Published online Jun 28, 2014. doi: 10.3748/wjg.v20.i24.7950
Revised: April 8, 2014
Accepted: April 28, 2014
Published online: June 28, 2014
Processing time: 128 Days and 12.1 Hours
AIM: To determine the significance of enterostomy in the emergency management of Fournier gangrene.
METHODS: The clinical data of 51 patients (49 men and 2 women) with Fournier gangrene who were treated at our hospital over the past 12 years were retrospectively analyzed. The patients were divided into two groups according the surgical technique performed: enterostomy combined with debridement (the enterostomy group, n = 28) or debridement alone (the control group, n = 23). Patients in the enterostomy group received thorough debridement during surgery and adequate local drainage after surgery, as well as administration of broad-spectrum antibiotics. The clinical data and outcomes in both groups were analyzed.
RESULTS: The surgical procedures were successful in both patient groups. In the enterostomy group, 10 (35.8%) patients required skin grafting with a total of six debridement procedures. While in the control group, six (26.1%) patients required four debridement procedures. However, this difference was not statistically significant. Following surgery, the time to normal body temperature (6 d vs 8 d, P < 0.05) and average length of hospital stay (14.3 ± 7.8 d vs 20.1 ± 8.9 d, P < 0.05) were shorter in the enterostomy group. The case fatality rate was lower in the enterostomy group than that in the control group (3.6% vs 21.7%, P < 0.05).
CONCLUSION: Enterostomy can decrease the case fatality rate of patients with Fournier gangrene.
Core tip: Fournier gangrene presents as a severe disease with a high mortality. In this study, 51 patients with Fournier gangrene who received enterostomy (enterostomy group) or not (control group) over the past 12 years were included. Their postoperative recovery and outcomes were compared. Ten patients in the enterostomy group and six patients in the control group required skin grafting. Compared with the control group, the time to normal body temperature and average length of hospital stay were significantly shorter, and the case fatality rate was lower, in the enterostomy group. Enterostomy can decrease the fatality rate of Fournier gangrene.
- Citation: Li YD, Zhu WF, Qiao JJ, Lin JJ. Enterostomy can decrease the mortality of patients with Fournier gangrene. World J Gastroenterol 2014; 20(24): 7950-7954
- URL: https://www.wjgnet.com/1007-9327/full/v20/i24/7950.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i24.7950
Fournier gangrene is a rare necrotizing fasciitis of the perianal, genitourinary and perineal region, characterized by perineal pain and swelling at onset with symptoms of severe systemic toxicity. The condition may also spread to the abdominal and retroperitoneal area, which is rare, but extremely dangerous[1-7]. Despite the improvement in outcomes with recent advances in resuscitation, antibiotics, and anesthesia, combined with adequate surgical debridement, the mortality rates from Fournier’s gangrene are still high, with reported rates of up to 75%[7,8].
In view of the acute onset and rapid progression of this disease, emergency debridement is the basic treatment, although the concurrent use of enterostomy is still controversial[9-12]. To identify the significance of enterostomy in the emergency management of Fournier gangrene, we retrospectively analyzed the clinical data of 51 patients with Fournier gangrene, who underwent emergency surgery at our hospital over the past 12 years, to compare the postoperative recovery and outcomes of patients who received enterostomy and those who did not.
Fifty-one patients (49 men and two women), with an average age of 51.6 years (range: 17-80 years), were admitted to our hospital from January 2002 to January 2013 suffering from Fournier gangrene and were included in this study. Of these patients, 41 had diabetes, 34 had liver cirrhosis, and five had renal failure. The initial infected regions were anal areas in 33 cases, urogenital areas in 12 cases, and unknown areas in seven cases. Fever was present in all patients to varying degrees, with prolonged high fever observed in some patients. Perineal and scrotal swelling was observed, and palpable fluctuations, skin surface darkening and necrosis, and ulceration and pus exudate in the perineal area were found in 33 cases.
Routine blood test, liver and kidney function, electrolytes, blood gas analysis and other laboratory tests were performed, and perineal color Doppler ultrasound was performed to assess testicular blood flow. Computed tomography (CT) scanning was carried out in patients with suspected abdominal and retroperitoneal infections. The Fournier’s Gangrene Severity Index[3] score was determined based on body temperature, heart rate, respiratory rate, serum sodium concentration, serum potassium concentration, serum creatinine, hematocrit, white blood cell count and blood bicarbonate concentration for each patient.
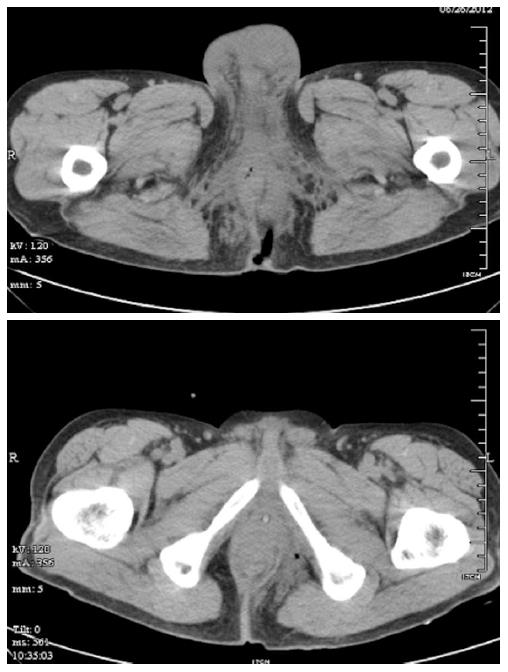
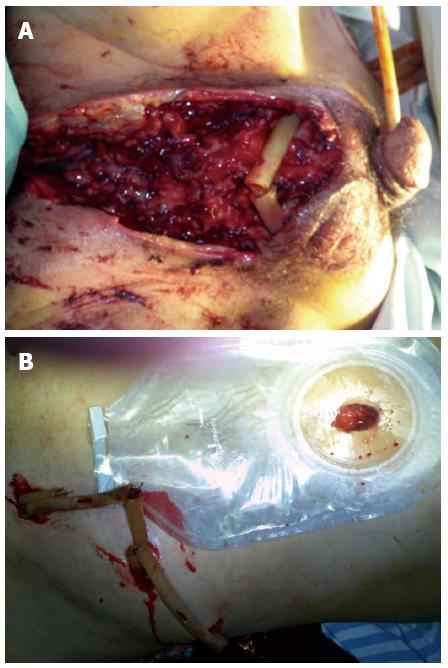
The 51 patients were divided into two groups according to the surgical technique performed: enterostomy combined with debridement (the enterostomy group n = 28), and debridement alone (the control group n = 23). Emergency surgery was performed on each patient following the routine administration of broad-spectrum antibiotics (carbapenems preferred), and an abdominal CT scan (including the perineal area) was performed to identify the extent of inflammation as a guide to surgery (Figure 1). All patients underwent general anesthesia in the lithotomy position. Multiple incisions were made at the lesion sites, with obvious swelling and inflammation detected by CT, to drain the pus, which was collected for bacterial culture. All necrotic fascial tissues and skin, up to the edge of normal structures with adequate blood supply, were removed. Perianal incisions parallel to the anal sphincter were made when testicular exposure was possible, and the testicles were fixed subcutaneously to the upper or lateral skin during the procedure. The surgical wounds were rinsed with plenty of hydrogen peroxide and povidone iodine. A drainage tube was placed in the subcutaneous tunnel between two adjacent incisions for contra-aperture drainage. End-to-end enterostomy was performed in the enterostomy group, with a normal saline flush toward the distal colorectal area during surgery. Postoperatively, the drainage tube was rinsed with iodophor each day. A repeat debridement was performed immediately when inflammation was observed in the surrounding tissues. Antibiotics were given based on drug susceptibility tests. The use of carbapenems was continued until the bacterial culture became negative. Enteral nutrition was administered soon after the surgery.
All data were analyzed using SPSS 16.0 software. Measurement data were presented as means ± SD. Inter-group comparisons were performed using the t test or Fisher’s exact test. A value of P < 0.05 was considered statistically significant.
Of the 51 patients, 28 underwent enterostomy and 23 did not (Table 1). The average FSGI score was 5.4 for all patients, 5.3 for patients in the enterostomy group and 5.6 for patients in the control group (P > 0.05). Diabetes was the most common complication (n = 41); however, there was no statistically significant difference in the incidence of diabetes between the two groups (P > 0.05).
| Enterostomy group (n = 28) | Control group (n = 23) | P value | |
| Gender (M/F) | 27/1 | 21/1 | > 0.05 |
| Age (yr) | 49.3 ± 7.8 | 50.1 ± 8.9 | > 0.05 |
| Disease course (from the onset to surgery) | 5.0 | 4.7 | > 0.05 |
| FGSI score | 5.3 | 5.6 | > 0.05 |
| Predisposing factors | |||
| Diabetes | 22 (78.6) | 19 (82.6) | > 0.05 |
| Cirrhosis | 19 (67.9) | 15 (54.3) | > 0.05 |
| Renal failure | 3 (10.7) | 2 (8.7) | > 0.05 |
| Initially infected regions | |||
| Anal areas | 22 (89.3) | 11 (47.8) | > 0.05 |
| Urogenital areas | 3 (10.7) | 9 (52.2) | > 0.05 |
| Unknown | 3 (10.7) | 3 (13.0) | > 0.05 |
The surgical procedures were successful in both treatment groups (Figure 2). In the enterostomy group, 10 (35.8%) patients required skin grafting with six debridement procedures in total, while in the control group, six (26.1%) patients required four procedures. Following surgery, the time to normal body temperature (6 d vs 8 d, P < 0.05) and average length of hospital stay (14.3 ± 7.8 d vs 20.1 ± 8.9 d, P < 0.05) were significantly shorter in the enterostomy group. Only one death was recorded in the enterostomy group, compared with five in the control group (fatality rate: 3.6% vs 21.7%, P < 0.05, Table 2).
| Enterostomy group (n = 28) | Control group (n = 23) | P value | |
| Cases requiring skin grafting | 10 (35.8) | 6 (26.1) | > 0.05 |
| Cases requiring debridement procedures | > 6 | > 4 | > 0.05 |
| Time to normal body temperature (d) | > 6 | > 8 | > 0.05 |
| Average length of hospital stay (d) | 14.3 ± 7.8 | 20.1 ± 8.9 | > 0.05 |
| Case fatality rate | > 3.6% | > 21.7% | > 0.05 |
According to the bacterial culture results, there were 25 aerobic isolates, including Escherichia. coli, Streptococcus, Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis; and nine anaerobic isolates, including Clostridium perfringens and Bacteroides fragilis. Growth of both types of bacteria was found in eight (15.7%) patients. Bacterial cultures were negative in the specimens from 17 (33.3%) patients.
Although various definitions of Fournier gangrene have been used in previous reports[1,7,8], in the present study, this condition is referred to as necrotizing fasciitis of the perianal, perineal and external genital regions, which constituted a broader area of the perineum, including the urinary, genital and anal areas. Fournier gangrene mainly occurs in 40- to 50-year-old men[13,14]. While primarily originating in the rectum and urogenital areas, some cases can also be derived from subcutaneous infection or local trauma, surgery and infection, with unknown origins of infection in particular cases. A systemic immunocompromised state may exist before the onset of Fournier gangrene, such as diabetes, cirrhosis, renal failure and the use of steroids[10,11]. In the present cohort, 80% of patients had diabetes, which was significantly higher than the incidence reported in previous publications[1,11]. On the other hand, 66.7% of patients had cirrhosis. While the development of Fournier gangrene has been associated with cirrhosis[8-10] and alcohol consumption itself may give rise to alcoholic cirrhosis, the incidence of cirrhosis is significantly lower in other countries compared with China, which could be related to the relatively high incidence of hepatitis in China.
Fournier gangrene is generally the result of infection with a combination of multiple aerobic and anaerobic bacteria[15,16]. The most common strains include Streptococci, Staphylococci, gastrointestinal Streptococcus spp, Bacteroides, Clostridium and Candida. Two or more species of bacteria can often be isolated from a single case. In this study, however, only 15.7% of patients were positive for at least two species, which was lower than the reported rate in other studies; negative specimens accounted for up to 33.3%, which could be related to the early use of broad-spectrum antibiotics.
Our empirical principles for treating Fournier gangrene include: early surgery to open the lesions, reduce tension and eliminate the anaerobic environment; removal of necrotic tissues to minimize absorbable toxins and collect specimens for bacterial culture; topical rinsing with 3% hydrogen peroxide; complete hemostasis of wounds and drainage placement, including both a drainage strip and tube connecting the deep lesions to ensure patent drainage; and postoperative nutritional support and anti-infection treatment, with carbapenems as the drug of choice, which can be adjusted according to the bacterial culture and sensitivity results. Repeat debridement is warranted when necrotic tissue or fluid is found on postoperative ultrasonography or CT.
Although the need for enterostomy for feces diversion remains controversial[10,15,17-26], 54.9% of the present cohort underwent enterostomy in our hospital. The results showed significantly lower mortality in the enterostomy group compared with the control group. Possible explanations for this may include: (1) fecal diversion could help reduce perineal wound contamination during the acute inflammatory phase, thereby bringing the source of infection under control and preventing any further spread; and (2) the nutritional status is essential for containing inflammation. As enterostomy helps reduce wound contamination, the systemic inflammatory response is alleviated and the body burden is thus reduced, enabling more effective support by the provision of early enteral nutrition resulting in rapid improvement of nutritional status. Another advantage of choosing enterostomy is that, when patients survive the acute phase, the diversion results in clean, fresh wounds for large areas requiring skin grafts during the later phases, which will ultimately improve the skin graft survival rate. In our hospital, the principles for performing enterostomy include: (1) a transverse colostomy is always preferred because it yields solid and formed stools that are easily restricted and introduces less contamination to the surrounding skin. On the other hand, an intestinal stoma, which results in liquid stools, could easily lead to dermatitis and cause new infection of the peripheral area in the event of overflow, generating a new source of infection that could aggravate the existing condition; (2) the upper abdomen above the umbilicus is an ideal area for enterostomy because perineal necrotizing fasciitis often affects the lower abdominal wall with non-localized infections that require repeated debridement. With an initial enterostomy in the lower abdominal area, a subsequent debridement series because of extensive inflammation will complicate the situation; and (3) a single stoma is created to achieve complete fecal diversion.
In conclusion, the use of enterostomy in the treatment of Fournier gangrene significantly reduced the mortality of this disease. The limited number of patients included in this study meant that further research with a larger sample size is needed to identify the efficacy of enterostomy in the treatment of Fournier gangrene.
Fournier gangrene is a rare, necrotizing fasciitis of the perianal, genitourinary and perineal region, characterized by perineal pain and swelling at onset, with symptoms of severe systemic toxicity. The condition may also spread to the abdominal and retroperitoneal area, causing soft tissue necrosis and sepsis. Despite recent advances in surgical techniques, critical care and development of newer antibiotics, the mortality rates caused by Fournier’s gangrene are still high, with reported rates of up to 75%. Emergency debridement remains the basic treatment; however, the concurrent use of enterostomy is still controversial.
In the diagnosis of Fournier gangrene, besides clinical examination, radiological examinations are useful to establish the extent of the necrotic process. Treatment of Fournier gangrene involves several modalities. Surgery is necessary for definitive diagnosis and excision of necrotic tissue. Earlier surgical intervention has been associated with reduced mortality. Emergency treatment includes aggressive resuscitation in anticipation of surgery. Early broad-spectrum antibiotics are indicated. Tetanus prophylaxis is indicated if soft-tissue injury is present. Any underlying comorbid conditions must ultimately be addressed, because failure to adequately manage the comorbid conditions may threaten the success of even the most appropriate interventions to resolve the infectious disease.
The mainstay of treatment of Fournier gangrene is aggressive and repeated radical surgical debridement, intravenous antibiotic therapy and, sometimes, intensive care. The need for colostomy diversion and multiple surgical debridement have a significant impact on survival. To identify the significance of enterostomy in the emergency management of Fournier gangrene, the authors retrospectively analyzed clinical data from 51 patients with Fournier gangrene who underwent emergency surgery to compare the postoperative recovery and outcomes of patients who received enterostomy with those who did not.
The use of enterostomy in the treatment of Fournier gangrene significantly reduced the mortality of this disease. Compared with the control group, the time to normal body temperature and average length of hospital stay were significantly shorter, and the case fatality rate was lower in the enterostomy group.
Fournier gangrene is defined as a polymicrobial necrotizing fasciitis of the perineal, perianal or genital areas. The condition may also spread to the abdominal and retroperitoneal area, causing soft tissue necrosis and sepsis.
This article presented surgical methods for the treatment of Fournier’s gangrene with adequate discussion, introduction and references. The subject is important. The article is well written, clear and concise. The conclusions are supported by the results. The article concerns a controversial, yet very important topic. Enterostomy in Fournier’s gangrene still needs further clear evidence of its usefulness.
P- Reviewers: Hrgovic Z, Indrasena B, Ribeiro-Silva A S- Editor: Qi Y L- Editor: Stewart G E- Editor: Zhang DN
| 1. | Yaghan RJ, Al-Jaberi TM, Bani-Hani I. Fournier’s gangrene: changing face of the disease. Dis Colon Rectum. 2000;43:1300-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Paty R, Smith AD. Gangrene and Fournier’s gangrene. Urol Clin North Am. 1992;19:149-162. [PubMed] |
| 4. | Eke N. Fournier’s gangrene: a review of 1726 cases. Br J Surg. 2000;87:718-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 452] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Ullah S, Khan M, Asad Ullah Jan M. Fournier’s gangrene: a dreadful disease. Surgeon. 2009;7:138-142. [PubMed] |
| 6. | Morpurgo E, Galandiuk S. Fournier’s gangrene. Surg Clin North Am. 2002;82:1213-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Kuo CF, Wang WS, Lee CM, Liu CP, Tseng HK. Fournier’s gangrene: ten-year experience in a medical center in northern Taiwan. J Microbiol Immunol Infect. 2007;40:500-506. [PubMed] |
| 8. | Sroczyński M, Sebastian M, Rudnicki J, Sebastian A, Agrawal AK. A complex approach to the treatment of Fournier’s gangrene. Adv Clin Exp Med. 2013;22:131-135. [PubMed] |
| 9. | Estrada O, Martinez I, Del Bas M, Salvans S, Hidalgo LA. Rectal diversion without colostomy in Fournier’s gangrene. Tech Coloproctol. 2009;13:157-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Ozturk E, Sonmez Y, Yilmazlar T. What are the indications for a stoma in Fournier’s gangrene? Colorectal Dis. 2011;13:1044-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Yanar H, Taviloglu K, Ertekin C, Guloglu R, Zorba U, Cabioglu N, Baspinar I. Fournier’s gangrene: risk factors and strategies for management. World J Surg. 2006;30:1750-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Ruiz-Tovar J, Córdoba L, Devesa JM. Prognostic factors in Fournier gangrene. Asian J Surg. 2012;35:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | D’Arena G, Pietrantuono G, Buccino E, Pacifico G, Musto P. Fournier’s Gangrene Complicating Hematologic Malignancies: a Case Report and Review of Licterature. Mediterr J Hematol Infect Dis. 2013;5:e2013067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Sarwar U, Akhtar N. Fournier’s gangrene developing secondary to infected hydrocele: A unique clinical scenario. Urol Ann. 2012;4:131-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Payton S. Infection: Scoring Fournier’s gangrene. Nat Rev Urol. 2012;9:292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Thwaini A, Khan A, Malik A, Cherian J, Barua J, Shergill I, Mammen K. Fournier’s gangrene and its emergency management. Postgrad Med J. 2006;82:516-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Lee SH, Rah DK, Lee WJ. Penoscrotal reconstruction with gracilis muscle flap and internal pudendal artery perforator flap transposition. Urology. 2012;79:1390-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Goyette M. Group A streptococcal necrotizing fasciitis Fournier’s gangrene--Quebec. Can Commun Dis Rep. 1997;23:101-103. [PubMed] |
| 19. | Chen SY, Fu JP, Wang CH, Lee TP, Chen SG. Fournier gangrene: a review of 41 patients and strategies for reconstruction. Ann Plast Surg. 2010;64:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Lee LH, Chalmers R, Muir T. Diagnosis, management and outcomes of necrotising soft tissue infection within a Plastic and Reconstructive Surgery unit. Scott Med J. 2014;59:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Bakari AA, Ali N, Gadam IA, Gali BM, Tahir C, Yawe K, Dahiru AB, Mohammed BS, Wadinga D. Fistula-in-Ano Complicated by Fournier’s Gangrene Our Experience in North-Eastern Region of Nigeria. Niger J Surg. 2013;19:56-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Akilov O, Pompeo A, Sehrt D, Bowlin P, Molina WR, Kim FJ. Early scrotal approximation after hemiscrotectomy in patients with Fournier’s gangrene prevents scrotal reconstruction with skin graft. Can Urol Assoc J. 2013;7:E481-E485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | D’Arena G, Cammarota A, Musto P. Fournier’s gangrene complicating thrombocytopenia treated with steroids. Lancet. 2014;383:1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Lin TY, Ou CH, Tzai TS, Tong YC, Chang CC, Cheng HL, Yang WH, Lin YM. Validation and simplification of Fournier’s gangrene severity index. Int J Urol. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Vyas HG, Kumar A, Bhandari V, Kumar N, Jain A, Kumar R. Prospective evaluation of risk factors for mortality in patients of Fournier’s gangrene: A single center experience. Indian J Urol. 2013;29:161-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Czymek R, Kujath P, Bruch HP, Pfeiffer D, Nebrig M, Seehofer D, Guckelberger O. Treatment, outcome and quality of life after Fournier’s gangrene: a multicentre study. Colorectal Dis. 2013;15:1529-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |