Published online Jun 28, 2014. doi: 10.3748/wjg.v20.i24.7964
Revised: March 27, 2014
Accepted: April 28, 2014
Published online: June 28, 2014
Processing time: 178 Days and 13.9 Hours
AIM: To compare apparent diffusion coefficient (ADC) values on diffusion-weighted imaging (DWI) of hepatic fibrosis patients with those of healthy controls and to identify their correlations with serum indices of liver fibrosis.
METHODS: Hyaluronic acid (HA), laminin (LN), type III procollagen (PCIII), and collagen type IV (IV-C) were measured in 54 hepatic fibrosis patients and 23 normal controls, and ADC values were determined on DWI at different b values (b = 300, 500, 700 s/mm2). Correlations between serum indices and ADC values at different liver fibrosis stages were examined, and each index variation of liver fibrosis in different stages were compared, and correlation analysis of each index and the staging of liver fibrosis carried out, and the correlation of each index performed.
RESULTS: With progressive liver fibrosis, HA, PCIII, and IV-C levels increased (P < 0.01). As the b value increased, the ADC value decreased gradually with the hepatic fibrosis stages. In different groups with b values of 500 s/mm2 and 700 s/mm2, the ADC value decreased significantly as liver fibrosis progressed (P < 0.01). With b values of 500 s/mm2 and 700 s/mm2, there were negative correlations between ADC and LN, PCIII, HA, and IV-C. This pattern was observed only for HA and IV-C at a b value of 300 s/mm2.
CONCLUSION: Serum indices of liver fibrosis and ADC values are useful for diagnosing liver fibrosis, with some correlations among them.
Core tip: In our present study, we aimed to evaluate the effectiveness of diffusion-weighted imaging scanning in cooperation with serological examinations for making the diagnosis of hepatic fibrosis and to examine their correlations in order to aid in the selection and reasonable application of noninvasive techniques to improve the evaluation and diagnosis of hepatic fibrosis.
- Citation: Hu XR, Cui XN, Hu QT, Chen J. Value of MR diffusion imaging in hepatic fibrosis and its correlations with serum indices. World J Gastroenterol 2014; 20(24): 7964-7970
- URL: https://www.wjgnet.com/1007-9327/full/v20/i24/7964.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i24.7964
Chronic liver disease is a common disease with complex causative agents, primarily type B and type C hepatitis virus infections. Hepatic fibrosis is a body repair response to chronic liver injury that can be attributed to a variety of causes[1]. In most cases, chronic liver disease progresses to its ultimate culmination in cirrhosis, and liver cirrhosis and its complications pose a serious global health threat[1-3]. At present, the main diagnostic approaches for liver fibrosis are serology, pathology, and imaging[4,5]. In recent years, instead of only conventional liver biopsy, novel molecular markers and imaging diagnostic techniques have begun to provide accurate evidence for the clinical diagnosis of liver fibrosis, but more sensitive and specific indices of morphology in liver fibrosis have not been identified using traditional imaging methods that reflect anatomical structure[6,7]. Diffusion weighted imaging (DWI) is an advanced application of magnetic resonance imaging (MRI) used in evaluating the microscopic structure of tissues, which relies on quantification of the diffusion of water molecules inside tissues[8,9]. Several publications had indicated the efficacy of quantitative apparent diffusion coefficient (ADC) measurement with this imaging modality in liver fibrosis, especially combining with other noninvasive tests[10,11].
In our present study, we aimed to evaluate the effectiveness of DWI scanning in cooperation with serological examinations for making the diagnosis of hepatic fibrosis and to examine their correlations in order to aid in the selection and reasonable application of noninvasive techniques to improve the evaluation and diagnosis of hepatic fibrosis.
A total of 54 consecutive patients (38 males, 16 females) with hepatitis (49 hepatitis B and 5 hepatitis C) were prospectively enrolled and diagnosed by liver biopsy from September 2008 to October 2010 in our hospital. Their average age was 41.27 ± 11.59 years (range, 19-61 years). The data of a control group consisting of 23 adult volunteers with no evident hepatic pathology (15 males, 8 females; average age 43.28 ± 10.97 years; age range 27-56 years) were also collected. All subjects provided their written, informed consent. This study was approved by IRB from our hospital.
Biopsies of areas of liver fibrosis were obtained under computed tomography (CT) guidance using the following steps. (1) before performing liver puncture, routine blood and coagulation tests were performed, respiratory training was completed, and written informed consent was obtained; (2) patients were placed in the supine position, and the puncture point was located using a Toshiba Xvision/EX spiral CT (Tokyo, Japan), and a surface marker was placed using a self-made positioning grid strip; this was followed by thin layer scanning (with a slice thickness and interstice gap of 3 or 5 mm) to choose the best entry point for the biopsy of a site with a known ADC value; (3) a routine disinfection towel was placed in the area of the puncture point, and under local anesthesia, the liver puncture was performed according to the preset angle and depth of puncture using an 18-G automatic biopsy gun needle (Cook Company, Bloomington, IN, United States). Scanning was repeated to confirm the correct position of the tip, and the patient was asked to hold his breath during quiet breathing, while the liver biopsy was taken, usually involving 2 to 3 draws to obtain tissue strips of greater than 1.0 cm. The specimens were then fixed with 10% formaldehyde; and (4) after the puncture was completed, pressure was applied to the puncture point, and it was bandaged. Local conventional CT scanning was performed, the patient’s vital signs were monitored, and the patient was observed for complications such as bleeding. The liver biopsy specimens were stained with hematoxylin and eosin (HE) and Masson stain.
The classification criteria for liver fibrosis (S0-S4) published in the revised “viral hepatitis prevention program” of the 2000 conference of Xi’an were adopted[12]: (1) S0, no fibrosis; (2) S1, expanding periportal fibrosis, and limited protrusion and expanding intralobular fibrosis; (3) S2, fiber septum formation and lobular body retention around the periportal fibrosis; (4) S3, disordered fiber structure and no liver cirrhosis; and (5) S4, early liver cirrhosis. This classification was commonly used in China, which is similar to the METAVIR system[13].
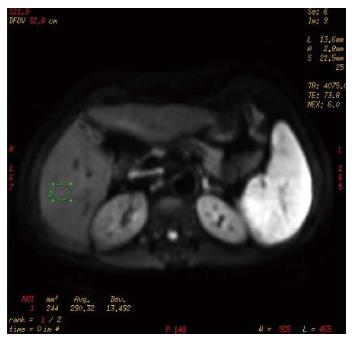
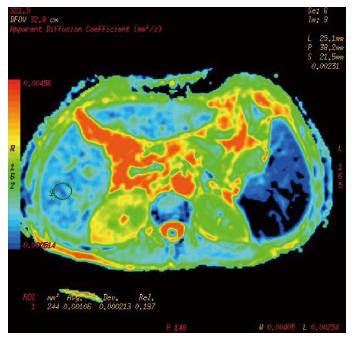
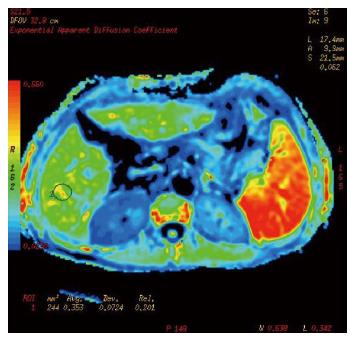
Cases and controls were scanned from the dome of the diaphragm to the liver margin with fat suppression sequences of conventional axial spoiled gradient recalled-T1 weighted imaging (SPGRT1WI) and fast spin echo-T2 weighted imaging (FSET2WI), using a GE Signa HDe 1.5-T MR scanner and body coil (General Electric, Milwaukee, WI, United States). DWI was performed using spin echo-echo planar imaging (SE-EPI) and 3 different b values applied to the X, Y, and Z directions. The scanning parameters were: TR/TE 4075 ms/73.9 ms, FOV 40 × 40, thickness/interstice gap 8 mm/2 mm, array 128 × 128, Next 6, and b values of 300 s/mm2, 500 s/mm2, and 700 s/mm2. The chemical shift artifact was removed with fat saturation (FATSTA), and, during scanning, for each saturated zone in the liver above and below; FATSTA was applied to eliminate the effects of pulmonary gas, gastrointestinal tract gas, the heartbeat, and gastrointestinal peristalsis on image quality. Diffusion weighted imaging (DWI) post-processing was completed using the Functool 2 software belonging to the GE ADW4.3 workstation, and ADC values and pseudo-color pictures of the exponential apparent diffusion coefficient (EADC) were automatically generated by the above software. Three levels in the left and right lobes of the liver were chosen as regions of interest (ROIs), avoiding big blood vessel branching and artifacts. Each ROI was a circle of about 1 cm in diameter. The ADC value of each ROI was obtained directly, and the average value of the measured area was calculated.
All venous blood samples were collected in serum separation tubes after overnight fasting before liver biopsy. Serum values of hyaluronic acid (HA), laminin (LN), type III procollagen (PCIII), and type IV collagen (IV-C) were detected by radio-immunoassay following the manufacturer’s instructions.
All measurement results are expressed as means ± SD. Comparisons between groups for continuous data were made using one-way analysis of variance. Spearman rank correlation analysis was used to test the correlation between the ADC and serum indices of liver fibrosis at different b values. All data were processed using SPSS14.0 statistical analysis software (SPSS, Chicago, IL, United States). A probability value of P < 0.05 was considered statistically significant.
Based on HE and Mason staining of the liver biopsy specimens, the liver fibrosis stage was S0 in 13 patients, S1 in 14, S2 in 9, S3 in 11, and S4 in 7.
Serum HA, PC III, and IV-C values of the cases and controls (Table 1) and the liver fibrosis stages showed significant differences (P < 0.01); LN was not significantly different (F = 2.699, P = 0.0573).
| Stage of liver fibrosis | Cases (n) | Serum indices | |||
| HA (μg/L) | LN (μg/L) | PCIII (μg/L) | IV-C (μg/L) | ||
| Control | 23 | 58.12 ± 13.51 | 96.67 ± 21.53 | 82.63 ± 11.74 | 57.54 ± 19.76 |
| S0 | 13 | 62.72 ± 23.24 | 97.47 ± 23.41 | 87.23 ± 24.17 | 56.59 ± 21.29 |
| S1 | 14 | 84.29 ± 32.96 | 111.89 ± 26.35 | 106.37 ± 25.45 | 61.70 ± 24.83 |
| S2 | 9 | 145.46 ± 87.01 | 115.00 ± 43.14 | 117.41 ± 28.57 | 78.93 ± 46.37 |
| S3 | 11 | 215.36 ± 96.08 | 128.64 ± 44.30 | 149.33 ± 37.11 | 99.65 ± 40.61 |
| S4 | 7 | 300.05 ± 121.95 | 129.94 ± 36.36 | 168.17 ± 34.27 | 131.17 ± 52.51 |
| F | 25.62 | 2.699 | 20.24 | 8.102 | |
| P value | 0.0000 | 0.0573 | 0.0000 | 0.0000 | |
Conventional upper abdominal MR and DWI scanning of 77 cases, including liver fibrosis patients and the control group, was completed, and ADC values and EADC pseudo-color images were obtained (Figures 1, 2 and 3). As the b value increased, the signal to noise ratio (SNR) of the DWI image decreased, deformation of artifacts was aggravated, the liver contour and intrahepatic bile were not clear, the ADC value and the image signal intensity of EADC decreased, and the contrast of the liver and intrahepatic duct system became weak. The ADC values of cases and controls are shown in Table 2. As the b value increased, the ADC values for different stages of hepatic fibrosis in the same group decreased gradually; with b values of 300 s/mm2, 500 s/mm2, and 700 s/mm2 in the different groups, as the liver fibrosis stage increased, the ADC value decreased, and the difference was significant (P < 0.01). With a b value of 300 s/mm2, the ADC value and liver fibrosis stage showed no significant correlation (r = -0.771, P = 0.072). When two groups were compared, with a b value of 300 s/mm2, both S0-S3 and S4 could be distinguished based on the ADC value; with a b value of 500 s/mm2, S2-S4 and S0, S2-S4 and S0, and S1 and S2 could be distinguished; with a b value of 700 s/mm2, S2-S4 and S0, S0 + S1 and S4, S0 + S1 and S3, S2 and S0, and S2 and S1 could be distinguished.
| Stage of liver fibrosis | Cases (n) | ADC value (× 10-3 mm2/s) | ||
| b1 = 300 s/mm2 | b2 = 500 s/mm2 | b3 =700 s/mm2 | ||
| Control | 23 | 1.80 ± 0.34 | 1.69 ± 0.39 | 1.51 ± 0.34 |
| S0 | 13 | 1.93 ± 0.25 | 1.63 ± 0.19 | 1.49 ± 0.11 |
| S1 | 14 | 1.83 ± 0.25 | 1.60 ± 0.17 | 1.43 ± 0.14 |
| S2 | 9 | 1.71 ± 0.27 | 1.44 ± 0.18 | 1.26 ± 0.13 |
| S3 | 11 | 1.79 ± 0.26 | 1.36 ± 0.17 | 1.20 ± 0.13 |
| S4 | 7 | 1.39 ± 0.21 | 1.18 ± 0.16 | 1.15 ± 0.22 |
| F | 3.648 | 5.926 | 5.758 | |
| P | 0.0054 | 0.0001 | 0.0002 | |
When the b value was 300 s/mm2, the ADC value was not correlated with LN and PCIII values, and it was negatively correlated with HA and IV-C values. When the b value was 500 s/mm2 and 700 s/mm2, the ADC value was negatively correlated with LN, PCIII, HA, and IV-C values (Table 3).
| Serum index | b1 = 300 s/mm2 | b2 = 500 s/mm2 | b3 = 700 s/mm2 | |||
| r value | P value | r value | P value | r value | P value | |
| HA | -0.853 | 0.031 | -0.991 | 0.000 | -0.951 | 0.003 |
| LN | -0.686 | 0.132 | -0.926 | 0.008 | -0.956 | 0.003 |
| PCIII | -0.486 | 0.328 | -0.976 | 0.001 | -0.959 | 0.002 |
| IV-C | -0.946 | 0.004 | -0.981 | 0.001 | -0.925 | 0.008 |
Early appearance of the pathological changes of liver fibrosis in chronic liver diseases is related to an imbalance between formation and degradation of liver fibers, resulting in excessive collagen deposition in the liver. There is a middle stage that all chronic liver diseases go through to become cirrhosis and even liver cancer, and there is an early reversible stage of cirrhosis, that, if not treated in time, may progress to become decompensated cirrhosis with the complications of end-stage liver disease[14,15]. The underlying pathological mechanism involves cytokine activation of resting hepatic satellite cells (HSCs), which transforms them into muscle fibroblasts, resulting in excessive proliferation of myofibroblasts, while a large amount of collagen, such as extracellular matrix (ECM), is synthesized in the liver, leading to diffuse and excess ECM within the liver, particularly collagen deposition, since its degradation is inadequate[16]. Early diagnosis and treatment can significantly improve the prognosis, but the diagnosis of liver fibrosis and early cirrhosis and its staging has been a difficult clinical problem. Although liver biopsy is the gold standard of hepatic fibrosis staging and for understanding the extent of liver lesions, in the clinical diagnosis of liver fibrosis and early cirrhosis, comprehensive use of molecular markers and imaging techniques provides an accurate diagnosis without resorting to the traditional technology, and it gives patients with chronic liver disease with fibrosis new hope for the diagnosis, treatment, and rehabilitation[17,18].
Liver fibrosis formation is a very complicated process that includes liver cell apoptosis, mesenchymal cell proliferation, and deposition of type I and type III collagens in the ECM[16,19]. ECM deposition disrupts the normal histological structure of the liver, ultimately leading to cirrhosis. Many studies have shown that the available serum biomarkers, HA, LN, PCIII, and IV-C, which belong to elements of the ECM, can reflect hepatic ECM degradation. Moreover, the values of the serum indices (HA, LN, PCIII, and IV-C) increase gradually as the stage of liver fibrosis progresses, and correlation analyses have shown that HA, PCIII, and IV-C levels have significant positive correlations with the pathological stage of hepatic fibrosis, reflecting the dynamic evolution process of liver fibrosis[20]. HA is the most important glycosaminoglycan component of the ECM, which is secreted by activated HSC cells and enters into the tubular sinus; it directly reflects the degree of HSC cell activity and indirectly reflects liver fibrosis progression[19]. The serum markers in different studies have been shown to fluctuate within a certain range[20-22]. The serum PC III content reflects the degree of type III collagen metabolism and liver fibrosis; IV-C is the major component of the basement membrane, whose synthesis and deposition is increased in liver fibrosis; and LN is a major non-collagen glycoprotein of the ECM, which forms a basement membrane-like structure with IV-C binding and results in capillarization of hepatic sinusoids. The present study demonstrated that, although the LN value increased as liver fibrosis progressed, the changes seen were not statistically significant for the diagnosis of liver fibrosis (P > 0.05). Similar changes may be seen in non-specific markers of liver fibrosis, as these markers also increase in certain autoimmune diseases, kidney diseases, and pulmonary fibrosis.
In recent years, with the development of MR technology, MRI techniques have been used in the detection of liver disease[23]. In particular, use of the DWI technique for abdominal MRI scanning has become useful for the diagnosis of liver fibrosis[10,24]. DWI is a functional imaging method that highlights water molecule diffusion effects for image contrast. It is a new field compared with traditional MRI and detects the state of motion of tissue water molecules, which reflects the structural characteristics of tissue organization by linking the incoherent micro motions of tissue protons or water molecules and the MR signal, since the motions of water molecules differ between living tissue and water. The motion of water in living tissue is limited by biological membranes and macromolecules in body fluids, while water molecules diffuse freely in water. In addition, there are differences in the degree of motion of water molecules in normal tissue and pathological tissue. These differences allow one to evaluate the structure, metabolism, and function of liver fibrosis with DWI. Liver imaging reflects the motions of molecules, including diffusion movement of water molecules and the perfusion function of the microcirculation or capillary network in vivo. Determination of the ADC value is affected by the intracellular and extracellular spaces, and when liver fibrosis develops, increases are seen in the impermeable structures of the cell membrane between cell gaps, nuclear membrane, organelles, cell scaffold structure, and matrix. There is also an expansion of the volume ratio of the intracellular to the extracellular fluid caused by cytotoxic intracellular edema, and intracellular proton movement decreases. The liver fibrosis leads to limitation of water molecule diffusion in the extracellular space, and with progression of fibrosis, the collagen fiber content increases, further limiting proton movement and decreasing the ADC value. Therefore, the choice of the value of the diffusion coefficient (b value) can directly affect the changes in the ADC value. When the b value is low, microcirculation perfusion has a significant effect on the ADC value, increasing the ADC value; as the b value is increased, the effect of perfusion weakens, resulting in the ADC value approaching its true value. However, as the b value is increased, TE prolongation and T2-weighted aggravation and signal strength weakening can affect image quality. The results of the present study demonstrated that the ADC value in different stages of hepatic fibrosis in the same group decreased as the b value increased. When the b value was 300 s/mm2, the correlation between the ADC value and the stage of liver fibrosis was not significant (r = -0.771, P = 0.072), but when the b values were 500 and 700 s/mm2, as liver fibrosis increased, the ADC value decreased, and this was significant (P < 0.01). However, when comparing the two groups when the b value was 700 s/mm2, the ADC value was found to provide more information for staging liver fibrosis; this was consistent with the results of many local and international studies[10,11,25-27]. The present study also showed that, with a b value of 700 s/mm2, the image quality was better, and that, with increasing liver fibrosis limiting the movement of water molecules within the liver, reducing their diffusion, DWI could be used to quantitatively analyze the severity of hepatic fibrosis. This is consistent with the changes that occur during formation of hepatic collagen fibers and reticular fibers, hepatic sinusoid capillarization, and increased deposition of sinus space fibers.
The serum markers of hepatic fibrosis and DWI are both capable of detecting and quantifying liver fibrosis, and there are significant correlations between several markers and the stage of liver fibrosis. In fact, LN, PCIII, HA, IV-C, and the ADC values on DWI were capable of diagnosing liver fibrosis and monitoring its dynamic changes. It was also found that different diagnostic indices based on different diagnostic methods have different significances, but whether there is a correlation with various diagnostic indices or an inherent relationship remains to be determined. Koinuma et al[28] showed a significant relationship between the serum HA value and the ADC value on DWI, but correlations between ADC values and other serum markers have rarely been reported. In the present study, with b values of 300 s/mm2, 500 s/mm2, and 700 s/mm2, negative correlations were seen between the ADC values and HA and IV-C values, demonstrating decreased diffusion in liver tissue as liver fibrosis progressed. With a b value of 300 s/mm2, there were no correlations between the ADC value and LN and PCIII values. However, with b values of 500 and 700 s/mm2, the ADC value and HA and IV-C values showed negative correlations, demonstrating that the main factor affecting DWI is microcirculation perfusion when these b values are selected, and that the ADC value cannot reflect the diffusion characteristics of water molecules in tissue. When liver fibrosis develops, the dysplasia and deposition of ECM and the increased ECM metabolites in the blood increase the serum HA, LN, PCIII, and IV-C values. With more ECM deposition, the barrier structure of the liver extracellular space and the limitation of water molecule motion within the liver reduce diffusion capacity. The correlations between the serum markers of hepatic fibrosis and the ADC value reflect the common pathological and physiological changes that occur with liver fibrosis and the variations in the contents of ECM metabolites.
Hepatic fibrosis is abnormal hyperplasia and deposition of fibrous connective tissue in the liver, and a necessary stage in the progression of various chronic liver diseases to cirrhosis, and the early diagnosis and evaluating severity of liver fibrosis are of vital significance for determining early treatment and prognosis for chronic liver disease. The method of noninvasive assessment for liver fibrosis has become a focus of research for hepatology.
Because of more sensitive and specific indices of imaging methods for liver fibrosis based on various morphology not having been found, image study of liver fibrosis has been the focus of many scholars. In recent years, functional magnetic resonance imaging method has brought hope for early diagnosis of liver fibrosis. Change of structure and metabolic function at the molecular level in liver fibrosis before morphological change of hepatic fibrosis structure was evaluated by detecting change of micro water molecules within the organization with diffusion weighted imaging.
The diagnostic efficacy for serum index of liver fibrosis, diffusion-weighted imaging (DWI) and histopathology of live fibrosis and its correlation was performed with this method, and the degree of correlation between index of serology and DWI were found and its correlation is a reflection of common pathological and physiological basis.
The correlation of serum indices, apparent diffusion coefficient (ADC) value and staging for liver fibrosis was analyzed by determining ADC value with serum index of liver fibrosis and magnetic resonance diffusion weighted imaging and its correlation of each index carried out.
Diffusion weighted magnetic resonance imaging DWI is an imaging method of image contrast highlighting the dispersion effect of water molecules, and ADC is the most commonly used quantitative indictor of DWI imaging, which reflects movement speed of water molecules within tissue.
In this study, liver fibrosis in our country being researched with advanced MR technology, serum hyaluronic acid, type III procollagen, collagen type IV and laminin being compared with it, the degree of liver fibrosis being explored with DWI technology and serological indicators were provided with advanced methods and vital clinical significance. This topic was designed reasonably and complied with ethics; the statistics were correct, and data reliable, and the conclusion objective and true, and had important clinical value.
P- Reviewer: Schmidt CM S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Zhang DN
| 1. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1563] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 2. | Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756-762. [PubMed] |
| 3. | Heidelbaugh JJ, Sherbondy M. Cirrhosis and chronic liver failure: part II. Complications and treatment. Am Fam Physician. 2006;74:767-776. [PubMed] |
| 4. | Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134:1670-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. [PubMed] |
| 6. | Kisseleva T, Brenner DA. Anti-fibrogenic strategies and the regression of fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:305-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] |
| 8. | Laun FB, Fritzsche KH, Kuder TA, Stieltjes B. [Introduction to the basic principles and techniques of diffusion-weighted imaging]. Radiologe. 2011;51:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45:169-184. [PubMed] |
| 10. | Lewin M, Poujol-Robert A, Boëlle PY, Wendum D, Lasnier E, Viallon M, Guéchot J, Hoeffel C, Arrivé L, Tubiana JM. Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology. 2007;46:658-665. [PubMed] |
| 11. | Taouli B, Tolia AJ, Losada M, Babb JS, Chan ES, Bannan MA, Tobias H. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol. 2007;189:799-806. [PubMed] |
| 12. | Institute of Infectious Disease and Parasites of the Chinese Medical Association SOH. Management of viral hepatitis. Zhonghua Chuanranbing Zazhi. 2001;19:56-62. |
| 13. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] |
| 14. | Shah AM, Malhotra A, Kothari S, Baddoura W, Depasquale J, Spira R. Reversal of liver cirrhosis in autoimmune hepatitis. Hepatogastroenterology. 2011;58:2115-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Ismail MH, Pinzani M. Reversal of liver fibrosis. Saudi J Gastroenterol. 2009;15:72-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728-1734. [PubMed] |
| 17. | Taouli B, Ehman RL, Reeder SB. Advanced MRI methods for assessment of chronic liver disease. AJR Am J Roentgenol. 2009;193:14-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Baranova A, Lal P, Birerdinc A, Younossi ZM. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 19. | Acalovschi M. Retraction. Parsian H, Rahimipour A, Nouri M, Somi MH, Qujeq D, Fard MK, Agcheli K. Serum hyaluronic acid and laminin as biomarkers in liver fibrosis. J Gastrointestin Liver Dis 2010; 19 (2): 169-174. J Gastrointestin Liver Dis. 2012;21:118. [PubMed] |
| 20. | Plebani M, Basso D. Non-invasive assessment of chronic liver and gastric diseases. Clin Chim Acta. 2007;381:39-49. [PubMed] |
| 21. | Zheng M, Cai W, Weng H, Liu R. Determination of serum fibrosis indexes in patients with chronic hepatitis and its significance. Chin Med J (Engl). 2003;116:346-349. [PubMed] |
| 22. | Castera L, Bedossa P. How to assess liver fibrosis in chronic hepatitis C: serum markers or transient elastography vs. liver biopsy? Liver Int. 2011;31 Suppl 1:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Faria SC, Ganesan K, Mwangi I, Shiehmorteza M, Viamonte B, Mazhar S, Peterson M, Kono Y, Santillan C, Casola G. MR imaging of liver fibrosis: current state of the art. Radiographics. 2009;29:1615-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Wagner M, Doblas S, Daire JL, Paradis V, Haddad N, Leitão H, Garteiser P, Vilgrain V, Sinkus R, Van Beers BE. Diffusion-weighted MR imaging for the regional characterization of liver tumors. Radiology. 2012;264:464-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Zhu NY, Chen KM, Chai WM, Li WX, Du LJ. Feasibility of diagnosing and staging liver fibrosis with diffusion weighted imaging. Chin Med Sci J. 2008;23:183-186. [PubMed] |
| 26. | Ståhlberg F, Brockstedt S, Thomsen C, Wirestam R. Single-shot diffusion-weighted echo-planar imaging of normal and cirrhotic livers using a phased-array multicoil. Acta Radiol. 1999;40:339. [PubMed] |
| 27. | Fujimoto K, Tonan T, Azuma S, Kage M, Nakashima O, Johkoh T, Hayabuchi N, Okuda K, Kawaguchi T, Sata M. Evaluation of the mean and entropy of apparent diffusion coefficient values in chronic hepatitis C: correlation with pathologic fibrosis stage and inflammatory activity grade. Radiology. 2011;258:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Koinuma M, Ohashi I, Hanafusa K, Shibuya H. Apparent diffusion coefficient measurements with diffusion-weighted magnetic resonance imaging for evaluation of hepatic fibrosis. J Magn Reson Imaging. 2005;22:80-85. [PubMed] |