||||||
| Absent (n = 138) | 10 (0-60) | 0.159 | 60 (0-180) | 0.020 | 180 (120-240) | 0.942 |
| Present (n = 162) | 10 (0-80) | 110 (10-210) | 180 (120-240) | |||
| Depth of invasion | ||||||
| T1 (n = 8) | 80 (13-130) | 0.090 | 130 (20-225) | 0.082 | 185 (128-240) | 0.424 |
| T2 (n = 38) | 20 (0-98) | 60 (0-180) | 180 (50-240) | |||
| T3 (n = 223) | 10 (0-70) | 90 (10-210) | 180 (120-240) | |||
| T4 (n = 31) | 10 (0-20) | 40 (0-140) | 180 (120-240) | |||
| Lymph metastasis | ||||||
| N0 (n = 65) | 30 (0-80) | 0.001 | 60 (0-170) | 0.503 | 180 (100-240) | 0.166 |
| N1 (n = 92) | 0 (0-28) | 60 (0-203) | 210 (140-240) | |||
| N2 (n = 78) | 20 (0-93) | 110 (5-188) | 180 (120-240) | |||
| N3 (n = 65) | 20 (0-70) | 90 (15-180) | 180 (145-255) | |||
| Distant metastasis | ||||||
| Negative (n = 265) | 10 (0-80) | 0.938 | 80 (5-180) | 0.679 | 195 (120-240) | 0.285 |
| Positive (n = 35) | 10 (0-60) | 90 (0-210) | 160 (90-240) | |||
| Survival | ||||||
| Survival (n = 180) | 10 (0-80) | 0.656 | 60 (0-180) | 0.009 | 180 (120-240) | 0.441 |
| Death (n = 120) | 10 (0-60) | 120 (13-210) | 210 (120-240) |
Follow-up information was available for all 300 patients, covering periods ranging from 3 to 140 mo (median 41 mo). No patient died of postoperative complications within 30 d of the beginning of the study period, and 120 (40.0%) patients had died during the follow-up. The overall survival time was significantly longer in the DNMT3a negative group than in the DNMT3a positive group (Figure 2B, Log-rank test, P = 0.032). However, expression of DNMT1 and DNMT3b was not significantly associated with survival (Figure 2A, 2C, P = 0.289, P = 0.347). The analysis also showed that TNM stage was significantly related to postoperative survival (P < 0.001).
After adjusted for gender, age, TNM stage and lymph-vascular invasion, patients with DNMT3a positive expression showed a significant difference in risk of gastric cancer-related deaths compared to those who were negative for DNMT3a expression (OR = 1.60, 95%CI: 1.06-2.40, P = 0.025). In multivariate analyses, DNMT3a expression and TNM stage were independent prognostic factors of poor patient survival in gastric cancer. However, expression of DNMT1and DNMT3b was not associated with prognosis of gastric cancer (P = 0.542, 0.620) (Table 3).
| HR (95%CI) | P value1 | |
| DNMT1 expression | ||
| Negative (n = 180) | Reference | 0.542 |
| Positive (n = 120) | 0.89 (0.60-1.31) | |
| DNMT3a expression | ||
| Negative (n = 106) | Reference | 0.025 |
| Positive (n = 194) | 1.60 (1.06-2.40) | |
| DNMT3b expression | ||
| HSCORE ≤ 200 (n = 154) | Reference | 0.620 |
| HSCORE > 200 (n = 146) | 1.17 (0.63-2.18) | |
| Lymph-vascular invasion | ||
| Absent (n = 138) | Reference | 0.130 |
| Present (n = 162) | 1.36 (0.91-2.03) | |
| TNM stage | ||
| I (n = 22) | Reference | |
| II (n = 51) | 1.78 (0.50-6.37) | 0.379 |
| III (n = 195) | 4.11 (1.30-13.05) | 0.016 |
| IV (n = 32) | 14.55 (4.38-48.31) | < 0.001 |
A great number of genes with promoter methylation have been observed in gastric cancer. It is believed that increased expression of DNMTs may contribute to the excessive methylation[16-18]. Recently, the DNMT activity of DNMT1, DNMT3a and DNMT3b has been confirmed. Several studies have focused on the clinicopathology and DNMT expression in human cancers[19-21]. However, the prognostic significance of DNMT expression in gastric carcinoma has not been explored thoroughly.
DNMT1 is a major and best known DNMT, and it can transfers methyl groups from S-adenosylmethionine to cytosines. DNMT1 can interact with the DNMT1-associated protein 1, histone deacetylases 1 and 2 and Rb and repress gene transcription. Etoh et al[22] reported that DNMT1 plays an important role in the development of poorly differentiated gastric cancer, by inducing frequent DNA hypermethylation in multiple CpG islands. Kanai et al[23] reported DNMT1 protein expression and DNA methylation status of CpG islands in tumor-related genes during multistage carcinogenesis of the pancreas. They found that the average number of methylated genes in ductal carcinomas was significantly correlated with DNMT1 protein expression level (P < 0.0093). Mutze et al[24] analyzed DNMT1/3b expression immunohistochemically in 127 pre-therapeutic biopsies from neoadjuvant-treated gastric cancer patients. They found that DNMT1 was a predictive biomarker and potential target for chemotherapy in gastric cancer. In the present study, DNMT1 expression was significantly higher in cancer tissue compared with the paired non-cancer mucosa. However, there was no difference between prognosis and expression levels. The results consist with a previous study[12]. Thus, it can be speculated that DNMT1 protein expression may play an essential effect in the carcinogenesis, but it was not associated with prognosis of gastric cancer.
DNMT3a and DNMT3b do show de novo DNA methylation activity in vitro, and they are responsible for the creation of methylation patterns at an early stage of embryogenesis. In 2008, Ding et al[12] investigated 38 gastric cancer patients and they failed to find the positive association between immunoreactivity of DNMT3a and 3b and clinical parameters in gastric cancer tissues. Yang et al[13] examined expression of DNMT1, DNMT3a and DNMT3b in 54 gastric cancer patients, and they reported that co-expression of DNMT1 and DNMT3a was significantly associated with lymph node metastasis. In this study, we only found expression of DNMT1 was significantly associated with lymph node metastasis. However, DNMT3a expression was significantly associated with lymph-vascular invasion. Furthermore, in the follow-up study, expression of DNMT3a was detected as an independent prognostic marker. The inconsistent results from above studies may be caused by different study subjects and environmental backgrounds.
H. pylori infection is believed to be involved in gastric carcinogenesis, and has also been reported to strongly promote regional DNA hypermethylation. However, in the current study, H. pylori infection did not induce expression of DNMTs. Recently, Huang et al[25] found that there were no significant alterations in the total DNMT activities in mice challenged with H. pylori. Thus, although H. pylori infection alters DNA methylation, it did not induce expression of DNMTs. The mechanism is still unclear and warrants further investigation[26].
Most epigenetic modifications are post-transcriptional, and inhibition of these mechanisms could be advantageous in the treatment of gastric cancer. As a consequence the role of epigenetic regulators like DNMT inhibitors as a treatment for gastric cancer is under evaluation. Niwa et al[27] reported that treatment with 5-aza-dC, a DNA demethylating agent, can prevent H. pylori-induced gastric cancer. The result also suggested that removal of induced DNA methylation and/or suppression of DNA methylation induction can become a target for prevention of chronic inflammation-associated cancer.
In conclusion, aberrant expression of DNMT3a plays a crucial role in gastric carcinogenesis. Expression of DNMT3a was associated with poor survival in gastric carcinoma. This suggested that DNMT3a is clinically useful for prediction of prognosis of gastric cancer, and could be useful as a therapeutic target. Since the function of DNMT3a in gastric carcinogenesis is still unclear, future research is needed[28-32].
DNA methylation mediated by DNA methyltransferases (DNMTs) plays an important role in cancer. Few studies have investigated the relationship between expression of the DNMTs and prognosis in gastric cancer.
Previous studies reported the clinical significance of the expression of DNMT proteins. However, the results were controversial.
In this study, DNMT3a expression was detected as a new independent prognostic marker in gastric cancer.
Expression of DNMT3a was associated with poor survival in gastric cancer. This suggested that DNMT3a is clinically useful for prediction of prognosis of gastric cancer, and it was considered as a potential therapeutic target.
DNA methyltransferases 3a (DNMT3a) is an enzyme that catalyzes the transfer of methyl groups to specific CpG structures in DNA, a process called DNA methylation.
Generally, the authors made a significant research of gastric cancer. With the basic study, the authors demonstrated that DNMT3a, which was a methyltransferase of DNA, was an independent factor of OS in patients with gastric cancer.
P- Reviewer: Liang H S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Sasako M, Inoue M, Lin JT, Khor C, Yang HK, Ohtsu A. Gastric Cancer Working Group report. Jpn J Clin Oncol. 2010;40 Suppl 1:i28-i37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Daniel FI, Cherubini K, Yurgel LS, de Figueiredo MA, Salum FG. The role of epigenetic transcription repression and DNA methyltransferases in cancer. Cancer. 2011;117:677-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Leclerc D, Lévesque N, Cao Y, Deng L, Wu Q, Powell J, Sapienza C, Rozen R. Genes with aberrant expression in murine preneoplastic intestine show epigenetic and expression changes in normal mucosa of colon cancer patients. Cancer Prev Res (Phila). 2013;6:1171-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 5. | Previati M, Manfrini M, Galasso M, Zerbinati C, Palatini J, Gasparini P, Volinia S. Next generation analysis of breast cancer genomes for precision medicine. Cancer Lett. 2013;339:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Chen XY, He QY, Guo MZ. XAF1 is frequently methylated in human esophageal cancer. World J Gastroenterol. 2012;18:2844-2849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Chik F, Szyf M. Effects of specific DNMT gene depletion on cancer cell transformation and breast cancer cell invasion; toward selective DNMT inhibitors. Carcinogenesis. 2011;32:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | He S, Wang F, Yang L, Guo C, Wan R, Ke A, Xu L, Hu G, Xu X, Shen J. Expression of DNMT1 and DNMT3a are regulated by GLI1 in human pancreatic cancer. PLoS One. 2011;6:e27684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Calcagno DQ, Gigek CO, Chen ES, Burbano RR, Smith Mde A. DNA and histone methylation in gastric carcinogenesis. World J Gastroenterol. 2013;19:1182-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 10. | Psofaki V, Kalogera C, Tzambouras N, Stephanou D, Tsianos E, Seferiadis K, Kolios G. Promoter methylation status of hMLH1, MGMT, and CDKN2A/p16 in colorectal adenomas. World J Gastroenterol. 2010;16:3553-3560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Kim SJ, Zhao H, Hardikar S, Singh AK, Goodell MA, Chen T. A DNMT3A mutation common in AML exhibits dominant-negative effects in murine ES cells. Blood. 2013;122:4086-4089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Ding WJ, Fang JY, Chen XY, Peng YS. The expression and clinical significance of DNA methyltransferase proteins in human gastric cancer. Dig Dis Sci. 2008;53:2083-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Yang J, Wei X, Wu Q, Xu Z, Gu D, Jin Y, Shen Y, Huang H, Fan H, Chen J. Clinical significance of the expression of DNA methyltransferase proteins in gastric cancer. Mol Med Rep. 2011;4:1139-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Jiang J, Jia Z, Cao D, Jin MS, Kong F, Suo J, Cao X. Polymorphisms of the DNA methyltransferase 1 associated with reduced risks of Helicobacter pylori infection and increased risks of gastric atrophy. PLoS One. 2012;7:e46058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Cao XY, Jia ZF, Jin MS, Cao DH, Kong F, Suo J, Jiang J. Serum pepsinogen II is a better diagnostic marker in gastric cancer. World J Gastroenterol. 2012;18:7357-7361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Kim H, Park J, Jung Y, Song SH, Han SW, Oh DY, Im SA, Bang YJ, Kim TY. DNA methyltransferase 3-like affects promoter methylation of thymine DNA glycosylase independently of DNMT1 and DNMT3B in cancer cells. Int J Oncol. 2010;36:1563-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8:286-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1515] [Cited by in RCA: 1500] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 18. | Arai E, Nakagawa T, Wakai-Ushijima S, Fujimoto H, Kanai Y. DNA methyltransferase 3B expression is associated with poor outcome of stage I testicular seminoma. Histopathology. 2012;60:E12-E18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Qu Y, Mu G, Wu Y, Dai X, Zhou F, Xu X, Wang Y, Wei F. Overexpression of DNA methyltransferases 1, 3a, and 3b significantly correlates with retinoblastoma tumorigenesis. Am J Clin Pathol. 2010;134:826-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Rahman MM, Qian ZR, Wang EL, Yoshimoto K, Nakasono M, Sultana R, Yoshida T, Hayashi T, Haba R, Ishida M. DNA methyltransferases 1, 3a, and 3b overexpression and clinical significance in gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2010;41:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Gu Y, Yang P, Shao Q, Liu X, Xia S, Zhang M, Xu H, Shao Q. Investigation of the expression patterns and correlation of DNA methyltransferases and class I histone deacetylases in ovarian cancer tissues. Oncol Lett. 2013;5:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Etoh T, Kanai Y, Ushijima S, Nakagawa T, Nakanishi Y, Sasako M, Kitano S, Hirohashi S. Increased DNA methyltransferase 1 (DNMT1) protein expression correlates significantly with poorer tumor differentiation and frequent DNA hypermethylation of multiple CpG islands in gastric cancers. Am J Pathol. 2004;164:689-699. [PubMed] |
| 23. | Kanai Y, Hirohashi S. Alterations of DNA methylation associated with abnormalities of DNA methyltransferases in human cancers during transition from a precancerous to a malignant state. Carcinogenesis. 2007;28:2434-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Mutze K, Langer R, Schumacher F, Becker K, Ott K, Novotny A, Hapfelmeier A, Höfler H, Keller G. DNA methyltransferase 1 as a predictive biomarker and potential therapeutic target for chemotherapy in gastric cancer. Eur J Cancer. 2011;47:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Huang FY, Chan AO, Lo RC, Rashid A, Wong DK, Cho CH, Lai CL, Yuen MF. Characterization of interleukin-1β in Helicobacter pylori-induced gastric inflammation and DNA methylation in interleukin-1 receptor type 1 knockout (IL-1R1(-/-)) mice. Eur J Cancer. 2013;49:2760-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Huang FY, Chan AO, Rashid A, Wong DK, Cho CH, Yuen MF. Helicobacter pylori induces promoter methylation of E-cadherin via interleukin-1β activation of nitric oxide production in gastric cancer cells. Cancer. 2012;118:4969-4980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Niwa T, Toyoda T, Tsukamoto T, Mori A, Tatematsu M, Ushijima T. Prevention of Helicobacter pylori-induced gastric cancers in gerbils by a DNA demethylating agent. Cancer Prev Res (Phila). 2013;6:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Tang H, Deng M, Tang Y, Xie X, Guo J, Kong Y, Ye F, Su Q, Xie X. miR-200b and miR-200c as prognostic factors and mediators of gastric cancer cell progression. Clin Cancer Res. 2013;19:5602-5612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Zhang JJ, Zhu Y, Zhu Y, Wu JL, Liang WB, Zhu R, Xu ZK, Du Q, Miao Y. Association of increased DNA methyltransferase expression with carcinogenesis and poor prognosis in pancreatic ductal adenocarcinoma. Clin Transl Oncol. 2012;14:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Qu Y, Dang S, Hou P. Gene methylation in gastric cancer. Clin Chim Acta. 2013;424:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 31. | Chihara Y, Kanai Y, Fujimoto H, Sugano K, Kawashima K, Liang G, Jones PA, Fujimoto K, Kuniyasu H, Hirao Y. Diagnostic markers of urothelial cancer based on DNA methylation analysis. BMC Cancer. 2013;13:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | He M, Fan J, Jiang R, Tang WX, Wang ZW. Expression of DNMTs and genomic DNA methylation in gastric signet ring cell carcinoma. Mol Med Rep. 2013;8:942-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |