Published online Jun 28, 2014. doi: 10.3748/wjg.v20.i24.7864
Revised: December 26, 2013
Accepted: January 3, 2014
Published online: June 28, 2014
Processing time: 251 Days and 21 Hours
Pancreatic cancer (PC) remains one of the deadliest cancers worldwide, and has a poor, five-year survival rate of 5%. Although complete surgical resection is the only curative therapy for pancreatic cancer, less than 20% of newly-diagnosed patients undergo surgical resection with a curative intent. Due to the lack of early symptoms and the tendency of pancreatic adenocarcinoma to invade adjacent structures or to metastasize at an early stage, many patients with pancreatic cancer already have advanced disease at the time of their diagnosis and, therefore, there is a high mortality rate. To improve the patient survival rate, early detection of PC is critical. The diagnosis of PC relies on computed tomography (CT) and/or magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP), or biopsy or fine-needle aspiration using endoscopic ultrasound (EUS). Although multi-detector row computed tomography currently has a major role in the evaluation of PC, MRI with MRCP facilitates better detection of tumors at an early stage by allowing a comprehensive analysis of the morphological changes of the pancreas parenchyma and pancreatic duct. The diagnosis could be improved using positron emission tomography techniques in special conditions in which CT and EUS are not completely diagnostic. It is essential for clinicians to understand the advantages and disadvantages of the various pancreatic imaging modalities in order to be able to make optimal treatment and management decisions. Our study investigates the current role and innovative techniques of pancreatic imaging focused on the detection of pancreatic cancer.
Core tip: To improve the survival rate of pancreatic cancer, early detection and optimal treatment with various imaging modalities is essential. Our study investigates the current role of pancreatic imaging, including computed tomography (CT), magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography, and biopsy/fine-needle aspiration using endoscopic ultrasound, focused on the pancreatic cancer. This study introduces rapidly-developing novel imaging techniques, including dual energy, low-tube-voltage CT techniques, iterative reconstruction CT algorithms, functional MRI methods, and hybrid positron emission tomography/MR, which are expected to become widely used and to show excellent performance for pancreatic cancer imaging in the near future.
- Citation: Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J Gastroenterol 2014; 20(24): 7864-7877
- URL: https://www.wjgnet.com/1007-9327/full/v20/i24/7864.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i24.7864
Pancreatic cancer is the fourth most common cause of cancer-related mortality worldwide, with an incidence rate equaling that of its mortality rate[1-3]. Whereas there have been great advances in the early detection and treatment of other malignancies such as colorectal cancer, breast cancer, and prostate cancer, the prognosis of pancreatic cancer is still bleak, as the five-year survival rate is less than 5% and the mortality rate has not declined over the last few decades[4,5]. Therefore, pancreatic cancer seems to remain one of the greatest challenges in the fight against cancer in the 21st century[6]. One of the main causes of the poor prognosis of pancreatic cancer is the difficulty of its early diagnosis. As pancreatic cancer typically develops with few symptoms in the early stage and there are not many specific, well-known risk factors aside from smoking and family history, the appropriate screening and early diagnosis of pancreatic cancer is quite challenging[7]. Therefore, only 10% to 20% of diagnosed patients have a chance of successful resection and possible cure, and even in patients with resectable disease, the survival rate is only 23%[3].
Despite the numerous obstacles detailed above, there is a continued effort to achieve early detection and to make the appropriate selection of surgical candidates with pancreatic cancer[8-12]. Furthermore, currently available pancreatic imaging has a key role in the characterization of pancreatic focal lesions, initial staging, surgical and therapeutic planning, and assessment of the treatment response using various imaging modalities, including ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and endoscopic ultrasonography (EUS)[8-18]. Multi-detector row computed tomography (MDCT) has a major role in the diagnosis and staging of pancreatic malignancies. MDCT of the pancreas is favorably complemented by EUS, which is more sensitive for the early detection of lesions, and allows relatively easy access to the pancreas for tissue diagnosis using fine-needle aspiration (FNA), as well as providing further important information for use in tumor staging[19].
MRI with magnetic resonance cholangiopancreatography (MRCP) and PET scanning can also have a successful role as a secondary imaging modality under special circumstances when CT and EUS are not diagnostic. Our study provides an overview of the current role and innovative techniques of pancreatic imaging for the detection and treatment of pancreatic cancer.
Although the average survival time of patients resected for PC is approximately 12 to 20 mo, and there is a high probability of relapse due to the highly adverse and aggressive nature of the evolving disease, the primary treatment offering the greatest potential for cure is the complete, curative, surgical resectioning of the primary carcinoma[20,21]. As surgical and oncological treatments for pancreatic cancer have continually become more aggressive and sophisticated, the role of imaging has become more important, not only for initial diagnosis and staging, but also for determining both the resectability and the optimal treatment monitoring of pancreatic cancer[16,17,22]. MDCT is currently the worldwide imaging modality of choice for evaluation of pancreatic cancer, although ultrasonography, endoscopic US, contrast-enhanced US, and MRI with MRCP provide complementary, sometimes even more detailed, information[10]. Each imaging modality has both its advantages and disadvantages according to the four, different aspects regarding pancreatic cancer imaging evaluation: (1) identification of the primary tumor; (2) local tumor resectability; (3) distant metastasis; and (4) treatment monitoring.
US is frequently the first-line diagnostic tool for patients presenting with jaundice or abdominal pain, as it is a non-invasive and cost-effective modality. A hypoechoic mass, dilatation of the pancreatic duct, and dilatation of the bile duct are typical imaging features of pancreatic head tumor when seen on US. However, in cases of pancreatic body and tail cancers, tumor detection is quite difficult due to the lack of biliary dilatation and the presence of gas bubbles in the stomach and transverse colon, which cause posterior shadowing. In this situation, oral administration of water or other contrast agents may help to delineate the entire organ. The sensitivity and accuracy of pancreatic US is also highly dependent on the operator’s experience, the degree of disease progression, and the body habitus of patients. For these reasons, the US sensitivity for detecting pancreatic cancer is controversial and has been reported as anywhere between 50%-90%[9,15,23-25]. Using US without contrast media, it is difficult to differentiate pancreatic cancer from other focal lesions, such as neuroendocrine tumor or chronic pancreatitis, as they show the same imaging features on conventional US. Overall, transabdominal US is an acceptable first-imaging method, although not a reliable method for a confident diagnosis or the exclusion of small pancreatic tumors, which are the only ones with even a slight chance for a cure[26].
In many medical institutions, MDCT is routinely used as the most important pre-operative examination in patients with suspected pancreatic cancer, as it has good spatial and temporal resolution with wide anatomic coverage, and thus permits both comprehensive local and distant disease assessment during a single session[10]. In particular, among the cross-sectional imaging modalities, MDCT has shown the best performance for the evaluation of vascular involvement, which is the most important factor for predicting the tumor resectability[27-33]. The reported positive predictive value, sensitivity, and specificity for predicting the resectability of pancreatic cancer were 89%, 100%, and 72%, respectively[34]. In terms of treatment monitoring following chemotherapy or surgery, MDCT is the primary imaging modality, and is used in conjunction with PET/CT[14,18]. However, MDCT may not depict small metastases to the liver or peritoneum[30], or even a primary pancreatic tumor showing isoattenuation[35].
As EUS offers excellent visualization of the pancreas from the duodenum or stomach and can produce high-resolution images of the pancreas, it has been considered one of the most accurate methods for the detection of pancreatic focal lesions, especially in patients with small tumors of 3 cm or less[36,37]. EUS also has the unique ability to obtain specimens for histopathological diagnosis using EUS-guided FNA. Since its early introduction in the early 1990s, EUS-FNA has emerged as a safe and accurate imaging technique for tissue diagnosis in patients with pancreaticobiliary disorders, particularly those with diagnosed pancreatic cancer. Furthermore, EUS-FNA has replaced endoscopic retrograde cholangiopancreatography (ERCP) with brush cytology as the endoscopic test of choice for tissue acquisition due to its higher success rates and decreased risk of post-procedural complications, especially in patients without obstructive jaundice[38]. Although EUS alone has shown slightly disappointing accuracy for differentiating pancreatic cancer from chronic pancreatitis (i.e., 76% for malignancy and 46% for focal inflammation[37]), the reported sensitivity and accuracy of conjoined EUS-FNA for detecting pancreatic malignancy usually exceeds 90%[39-44]. According to a recent meta-analysis covering the years between 1995 and 2008, the pooled sensitivity and specificity of EUS-FNA were 86.8% and 95.8%, respectively, for diagnosing a solid pancreatic mass[38].
In order to improve diagnostic accuracy of EUS, contrast-enhanced EUS and EUS elastography are valuable new techniques to be considered. By administrating micro-bubble agents, the diagnostic accuracy of EUS can be as high as 82% for pancreatic adenocarcinoma[45]. EUS elastography, one of the most recent advances in gastrointestinal endoscopy, is a non-invasive technique that measures tissue elasticity in real time using a dedicated probe and system. A number of recent investigations have shown promising results of EUS elastography for diagnosing pancreatic focal lesions[46-49].
Over the past few years, MRI scanners and imaging techniques have become more sophisticated, resulting in improvements in both imaging quality and diagnostic accuracy. Therefore, MRI with MRCP is currently used as a problem-solving tool for patients with pancreatic disease[50]. Given the greater soft-tissue contrast of MRI compared with that of CT, there are several specific situations in which MRI is superior to CT: small tumors, hypertrophied pancreatic head, isoattenuating pancreatic cancer, and focal fatty infiltration of the parenchyma[17]. Therefore, MRI has been proven to be outstanding for characterizing pancreatic masses. MRCP is also a very successful and classical MR technique for non-invasively delineating the pancreatic ductal system, as well as a valuable alternative to ERCP[51]. MRCP is also very useful for detecting subtle ductal narrowing that may suggest the presence of a small mass. Moreover, MRCP is very useful for delineating the presence of stones as an alternative cause of biliary or pancreatic ductal dilatation[17,52,53]. Although MDCT currently has a major role in the evaluation of PC, MRI with MRCP allows more successful tumor detection at an early stage by allowing a comprehensive analysis of the morphological changes of the pancreas parenchyma, as well as that of the pancreatic duct[20].
PET/CT is an established molecular imaging modality, with fluorine 18-fluorodeoxyglucose (FDG), a glucose analogue, being the most widely used radiotracer[14,54]. The reported sensitivity and specificity of FDG-PET for the depiction of pancreatic cancer are 46%-71% and 63%-100%, respectively[55]. However, FDG-PET is more sensitive for treatment monitoring following chemoradiotherapy and for depicting tumor recurrence after resection than MDCT[22,56-59]. Its wide anatomic coverage, which allows the depiction of all possible evidence of metastases in the entire body, is one of the advantages of PET/CT[18], while its inherently low spatial resolution and false-positive results caused by normal physiologic FDG uptake are its well-known limitations[60,61].
In our institution, EUS and PET/CT are not performed by radiologists. Therefore, we do not deal with the technical protocols of EUS and PET/CT in this section.
US examination of the pancreas is performed following a minimum fast of 6 h. The purposes of the fast are to improve visualization of the pancreas, limit bowel gas, and ensure an empty stomach. US scan plans include transvers, longitudinal, and oblique scans along the pancreatic duct. Bowel gas can be displaced by moving the transducer and applying compression when necessary. To obtain complete visualization of all the portions of the pancreatic gland it is possible, and sometimes convenient, to employ different scanning techniques, such as filling the stomach with water, examining the patient with suspended inspiration or expiration, and changing the patient’s position to erect, supine, and left and right decubitus. If the pancreas is poorly visualized, the water technique, using 100 to 300 mL of water through a straw, may be helpful[62].
A pancreas-specific protocol for pancreatic cancer typically utilizes a thin-section, multi-phase technique with pre-contrast images and early arterial phase (CT angiography phase) images of the aorta and the superior mesenteric artery (17-25 s after the start of contrast injection), pancreatic phase (35-50 s after the start of contrast injection), and portal venous phase images (55-70 s after the start of contrast injection). Pancreatic phase images show peak pancreatic parenchymal enhancement, and therefore provide the best lesion to pancreas contrast. Portal phase images are helpful to assess the extent of venous involvement and to identify possible liver metastases[34,63-66]. The bolus tracking technique is currently routinely used to adjust for variations in the cardiac circulation time[34]. With regard to post-processing, a variety of techniques have been described for pancreatic imaging. The most commonly-used techniques are multiplanar reformations (MPR), curved multiplanar reformations (CMPR), and minimum intensity projections (MinIP)[65,67]. Oblique coronal or sagittal MPR and CMPR along the pancreatic duct can clearly demonstrate the relationship between tumors and the pancreatic duct or adjacent major structures. MinIP images use the lowest density values along each ray and clearly show low-density structures such as pancreatic and bile ducts. The recommended MinIP slab thickness is 3 mm for the pancreatic duct[65,66,68]. Maximum intensity projections are also often used to evaluate the relationship between tumors and adjacent, enhanced vessels.
In our medical institution at the time of our study, quadruple-phase CT images were obtained according to our biliary-pancreas protocol. First, a baseline, unenhanced scan was obtained from the hepatic dome to the third portion of the duodenum. After unenhanced scanning, patients received 1.5 mL/kg of iopromide (Ultravist 370; Schering, Berlin, Germany) intravenously for 30 s using a power injector at a rate of 3-5 mL/s. Triple-phase, dynamic CT scans were then obtained. The scanning delay for the arterial, pancreatic, and portal-venous phases was approximately 25 s, 40 s, and 70 s, respectively, after the initiation of the contrast injection. For MDCT scanners, a bolus-tracking method was used. After reaching an enhancement of up to 100 Hounsfield units in the descending aorta, as measured using the bolus-tracking technique, the scanning delay for the arterial phase was 5-6 s for all MDCT scanners. The scanning delay for the pancreatic phase was 19-22 s and that for the portal-venous phase was 52-65 s following contrast injection. The time required to reach 100 Hounsfield units in the descending aorta ranged from 18 to 23 s. For clinical interpretation, the CT images were reconstructed with a slice thickness of 2.5-3.0 mm and a reconstruction interval of 1.5-2 mm for MDCT[69]. The minimum technical specifications for MDCT of the pancreas are summarized in Table 1.
| Feature | Specification | Comment |
| Scanner type | Multi-detector row scanner | |
| Detector type | Minimum of four detector rows | |
| Reconstructed slice thickness | Minimum of 5 mm | Thinner slices are preferable, especially in multiplanar reconstructions (MPR) |
| Injector | Power injector, preferably dual-chamber | Bolus tracking desirable |
| Contrast injection rate | No less than 3 mL/s of contrast, | A saline flush desirable |
| 300 mg I/mL or a higher concentration, | ||
| For a dose of 1.5 mL/kg of body weight | ||
| Mandatory dynamic phases | 1. Early arterial phase | MPR, |
| 2. Pancreatic phase | Curved MPR along the pancreatic duct, | |
| 3. Portal venous phase | Minimum intensity projections are helpful |
In many medical institutions, patients fast for four to six hours before MRI examination so that the gallbladder is distended and the signal from the overlying stomach and duodenum is decreased. For full evaluation of the pancreatic parenchyma and the pancreaticobiliary ductal system, obtaining the following MR sequences is recommended[50]: T1-weighted gradient-echo; T2-weighted axial and coronal sequences, usually turbo spin-echo; two dimensional (2D) and three dimensional (3D) MRCP; and T1-weighted 3D gradient-echo (GRE) before and after intravenous administration of gadolinium. Diffusion-weighted imaging (DWI) is currently becoming an increasingly used, optional sequence for the detection and characterization of pancreatic lesions[70]. The minimum technical specifications for MRI of the pancreas are summarized in Table 2. In our clinical practice at the time of our study, unenhanced T2-weighted images are usually obtained using a single-shot, fast SE sequence or a half-Fourier rapid acquisition with relaxation enhancement sequence. Unenhanced T1-weighted images are commonly acquired using in-phase and opposed-phase spoiled GRE (T1-weighted, dual-echo GRE) techniques. In addition, the following three MR cholangiographic methods were used to evaluate biliary anatomy: (1) the breath-hold, single-section, rapid acquisition with relaxation enhancement technique with fast or turbo SE sequences; (2) the breath-hold, multisection, single-shot, fast SE or half-Fourier rapid acquisition with relaxation enhancement technique; and (3) the respiratory-triggered, 3D, fast SE technique. Dynamic images were obtained using one of two fat-suppressed, 3D GRE sequences (i.e., LAVA [liver acquisition with volume acceleration], GE Medical Systems and VIBE [volume interpolation with breath-hold examination], Siemens Medical Solutions) before and after the administration of gadolinium-based contrast agents (Gd-BT-DO3A, Gadovist, Bayer Schering Pharma AG, Berlin, Germany) at a dose of 0.1 mmol per kilogram of body weight and with an injection rate of 1.5-2 mL/s (injection duration approximately 5-8 s). The arterial phase images were obtained five seconds after the gadolinium-containing bolus was detected in the abdominal aorta. Acquisition of 3D LAVA or VIBE data for each phase was completed during a single breath-hold at the end of expiration (mean time, 20 s; range, 18-21 s). Arterial, portal venous, and equilibrium phase images were obtained approximately 20-40 s, 45-65 s, and 3-5 min, respectively, after injection of the contrast agent. An additional, fat-suppressed LAVA or VIBE sequence was performed two minutes after the contrast-agent injection (between the portal venous and equilibrium phases) on the coronal plane and parallel to the portal vein bifurcation[71,72].
| Feature | Specification | Comment |
| Scanner type | 1.5-T or greater main magnetic field | Low-field magnets not suitable |
| Coil type | Phased-array, multichannel torso coil | Unless patient-related factors preclude the use |
| Gradient type | Current-generation, high-speed gradients (providing sufficient coverage of upper abdomen) | |
| Slice thickness | 5 mm or less for dynamic series, | |
| 8 mm or less for other imaging | ||
| Breath holding and matrix | Approximately 20 s of breath hold with a minimum matrix of 128 × 256 | Breath hold instructions are very important |
| Injector | Power injector, preferably dual-chamber | Bolus tracking/MR fluoroscopy desirable |
| Contrast injection rate | 1.5-2 mL/s of gadolinium chelate | Preferably resulting in the vendor-recommended total dose |
| Minimum sequences | T1-weighted, gradient echo (3D preferable) | |
| T2-weighted, turbo spin echo (axial, coronal) | ||
| MRCP (both 2D and 3D preferable) | ||
| Post-Gd, T1-weighted gradient echo | ||
| Mandatory dynamic phases | Arterial | |
| Portal-venous phase | ||
| Equilibrium phase | ||
| Dynamic timing | Arterial: 20-40 s | |
| Portal venous: 45-65 s | ||
| Equilibrium: 3-5 min | ||
| after contrast injection |
Pancreatic adenocarcinoma occurs most commonly in the pancreatic head (65%) and usually presents on US as a hypoechoic solid mass with ill-defined margins (Figure 1). Masses in the head of the pancreas cause ductal obstruction with secondary dilatation of both the common bile duct and the pancreatic duct, and result in the so-called double-duct sign[62]. On Doppler studies, pancreatic ductal adenocarcinoma shows poor vascularity[73], as well as poor enhancement on all phases of contrast-enhanced US[74]. This may be caused by marked desmoplasia, low mean vascular density, or the possible presence of necrosis and mucin[75].
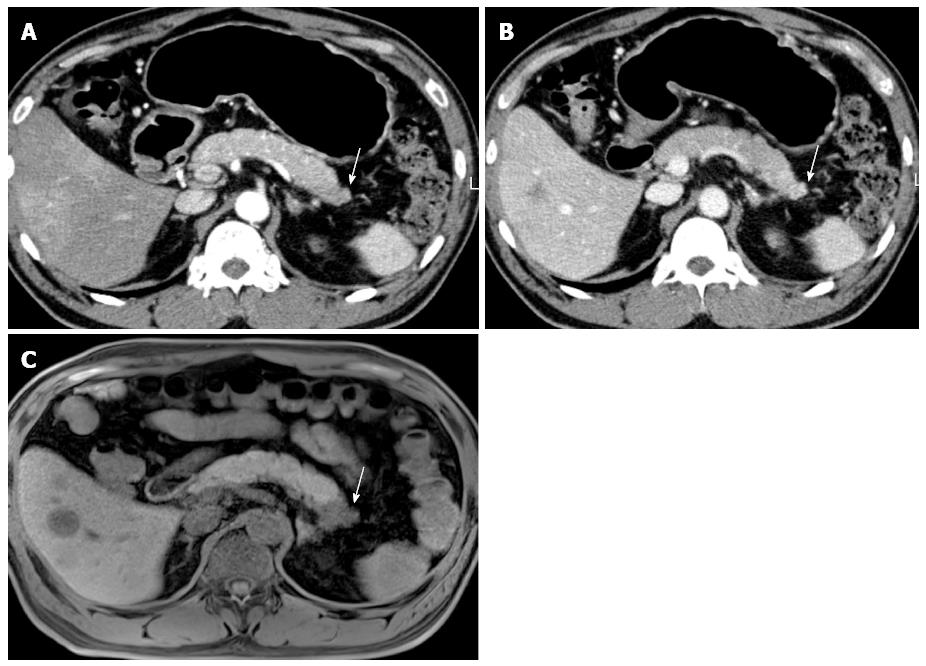
On CT, pancreatic adenocarcinomas most often appear as hypoattenuating masses (Figure 2)[76]. However, approximately 10% of pancreatic adenocarcinomas are isoattenuating relative to the background pancreatic parenchyma[35], especially in small tumors 2 cm or less[77], thus making diagnosis more difficult. In these situations, indirect (secondary) signs, such as upstream pancreatic duct dilation or the double-duct sign caused by pancreatic and common bile duct obstruction, are helpful for diagnosis[76,77]. In addition, the pancreas distal to the tumor usually also appears atrophic. As the tumor grows, it typically infiltrates the peripancreatic structures and may result in encasement of adjacent vasculature and in some cases adjacent organs. Pancreatic cancers can occasionally appear to be cystic or necrotic, and in rare cases they can contain calcium[78].
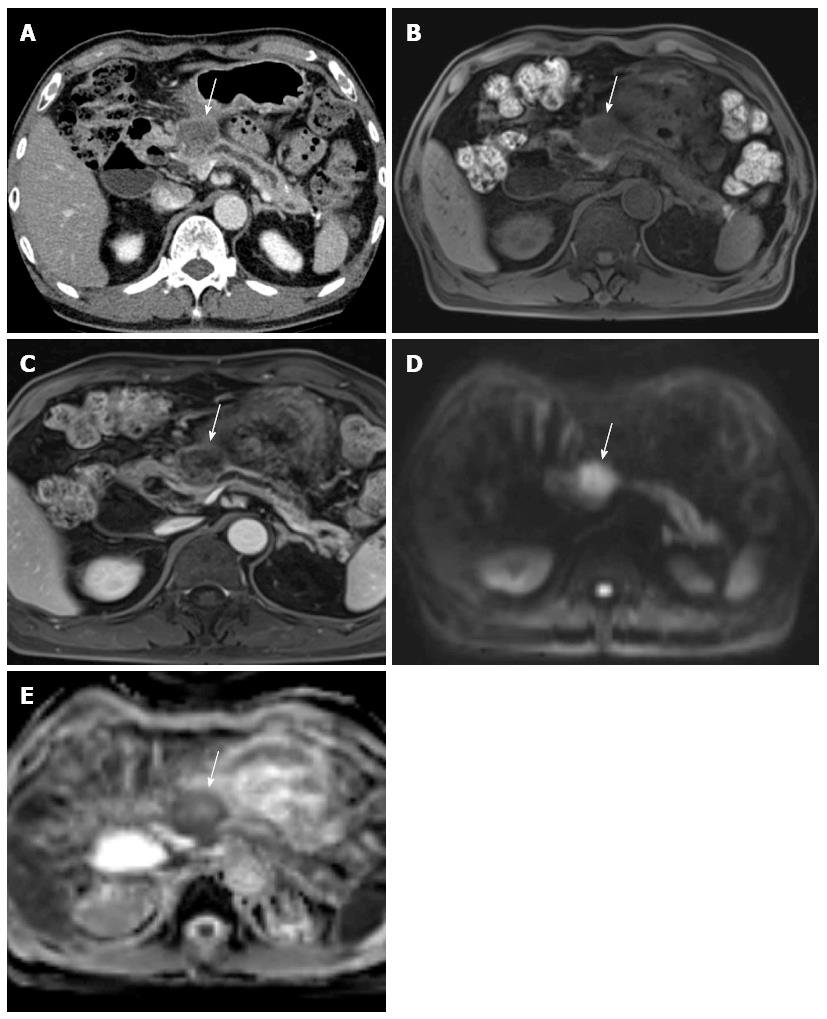
On MRI, pancreatic cancer typically appears hypointense on fat-suppressed, T1-weighted imaging (Figure 3) and on pancreatic parenchymal phase, dynamically enhanced, fat-suppressed, T1-weighted sequences, whereas it has a variable appearance on T2-weighted images[79]. Pancreatic cancer also has a variable appearance on diffusion-weighted images. In a recent study of 80 patients, 38 pancreatic cancers appeared hyperintense, 12 isointense, and 4 hypointense[80].
Pancreatic adenocarcinoma usually manifests as an area of increased uptake on PET/CT and appears as a “hot spot” within the pancreas. On the basis of tumor biology and the degree of desmoplastic response, pancreatic ductal adenocarcinoma may demonstrate a low level of FDG uptake or none at all[14]. The reported SUV (standardized uptake value) of pancreatic adenocarcinoma (3.50 ± 1.66) was found to be higher than that of benign lesions (1.91 ± 0.65) and of the normal pancreas[81]. In a recent study of patients with suspected pancreatic cancer, the FDG uptake of malignant tumors was also distinctly higher than that of benign lesions and in patients with chronic pancreatitis[55,82].
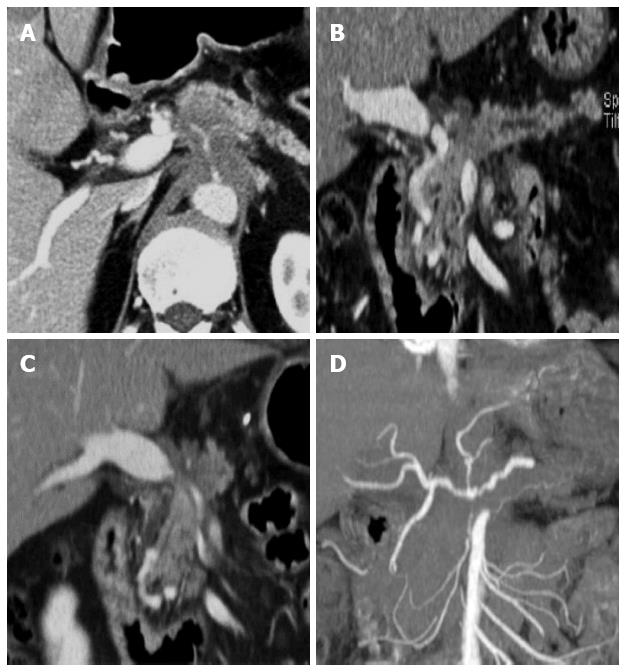
With the continuing substantial improvements in CT technology, the capacity of MDCT for the detection, diagnosis, and local staging of pancreatic cancer has increased. MDCT is very effective for detecting and staging adenocarcinoma, with a sensitivity of up to 90% for detection and an accuracy of 80%-90% for staging[26]. Determination of the extent of vascular involvement is usually made by identifying the extent to which the tumor involves the cross-sectional circumference of a vessel. This can be done by identifying, with regard to the circular cross-section of a vessel, the degrees of circumferential involvement, as described by Lu et al[31]. Since their study was published in 1997, the terms “abutment” and “encasement” have also been used; abutment refers to the involvement of 180° or less of a vessel’s circumference, and encasement refers to a greater than 180° vascular circumferential involvement[79]. As described above, as MDCT has shown the best performance for evaluating vascular involvement[27-33] (Figure 4), it is the most important factor for predicting tumor resectability. For example, four-section CT has been reported to have a 100% negative predictive value for vascular invasion and a 87% negative predictive value for overall tumor resectability[30,83]. Therefore, MDCT is still the modality of choice for diagnosis and local staging of patients with pancreatic cancer.
Recently, distinct advances in MR technology have caused great improvements in pancreatic cancer imaging. At the same time, several literature reports have been published describing the comparable diagnostic performance of MDCT and MR[84-88]. According to a recent report by Koelblinger et al[85], the mean sensitivity and specificity of 64-detector row CT and 3.0-T MR imaging for the detection of pancreatic cancer (mean sensitivity, 95% vs 96%, respectively; mean specificity, 96% for both) do not differ significantly.
Although MDCT has become the modality of choice for pancreatic cancer imaging and shows excellent performance regarding its diagnosis and staging, the detection of small pancreatic cancers < 2 cm in diameter or of isoattenuating tumors (which account for approximately 10% of all pancreatic adenocarcinomas) still remains challenging[35,77]. For those cases, we can improve the contrast-to-noise ratio between pancreatic cancer and normal parenchyma using the dual-energy or low-tube-voltage techniques[89]. A low-tube-voltage CT technique increases the X-ray absorption of iodine by increasing the gap between the mean effective energy of the X-ray spectrum and the k edge of iodine[90]. Clinically, this phenomenon results in improved contrast enhancement of normal pancreatic parenchyma in order to maximize the contrast to typically poorly vascularized pancreatic cancers[90,91]. Therefore, dual-energy CT and low-tube-voltage techniques offer increased detection rates for small or otherwise isoattenuating pancreatic tumors[89-93].
A new method for CT noise reduction based on iterative reconstruction (IR) algorithms has recently been developed. Since medical radiation exposure is generally increasing, one of the greatest concerns for radiologists, the use of this novel technique has recently been increasing due to its potential to preserve and enhance the diagnostic capability of CT with reduced radiation doses[94]. Currently, several IR methods have been proposed and are being commercially used for reducing radiation dose by decreasing the image noise during the reconstruction process (i.e., adaptive statistical iterative reconstruction (ASiR, GE Health-care), model-based iterative reconstruction (MBIR, GE Healthcare), iterative reconstruction in image space (IRIS, Siemens Healthcare), sinogram-affirmed iterative reconstruction (SAFIRE, Siemens Healthcare), and iDose (Philips Healthcare)[95]. Based on its development, many studies have continuously revealed the superiority of IR, compared with routine filtered back projection, across the whole body[94-107]. Regarding the variety of reconstructing algorithms, each method may show a detailed difference in image quality, as well as providing abnormal features such as a plastic, waxy, blotchy, or pixilated texture[104,107]. Considering the effects of IR techniques on reducing image noise, these techniques could be used for high spatial resolution pancreatic CT imaging, which may provide high quality, 1-2 mm, thin-slice CT images. Optimizing the IR technique using a study protocol is necessary to balance imaging distortion, radiation reduction, and image quality, as well as high spatial resolution, along the z-axis.
Although there is still technical complexity and room for improvement in terms of imaging resolutions regarding dynamic, contrast-enhanced (DCE) MR imaging, in previously published studies the quantitative analysis of the enhancement patterns and perfusion parameters using DCE-MR imaging has been shown to be both objective and helpful for the evaluation of malignant diseases regarding both their diagnosis and treatment monitoring[108-110]. In our preliminary data, the K(trans), K(ep), and iAUC values in patients with pancreatic cancer were significantly lower than those seen in patients with a normal pancreas (P < 0.05), and were, therefore, useful for differentiating pancreatic cancer from pancreatic neuroendocrine tumors[110].
DWI has also been used to characterize pancreatic lesions of various pathologic entities, such as cystic lesions, pancreatitis, and malignant tumors[70,111-113]. Although MR has the great advantage of excellent soft-tissue contrast for focal lesion detection, small or non-contour-deforming pancreatic adenocarcinomas may lack classic imaging features and thus may not be detected on conventional MRI. The use of diffusion-weighted imaging may allow earlier detection of pancreatic adenocarcinoma, as these neoplasms have increased signal intensity on diffusion-weighted images with high b values (b > 500 s/mm2), as well as relatively low ADC values because of their restricted diffusion associated with fibrosis[70]. The intravoxel incoherent motion (IVIM) model takes these two sources of signal decay into account, thus providing a theoretical framework from which to derive diffusion and perfusion parameters from DWI[114]. Recently, the IVIM-approach with multiple b-values has been applied to pancreatic imaging, and there have been several reports showing promising results regarding the differentiation of pancreatic cancer from a normal pancreas[112,115]. DWI may also show small metastases that are not so clearly seen using other sequences and which, therefore, suggest to radiologists, on the basis of the high-signal-intensity lesion seen on diffusion-weighted imaging, to more closely examine the images obtained on other sequences[70,116]. Gadoxetic acid-enhanced liver MR imaging is also regarded as one of the best imaging tools for detecting liver metastasis in patients with pancreatic cancer. The reported sensitivity of gadoxetic acid-enhanced liver MR is 85% for detecting liver metastasis in pancreatic cancer, which is significantly higher compared with that of CT (69%)[117].
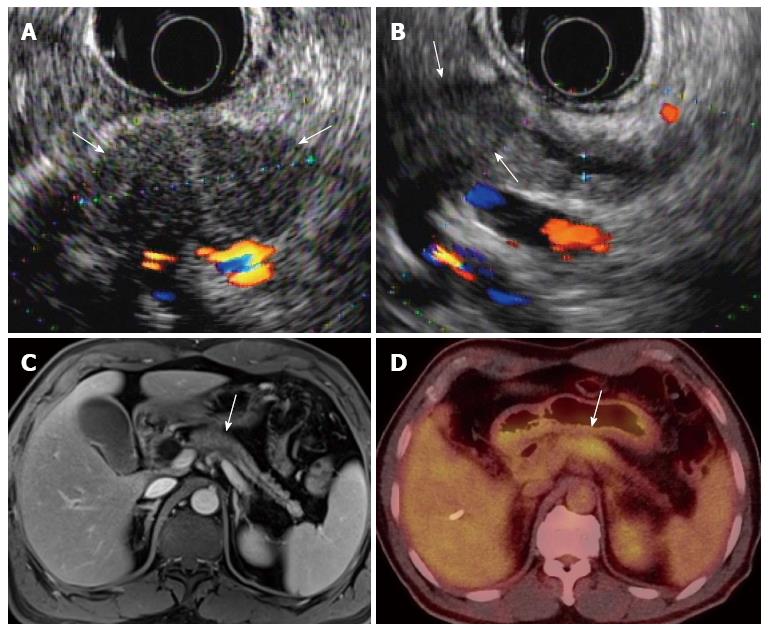
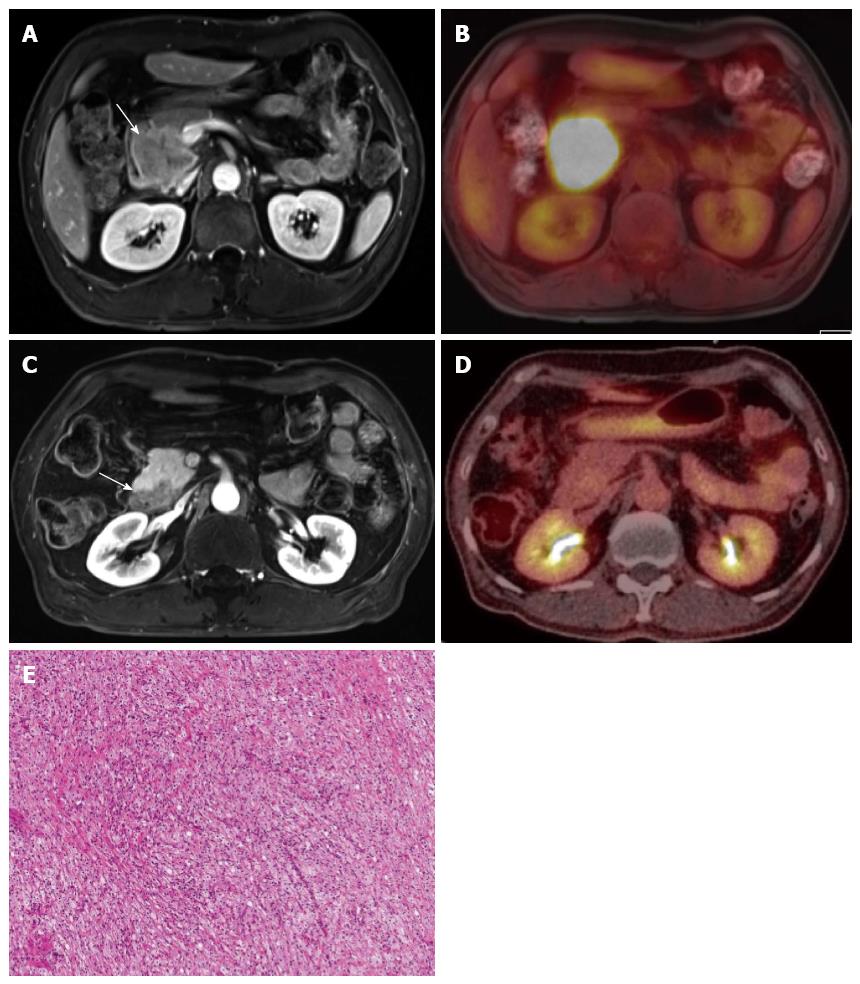
Integrated PET and MR (PET/MR) scanners have recently become available for use in humans. As MR has the inherent strength of superior soft-tissue contrast resolution, multiplanar imaging acquisition, and functional imaging capability, such as that seen in DCE-MR, DWI, MR spectroscopy, or elastography, PET/MR may exhibit superior diagnostic performance compared with that of PET/CT[118]. In our medical institution, PET/MR imaging is now being used for evaluation of staging in patients with locally advanced pancreatic cancers, as well as for evaluation of tumor response in patients with pancreatic cancer undergoing neoadjuvant chemoradiotherapy before and after treatment (Figure 5).
There have recently been notable improvements in pancreatic imaging using the multi-modality approach, although each imaging modality has its own role, advantages, and disadvantages, not only for diagnosis, but also for the treatment and follow-up of pancreatic cancer. Both radiologists and clinicians should be familiar with those characteristics of imaging modalities, and apply them whenever possible. Rapidly developing, novel imaging techniques, including dual energy, low-tube-voltage CT techniques, IR algorithms, functional MR imaging methods, and hybrid PET/MR, are expected to become widely-used and to show excellent performance for pancreatic cancer imaging in the near future.
P- Reviewers: Brisinda G, Casciaro S, Dietrich CF, Treglia G S- Editor: Cui XM L- Editor: Rutherford A E- Editor: Wang CH
| 1. | Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford). 2008;10:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2206] [Article Influence: 147.1] [Reference Citation Analysis (2)] |
| 3. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 4. | Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1544] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 5. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8225] [Article Influence: 483.8] [Reference Citation Analysis (0)] |
| 6. | Güngör C, Hofmann BT, Wolters-Eisfeld G, Bockhorn M. Pancreatic cancer. Br J Pharmacol. 2014;171:849-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: why, how, and who? Ann Surg. 2013;257:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Karmazanovsky G, Fedorov V, Kubyshkin V, Kotchatkov A. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging. 2005;30:488-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Miura F, Takada T, Amano H, Yoshida M, Furui S, Takeshita K. Diagnosis of pancreatic cancer. HPB (Oxford). 2006;8:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Brennan DD, Zamboni GA, Raptopoulos VD, Kruskal JB. Comprehensive preoperative assessment of pancreatic adenocarcinoma with 64-section volumetric CT. Radiographics. 2007;27:1653-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Morana G, Cancian L, Pozzi Mucelli R, Cugini C. Staging cancer of the pancreas. Cancer Imaging. 2010;10 Spec no A:S137-S141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Appel BL, Tolat P, Evans DB, Tsai S. Current staging systems for pancreatic cancer. Cancer J. 2012;18:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Fusaroli P, Kypraios D, Caletti G, Eloubeidi MA. Pancreatico-biliary endoscopic ultrasound: a systematic review of the levels of evidence, performance and outcomes. World J Gastroenterol. 2012;18:4243-4256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Sahani DV, Bonaffini PA, Catalano OA, Guimaraes AR, Blake MA. State-of-the-art PET/CT of the pancreas: current role and emerging indications. Radiographics. 2012;32:1133-1158; discussion 1158-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Conrad C, Fernández-Del Castillo C. Preoperative evaluation and management of the pancreatic head mass. J Surg Oncol. 2013;107:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Shrikhande SV, Barreto SG, Goel M, Arya S. Multimodality imaging of pancreatic ductal adenocarcinoma: a review of the literature. HPB (Oxford). 2012;14:658-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Raman SP, Horton KM, Fishman EK. Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J. 2012;18:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Dibble EH, Karantanis D, Mercier G, Peller PJ, Kachnic LA, Subramaniam RM. PET/CT of cancer patients: part 1, pancreatic neoplasms. AJR Am J Roentgenol. 2012;199:952-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Kinney T. Evidence-based imaging of pancreatic malignancies. Surg Clin North Am. 2010;90:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Bhat K, Wang F, Ma Q, Li Q, Mallik S, Hsieh TC, Wu E. Advances in biomarker research for pancreatic cancer. Curr Pharm Des. 2012;18:2439-2451. [PubMed] |
| 21. | Wray CJ, Ahmad SA, Matthews JB, Lowy AM. Surgery for pancreatic cancer: recent controversies and current practice. Gastroenterology. 2005;128:1626-1641. [PubMed] |
| 22. | Cameron K, Golan S, Simpson W, Peti S, Roayaie S, Labow D, Kostakoglu L. Recurrent pancreatic carcinoma and cholangiocarcinoma: 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT). Abdom Imaging. 2011;36:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Maringhini A, Ciambra M, Raimondo M, Baccelliere P, Grasso R, Dardanoni G, Lanzarone F, Cottone M, Sciarrino E, Pagliaro L. Clinical presentation and ultrasonography in the diagnosis of pancreatic cancer. Pancreas. 1993;8:146-150. [PubMed] |
| 24. | Karlson BM, Ekbom A, Lindgren PG, Källskog V, Rastad J. Abdominal US for diagnosis of pancreatic tumor: prospective cohort analysis. Radiology. 1999;213:107-111. [PubMed] |
| 25. | Rickes S, Unkrodt K, Neye H, Ocran KW, Wermke W. Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonography. Scand J Gastroenterol. 2002;37:1313-1320. [PubMed] |
| 26. | Schima W, Ba-Ssalamah A, Kölblinger C, Kulinna-Cosentini C, Puespoek A, Götzinger P. Pancreatic adenocarcinoma. Eur Radiol. 2007;17:638-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Lepanto L, Arzoumanian Y, Gianfelice D, Perreault P, Dagenais M, Lapointe R, Létourneau R, Roy A. Helical CT with CT angiography in assessing periampullary neoplasms: identification of vascular invasion. Radiology. 2002;222:347-352. [PubMed] |
| 28. | Zhao WY, Luo M, Sun YW, Xu Q, Chen W, Zhao G, Wu ZY. Computed tomography in diagnosing vascular invasion in pancreatic and periampullary cancers: a systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int. 2009;8:457-464. [PubMed] |
| 29. | Li H, Zeng MS, Zhou KR, Jin DY, Lou WH. Pancreatic adenocarcinoma: signs of vascular invasion determined by multi-detector row CT. Br J Radiol. 2006;79:880-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB. MDCT in Pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol. 2004;182:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 336] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | Manak E, Merkel S, Klein P, Papadopoulos T, Bautz WA, Baum U. Resectability of pancreatic adenocarcinoma: assessment using multidetector-row computed tomography with multiplanar reformations. Abdom Imaging. 2009;34:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Gusmini S, Nicoletti R, Martinenghi C, Del Maschio A. Vascular involvement in periampullary tumors: MDCT, EUS, and CDU. Abdom Imaging. 2009;34:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Zamboni GA, Kruskal JB, Vollmer CM, Baptista J, Callery MP, Raptopoulos VD. Pancreatic adenocarcinoma: value of multidetector CT angiography in preoperative evaluation. Radiology. 2007;245:770-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Prokesch RW, Chow LC, Beaulieu CF, Bammer R, Jeffrey RB. Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology. 2002;224:764-768. [PubMed] |
| 36. | Volmar KE, Vollmer RT, Jowell PS, Nelson RC, Xie HB. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc. 2005;61:854-861. [PubMed] |
| 37. | Rösch T, Lorenz R, Braig C, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347-352. [PubMed] |
| 38. | Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas. 2013;42:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 39. | Ardengh JC, Lopes CV, de Lima LF, de Oliveira JR, Venco F, Santo GC, Modena JL. Diagnosis of pancreatic tumors by endoscopic ultrasound-guided fine-needle aspiration. World J Gastroenterol. 2007;13:3112-3116. [PubMed] |
| 40. | Faigel DO, Ginsberg GG, Bentz JS, Gupta PK, Smith DB, Kochman ML. Endoscopic ultrasound-guided real-time fine-needle aspiration biopsy of the pancreas in cancer patients with pancreatic lesions. J Clin Oncol. 1997;15:1439-1443. [PubMed] |
| 41. | Chen J, Yang R, Lu Y, Xia Y, Zhou H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: a systematic review. J Cancer Res Clin Oncol. 2012;138:1433-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 42. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [PubMed] |
| 43. | Bhutani MS, Hawes RH, Baron PL, Sanders-Cliette A, van Velse A, Osborne JF, Hoffman BJ. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy. 1997;29:854-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 45. | Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629-34.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 46. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [PubMed] |
| 47. | Lee TH, Cha SW, Cho YD. EUS elastography: advances in diagnostic EUS of the pancreas. Korean J Radiol. 2012;13 Suppl 1:S12-S16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 49. | Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 50. | Sandrasegaran K, Lin C, Akisik FM, Tann M. State-of-the-art pancreatic MRI. AJR Am J Roentgenol. 2010;195:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Maccioni F, Martinelli M, Al Ansari N, Kagarmanova A, De Marco V, Zippi M, Marini M. Magnetic resonance cholangiography: past, present and future: a review. Eur Rev Med Pharmacol Sci. 2010;14:721-725. [PubMed] |
| 52. | Kalra MK, Maher MM, Mueller PR, Saini S. State-of-the-art imaging of pancreatic neoplasms. Br J Radiol. 2003;76:857-865. [PubMed] |
| 53. | Miller FH, Rini NJ, Keppke AL. MRI of adenocarcinoma of the pancreas. AJR Am J Roentgenol. 2006;187:W365-W374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Blodgett TM, Meltzer CC, Townsend DW. PET/CT: form and function. Radiology. 2007;242:360-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 55. | Kauhanen SP, Komar G, Seppänen MP, Dean KI, Minn HR, Kajander SA, Rinta-Kiikka I, Alanen K, Borra RJ, Puolakkainen PA. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 56. | Yoshioka M, Sato T, Furuya T, Shibata S, Andoh H, Asanuma Y, Hatazawa J, Shimosegawa E, Koyama K, Yamamoto Y. Role of positron emission tomography with 2-deoxy-2-[18F]fluoro-D-glucose in evaluating the effects of arterial infusion chemotherapy and radiotherapy on pancreatic cancer. J Gastroenterol. 2004;39:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Sperti C, Pasquali C, Bissoli S, Chierichetti F, Liessi G, Pedrazzoli S. Tumor relapse after pancreatic cancer resection is detected earlier by 18-FDG PET than by CT. J Gastrointest Surg. 2010;14:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Ruf J, Lopez Hänninen E, Oettle H, Plotkin M, Pelzer U, Stroszczynski C, Felix R, Amthauer H. Detection of recurrent pancreatic cancer: comparison of FDG-PET with CT/MRI. Pancreatology. 2005;5:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 59. | Kuwatani M, Kawakami H, Eto K, Haba S, Shiga T, Tamaki N, Asaka M. Modalities for evaluating chemotherapeutic efficacy and survival time in patients with advanced pancreatic cancer: comparison between FDG-PET, CT, and serum tumor markers. Intern Med. 2009;48:867-875. [PubMed] |
| 60. | Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 556] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 61. | von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology. 2006;238:405-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 391] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 62. | Martínez-Noguera A, D’Onofrio M. Ultrasonography of the pancreas. 1. Conventional imaging. Abdom Imaging. 2007;32:136-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Goshima S, Kanematsu M, Kondo H, Yokoyama R, Miyoshi T, Kato H, Tsuge Y, Shiratori Y, Hoshi H, Onozuka M. Pancreas: optimal scan delay for contrast-enhanced multi-detector row CT. Radiology. 2006;241:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Kondo H, Kanematsu M, Goshima S, Miyoshi T, Shiratori Y, Onozuka M, Moriyama N, Bae KT. MDCT of the pancreas: optimizing scanning delay with a bolus-tracking technique for pancreatic, peripancreatic vascular, and hepatic contrast enhancement. AJR Am J Roentgenol. 2007;188:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Tamm EP, Balachandran A, Bhosale P, Szklaruk J. Update on 3D and multiplanar MDCT in the assessment of biliary and pancreatic pathology. Abdom Imaging. 2009;34:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Salles A, Nino-Murcia M, Jeffrey RB. CT of pancreas: minimum intensity projections. Abdom Imaging. 2008;33:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Kim HC, Yang DM, Jin W, Ryu CW, Ryu JK, Park SI, Park SJ, Shin HC, Kim IY. Multiplanar reformations and minimum intensity projections using multi-detector row CT for assessing anomalies and disorders of the pancreaticobiliary tree. World J Gastroenterol. 2007;13:4177-4184. [PubMed] |
| 68. | Raptopoulos V, Prassopoulos P, Chuttani R, McNicholas MM, McKee JD, Kressel HY. Multiplanar CT pancreatography and distal cholangiography with minimum intensity projections. Radiology. 1998;207:317-324. [PubMed] |
| 69. | Sun HY, Kim SH, Kim MA, Lee JY, Han JK, Choi BI. CT imaging spectrum of pancreatic serous tumors: based on new pathologic classification. Eur J Radiol. 2010;75:e45-e55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Wang Y, Miller FH, Chen ZE, Merrick L, Mortele KJ, Hoff FL, Hammond NA, Yaghmai V, Nikolaidis P. Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics. 2011;31:E47-E64. [PubMed] |
| 71. | Kim JE, Lee JM, Kim SH, Baek JH, Moon SK, Yu IS, Kim SH, Lee JY, Han JK, Choi BI. Differentiation of intraductal growing-type cholangiocarcinomas from nodular-type cholangiocarcinomas at biliary MR imaging with MR cholangiography. Radiology. 2010;257:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Ryoo I, Lee JM, Chung YE, Park HS, Kim SH, Han JK, Choi BI. Gadobutrol-enhanced, three-dimensional, dynamic MR imaging with MR cholangiography for the preoperative evaluation of bile duct cancer. Invest Radiol. 2010;45:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Bertolotto M, D’Onofrio M, Martone E, Malagò R, Pozzi Mucelli R. Ultrasonography of the pancreas. 3. Doppler imaging. Abdom Imaging. 2007;32:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | D’Onofrio M, Zamboni G, Faccioli N, Capelli P, Pozzi Mucelli R. Ultrasonography of the pancreas. 4. Contrast-enhanced imaging. Abdom Imaging. 2007;32:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Oshikawa O, Tanaka S, Ioka T, Nakaizumi A, Hamada Y, Mitani T. Dynamic sonography of pancreatic tumors: comparison with dynamic CT. AJR Am J Roentgenol. 2002;178:1133-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Francis IR. Pancreatic adenocarcinoma: diagnosis and staging using multidetector-row computed tomography (MDCT) and magnetic resonance imaging (MRI). Cancer Imaging. 2007;7 Spec No A:S160-S165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Yoon SH, Lee JM, Cho JY, Lee KB, Kim JE, Moon SK, Kim SJ, Baek JH, Kim SH, Kim SH. Small (≤ 20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology. 2011;259:442-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 78. | Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 618] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 79. | Tamm EP, Bhosale PR, Vikram R, de Almeida Marcal LP, Balachandran A. Imaging of pancreatic ductal adenocarcinoma: State of the art. World J Radiol. 2013;5:98-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Fukukura Y, Takumi K, Kamimura K, Shindo T, Kumagae Y, Tateyama A, Nakajo M. Pancreatic adenocarcinoma: variability of diffusion-weighted MR imaging findings. Radiology. 2012;263:732-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 81. | Koyama K, Okamura T, Kawabe J, Nakata B, Chung KH, Ochi H, Yamada R. Diagnostic usefulness of FDG PET for pancreatic mass lesions. Ann Nucl Med. 2001;15:217-224. [PubMed] |
| 82. | Schick V, Franzius C, Beyna T, Oei ML, Schnekenburger J, Weckesser M, Domschke W, Schober O, Heindel W, Pohle T. Diagnostic impact of 18F-FDG PET-CT evaluating solid pancreatic lesions versus endosonography, endoscopic retrograde cholangio-pancreatography with intraductal ultrasonography and abdominal ultrasound. Eur J Nucl Med Mol Imaging. 2008;35:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Catalano C, Laghi A, Fraioli F, Pediconi F, Napoli A, Danti M, Reitano I, Passariello R. Pancreatic carcinoma: the role of high-resolution multislice spiral CT in the diagnosis and assessment of resectability. Eur Radiol. 2003;13:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 84. | Grenacher L, Klauss M, Dukic L, Delorme S, Knaebel HP, Düx M, Kauczor HU, Büchler MW, Kauffmann GW, Richter GM. [Diagnosis and staging of pancreatic carcinoma: MRI versus multislice-CT -- a prospective study]. Rofo. 2004;176:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Koelblinger C, Ba-Ssalamah A, Goetzinger P, Puchner S, Weber M, Sahora K, Scharitzer M, Plank C, Schima W. Gadobenate dimeglumine-enhanced 3.0-T MR imaging versus multiphasic 64-detector row CT: prospective evaluation in patients suspected of having pancreatic cancer. Radiology. 2011;259:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Lee JK, Kim AY, Kim PN, Lee MG, Ha HK. Prediction of vascular involvement and resectability by multidetector-row CT versus MR imaging with MR angiography in patients who underwent surgery for resection of pancreatic ductal adenocarcinoma. Eur J Radiol. 2010;73:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 87. | Park HS, Lee JM, Choi HK, Hong SH, Han JK, Choi BI. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging. 2009;30:586-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 88. | Schima W, Függer R. Evaluation of focal pancreatic masses: comparison of mangafodipir-enhanced MR imaging and contrast-enhanced helical CT. Eur Radiol. 2002;12:2998-3008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Chu AJ, Lee JM, Lee YJ, Moon SK, Han JK, Choi BI. Dual-source, dual-energy multidetector CT for the evaluation of pancreatic tumours. Br J Radiol. 2012;85:e891-e898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Marin D, Nelson RC, Barnhart H, Schindera ST, Ho LM, Jaffe TA, Yoshizumi TT, Youngblood R, Samei E. Detection of pancreatic tumors, image quality, and radiation dose during the pancreatic parenchymal phase: effect of a low-tube-voltage, high-tube-current CT technique--preliminary results. Radiology. 2010;256:450-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 91. | Heye T, Nelson RC, Ho LM, Marin D, Boll DT. Dual-energy CT applications in the abdomen. AJR Am J Roentgenol. 2012;199:S64-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 92. | Graser A, Johnson TR, Chandarana H, Macari M. Dual energy CT: preliminary observations and potential clinical applications in the abdomen. Eur Radiol. 2009;19:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 356] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 93. | Macari M, Spieler B, Kim D, Graser A, Megibow AJ, Babb J, Chandarana H. Dual-source dual-energy MDCT of pancreatic adenocarcinoma: initial observations with data generated at 80 kVp and at simulated weighted-average 120 kVp. AJR Am J Roentgenol. 2010;194:W27-W32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 94. | Silva AC, Lawder HJ, Hara A, Kujak J, Pavlicek W. Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. AJR Am J Roentgenol. 2010;194:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 424] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 95. | Yu MH, Lee JM, Yoon JH, Baek JH, Han JK, Choi BI, Flohr TG. Low tube voltage intermediate tube current liver MDCT: sinogram-affirmed iterative reconstruction algorithm for detection of hypervascular hepatocellular carcinoma. AJR Am J Roentgenol. 2013;201:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 96. | Baker ME, Dong F, Primak A, Obuchowski NA, Einstein D, Gandhi N, Herts BR, Purysko A, Remer E, Vachhani N. Contrast-to-noise ratio and low-contrast object resolution on full- and low-dose MDCT: SAFIRE versus filtered back projection in a low-contrast object phantom and in the liver. AJR Am J Roentgenol. 2012;199:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 97. | Goshima S, Kanematsu M, Nishibori H, Miyazawa D, Kondo H, Moriyama N, Bae KT. Image quality and radiation exposure in CT of the pancreas: 320-MDCT with and without adaptive iterative dose reduction versus 64-MDCT. Clin Radiol. 2013;68:e593-e600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Kalra MK, Woisetschläger M, Dahlström N, Singh S, Lindblom M, Choy G, Quick P, Schmidt B, Sedlmair M, Blake MA. Radiation dose reduction with Sinogram Affirmed Iterative Reconstruction technique for abdominal computed tomography. J Comput Assist Tomogr. 2012;36:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 99. | Leipsic J, Nguyen G, Brown J, Sin D, Mayo JR. A prospective evaluation of dose reduction and image quality in chest CT using adaptive statistical iterative reconstruction. AJR Am J Roentgenol. 2010;195:1095-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 100. | Marin D, Nelson RC, Schindera ST, Richard S, Youngblood RS, Yoshizumi TT, Samei E. Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm--initial clinical experience. Radiology. 2010;254:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 408] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 101. | Prakash P, Kalra MK, Ackman JB, Digumarthy SR, Hsieh J, Do S, Shepard JA, Gilman MD. Diffuse lung disease: CT of the chest with adaptive statistical iterative reconstruction technique. Radiology. 2010;256:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 102. | Schabel C, Fenchel M, Schmidt B, Flohr TG, Wuerslin C, Thomas C, Korn A, Tsiflikas I, Claussen CD, Heuschmid M. Clinical evaluation and potential radiation dose reduction of the novel sinogram-affirmed iterative reconstruction technique (SAFIRE) in abdominal computed tomography angiography. Acad Radiol. 2013;20:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 103. | Singh S, Kalra MK, Gilman MD, Hsieh J, Pien HH, Digumarthy SR, Shepard JA. Adaptive statistical iterative reconstruction technique for radiation dose reduction in chest CT: a pilot study. Radiology. 2011;259:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 272] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 104. | Singh S, Kalra MK, Hsieh J, Licato PE, Do S, Pien HH, Blake MA. Abdominal CT: comparison of adaptive statistical iterative and filtered back projection reconstruction techniques. Radiology. 2010;257:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 343] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 105. | Vardhanabhuti V, Loader RJ, Mitchell GR, Riordan RD, Roobottom CA. Image quality assessment of standard- and low-dose chest CT using filtered back projection, adaptive statistical iterative reconstruction, and novel model-based iterative reconstruction algorithms. AJR Am J Roentgenol. 2013;200:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 106. | Winklehner A, Karlo C, Puippe G, Schmidt B, Flohr T, Goetti R, Pfammatter T, Frauenfelder T, Alkadhi H. Raw data-based iterative reconstruction in body CTA: evaluation of radiation dose saving potential. Eur Radiol. 2011;21:2521-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 107. | Xu J, Mahesh M, Tsui BM. Is iterative reconstruction ready for MDCT? J Am Coll Radiol. 2009;6:274-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 108. | Akisik MF, Sandrasegaran K, Bu G, Lin C, Hutchins GD, Chiorean EG. Pancreatic cancer: utility of dynamic contrast-enhanced MR imaging in assessment of antiangiogenic therapy. Radiology. 2010;256:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 109. | Bali MA, Metens T, Denolin V, Delhaye M, Demetter P, Closset J, Matos C. Tumoral and nontumoral pancreas: correlation between quantitative dynamic contrast-enhanced MR imaging and histopathologic parameters. Radiology. 2011;261:456-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 110. | Kim JH, Lee JM, Park JH, Kim SC, Joo I, Han JK, Choi BI. Solid pancreatic lesions: characterization by using timing bolus dynamic contrast-enhanced MR imaging assessment--a preliminary study. Radiology. 2013;266:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 111. | Inan N, Arslan A, Akansel G, Anik Y, Demirci A. Diffusion-weighted imaging in the differential diagnosis of cystic lesions of the pancreas. AJR Am J Roentgenol. 2008;191:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 112. | Lee SS, Byun JH, Park BJ, Park SH, Kim N, Park B, Kim JK, Lee MG. Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses. J Magn Reson Imaging. 2008;28:928-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 113. | Fattahi R, Balci NC, Perman WH, Hsueh EC, Alkaade S, Havlioglu N, Burton FR. Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas. J Magn Reson Imaging. 2009;29:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 114. | Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505. [PubMed] |
| 115. | Lemke A, Laun FB, Klauss M, Re TJ, Simon D, Delorme S, Schad LR, Stieltjes B. Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters. Invest Radiol. 2009;44:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 116. | Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, Taouli B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246:812-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 415] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 117. | Motosugi U, Ichikawa T, Morisaka H, Sou H, Muhi A, Kimura K, Sano K, Araki T. Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology. 2011;260:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 118. | Torigian DA, Zaidi H, Kwee TC, Saboury B, Udupa JK, Cho ZH, Alavi A. PET/MR imaging: technical aspects and potential clinical applications. Radiology. 2013;267:26-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |