Published online Jun 28, 2014. doi: 10.3748/wjg.v20.i24.7785
Revised: November 29, 2013
Accepted: February 26, 2014
Published online: June 28, 2014
Processing time: 247 Days and 9 Hours
Pancreatic cystic lesions are increasingly recognised due to the widespread use of different imaging modalities. Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas represent a common, but also heterogeneous group of cystic tumors with a significant malignant potential. These neoplasms must be differentiated from other cystic tumors and properly classified into their different types, main-duct IPMNs vs branch-duct IPMNs. These types have a different malignant potential and therefore, different treatment strategies need to be implemented. Endoscopic ultrasound (EUS) offers the highest resolution of the pancreas and can aid in the differential diagnosis, classification and differentiation between benign and malignant tumors. The addition of EUS fine-needle aspiration can supply further information by obtaining fluid for cytology, measurement of tumor markers and perhaps DNA analysis. Novel techniques, such as the use of contrast and sophisticated equipment, like intraductal probes can provide information regarding malignant features and extent of these neoplasms. Thus, EUS is a valuable tool in the diagnosis and appropriate management of these tumors.
Core tip: This review shows that endoscopic ultrasound initially provides differential diagnosis of pancreatic cystic tumors and subsequently can classify intraductal papillary mucinous neoplasms of the pancreas into their different types. With the use of endoscopic ultrasound (EUS) fine-needle aspiration and other techniques, such as contrast enhancement, EUS can differentiate between benign and malignant neoplasms and help the clinician to implement the proper treatment strategy.
- Citation: Efthymiou A, Podas T, Zacharakis E. Endoscopic ultrasound in the diagnosis of pancreatic intraductal papillary mucinous neoplasms. World J Gastroenterol 2014; 20(24): 7785-7793
- URL: https://www.wjgnet.com/1007-9327/full/v20/i24/7785.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i24.7785
Intraductal papillary mucinous neoplasms of the pancreas (IPMNs) are a well-recognised disease entity since their first report by Ohashi et al[1] in 1982. They consist of pancreatic tumors characterised by papillary proliferation of the ductal epithelium which produces mucin and is accompanied by dilatation of the excretory pancreatic ducts. World Health Organization formally differentiated IPMNs from other mucin-producing cystic lesions of the pancreas in 1996, through a uniform classification scheme[2]. IPMNs have been reported with increased frequency, representing 21%-41% of all cystic neoplasms of the pancreas[3,4]. The increased detection of pancreatic cystic lesions, including IPMNs, is due to the widespread use of various abdominal imaging modalities, such as ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI)[5].
IPMNs represent a heterogeneous group of neoplasms that are classified according to their anatomic location into main duct vs branch duct IPMNs. There is also a mixed-type IPMN which is the combination of the above two types. IPMNs involving the main duct (MD) have a higher risk of associated carcinoma, compared to branch duct (BD) IPMNs[6]. IPMNs also comprise a wide histologic spectrum that ranges from adenoma to invasive carcinoma with different degrees of aggressiveness[7]. Early detection and precise anatomical and histological classification are therefore of paramount importance for the optimal management of these tumors.
Despite advances in CT and MRI, the ability of cross-sectional imaging modalities to characterize pancreatic IPMNs correctly, and to differentiate between benign and malignant lesions remains limited. Endoscopic ultrasound (EUS) is an ideal diagnostic tool for the imaging of pancreatic cystic lesions and therefore IPMNs, because of its high resolution, close proximity to the target-lesion and ability to take samples, by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA)[8].
This review focuses on the true prevalence of IPMNs, the role of EUS in the detection, differential diagnosis and classification of these neoplasms but also the impact of EUS/EUS FNA on the ultimate management of these tumors.
IPMNs account for 1%-3% of all exocrine pancreatic neoplasms and for 21%-41% of all cystic neoplasms of the pancreas[9-11]. The exact incidence of IPMNs, however, is not known because many of them are small and asymptomatic. Imaging studies revealed that asymptomatic cysts of the pancreas that presumably contain predominantly small IPMNs were found in 2.8% of 2832 consecutive CT scans performed in a single institution, and this figure rose to 8.7% in individuals aged > 80 years[12]. IPMNs are typically found in elderly people, with a median age at diagnosis of 65-70 years. As stated earlier, IPMNs may involve the main pancreatic duct (MD-IPMN), in either a diffuse or segmental manner, or may arise in a branch duct (BD-IPMN). Any combination of the above two types is designated as mixed-type IPMN. Most IPMNs arise from the main duct within the head or the uncinate process of the pancreas and progress along the duct[13]. BD-IPMNs are characterised by the presence of multifocal cystic lesions in different sites of the gland, sometimes with a complete involvement of the entire pancreas.
Microscopically, the epithelium is represented by tall, mucin-producing columnar cells that frequently form papillary projections and exhibit variable degrees of cellular atypia, even within an individual neoplasm. Noninvasive IPMN is graded according to the most atypical area as IPMN with low-grade dysplasia (adenoma), IPMN with moderate dysplasia (borderline), and IPMN with high-grade dysplasia (carcinoma in situ). If an invasive component is present, which occurs in 30%-50% of cases, the tumor is called an IPMN with an associated invasive carcinoma[9,14-16]. Progression from adenoma to carcinoma is estimated to occur within 5-6 years[15,16]. The frequency of malignancy (in situ and invasive carcinoma) in MD-IPMNs is high, ranging between 60% and 92%, with a mean of 70%[6,15-19]. By contrast, in BD-IPMNs the frequency of malignancy is significantly lower (between 6% and 46%, with a mean of 25%) and that of invasive cancer ranges from 0% to 30% (mean of 15%)[6,15,17,19-21].
Another unique feature of IPMNs is their association with malignancy in other organs. Breast, colorectal, lung and prostate cancers are the most common extra-pancreatic tumors. The rate of association of IPMNs with malignant neoplasms in extrapancreatic organs has been reported to range from 23.6% to 32%[22,23].
IPMNs occur most frequently in men during the age of 60-70 years, but they can sometimes concern younger patients[13,24]. Detection of IPMNs is usually done by other imaging modalities rather than EUS itself. The usual clinical scenario is that of a cyst found by abdominal ultrasound, CT, or MRI, either incidentally or during diagnostic work-up in patients presenting with abdominal pain, recurrent acute pancreatitis, or chronic obstructive pancreatitis. Pain and pancreatitis are both caused by mucin-induced obstruction of the pancreatic duct[24]. The reported prevalence of pancreatic cystic lesions is 1.2%-2.9%, when CT is used[12,25], and 13.5%-44.7%, when MRI is the imaging modality[26,27].
EUS is a more invasive diagnostic procedure which allows high-resolution imaging of the pancreas. Since the vast majority of patients will have undergone prior abdominal imaging before presenting for EUS, the latter is carried out as part of a multi-modality diagnostic evaluation[28].
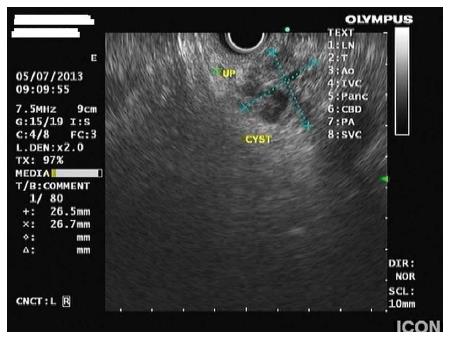
Cystic pancreatic lesions include pseudocysts, congenital and simple cysts and cystic neoplasms. Cystic neoplasms consist of serous cystadenoma (SCA), mucinous cystic neoplasm (MCN) and IPMN. In addition, there are pancreatic tumors that contain cystic spaces or cystic degeneration components, like solid-pseudopapillary neoplasm, cystic endocrine tumor and ductal adenocarcinoma). Certain morphological features have been used to predict particular types of pancreatic cysts. A cyst with accompanying features of pancreatitis, in the absence of septations and mural nodules, suggests a pseudocyst[29]. Multiple microcysts (< 3 mm) within a cystic lesion, occasionally with a honeycomb-like appearance in an asymptomatic patient strongly suggests serous cystadenoma. On the other hand, MCNs are usually cysts with septations of variable thickness, a visible wall and peripheral calcifications in up to 15% of cases[30]. IPMNs are usually macrocystic-type lesions, occasionally accompanied by parenchymal changes due to obstruction of the duct, which communicate with the pancreatic duct (Figure 1). If this communication can be identified by EUS, it definitely distinguishes IPMNs from MCNs or macrocystic SCAs, which both do not communicate with the ductal system[29].
The identification of a connection between the cystic lesion and the pancreatic ductal system is suggestive of the diagnosis of IPMN. This is usually feasible with the use of EUS, which due to its high resolution and proximity of the transducer to the pancreas, gives an excellent imaging of the ductal system.
EUS findings in IPMNs include segmental or diffuse, moderate to marked dilatation of the main pancreatic duct, often associated with intraductal nodules. Dilatation of the main pancreatic duct ≥ 1 cm strongly suggests MD-IPMN. However, according to “the international consensus guidelines 2012 for the mamagement of IPMN and mucinous cystic neoplasms of the pancreas”, the threshold of main duct dilatation has been lowered to > 5 mm without other causes of obstruction[31]. This change has increased the sensitivity for diagnosis of MD-IMPN without losing specificity.
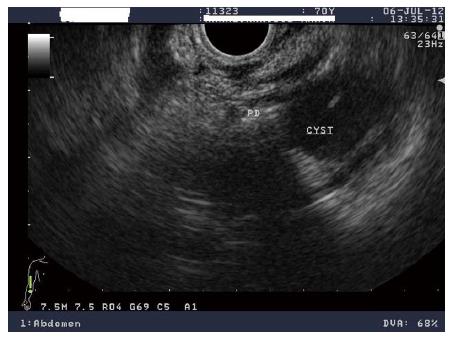
The presence of a cyst communicating with the pancreatic duct without main duct dilatation or with main duct diameter < 6 mm suggests BD-IPMN (Figure 2). The finding of multiple cysts supports the diagnosis of BD-IPMN, as these tumors are more frequently multifocal than MD-IPMNs. In mixed-type IPMNs, in addition to the presence of BD-IPMN, the main pancreatic duct contains papillary growth of columnar epithelium of various degrees of dysplasia. Pancreatic parenchymal atrophy is also frequently recognised in both MD-IPMNs and BD-IPMNs. Since, MD-IPMNs and BD-IPMNs have significant differences in prevalence of cancer, the correct classification has prognostic implications[24,31].
Invasive carcinoma has been reported in 33%-60% of cases in larger series of resected MD-IPMNs[16,19,32,33]. A pancreatic main duct > 1 cm in diameter, the presence of mural nodules and/or symptoms (especially jaundice and diabetes) are risk factors of invasive cancer[15,16,18,34]. A similar incidence of invasive cancer has been reported in main duct and mixed-type IPMN[34]. Hence, mixed-type IPMN should be considered as a main duct disease. Based on the high prevalence of malignancy, all patients fit for surgery with MD- or mixed type-IPMN should undergo resection[28].
BD-IPMNs harbor invasive carcinoma in 11%-30% of cases in large series[19,21,32,35]. The presence of mural nodules, dilatation of the main pancreatic duct > 6 mm, a growth rate over 2 mm/year, the presence of symptoms and elevated serum levels of CA 19-9 are all considered to be risk factors and indications for resection[36-42]. Cyst size greater than 3 cm has been shown to be associated with malignancy in a few studies[17]. However, later studies showed that cyst size alone was not a predictive factor of malignancy, since cancer was found in smaller lesions[43,44]. Furthermore, it was reported that even cysts larger than 3cm can be followed safely, as long as there are no other signs of malignancy[45]. Thus, dimension correlates with the risk of cancer, but there is no safe lower size limit that completely excludes malignancy[28].
The above data imply that EUS can differentiate benign from malignant IPMNs by accurately measuring the size of the cyst, the diameter of the main pancreatic duct and by detecting the presence of mural nodules. In a study done to investigate the value of EUS in differentiating malignant from benign IPMN, 51 patients with IPMN were preoperatively examined by EUS. The histopathological findings of the resected specimens were compared with the endosonographic findings. MD-IPMN with MD dilatation ≥ 10 mm, BD-IPMN (greater than 40 mm) with irregular septa, and large mural nodules (greater than 10 mm) strongly suggested malignancy on EUS[46].
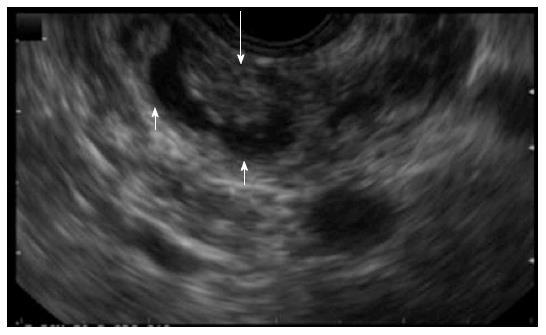
The presence of mural nodules in patients with IPMNs was shown to be a strong predictor of malignancy and may help determine whether the treatment strategy should be surgical resection or conservative management[20,21,45,47,48]. EUS is the most sensitive imaging modality used to detect mural nodules[29,49-51] (Figure 3). Most BD-IPMNs without mural nodules on EUS remained unchanged during long-term follow-up, suggesting that BD-IPMNs without mural nodules could be managed conservatively[37,45]. A study from Baba et al[50], comparing EUS with ultrasound, CT and magnetic resinance cholangiopancreatography (MRCP) concluded that EUS was the most effective in differentiating between benign IPMNs from malignant tumors, by assessing the height of protrusion of lesion within the cysts. In a study from Hara et al[52], using intraductal ultrasonography, 88% of lesions protruding 4mm or more were malignant. A recent study from Kim et al[53], showed that BD-IPMNs smaller than 16 mm, without main pancreatic duct dilatation can be safely followed-up with CT or MRCP, while BD-IPMNs greater than 16 mm or with main pancreatic duct dilatation need an initial EUS evaluation for detection of mural nodules. Recently, Kobayashi et al[54] studied 36 patients with BD-IPMN and found that the diameter of the mural nodule of papillary protrusions and the width diameter reliably distinguished low-risk from high-risk IPMNs (4.3 mm vs 16.4 mm and 5.7 mm vs 23.2 mm, respectively).
Contrast-enhanced harmonic EUS is often used to examine the microvasculature and perfusion in the pancreas. Contrast-enhanced EUS (CE-EUS) detects signals from microbubbles produced by intravenously administered contrast agents and filters signals originating from tissues by selectively detecting harmonic components. This technology can detect signals from microbubbles in vessels with very slow flow without Doppler-related artifacts and is used to characterize vascularity[55]. Different second-generation ultrasound contrast agents, designed and optimized with regard to their resistance to pressure, have been developed for CE-EUS. They consist of microbubbles which are filled with different chemicals, e.g., sulfur hexafluoride (Sonovue, Bracco United Kingdom Ltd., United Kingdom) or galactose (Levovist, Nihon Schering Co., Ltd., Tokyo, Japan), etc. After intravenous injection of these agents, vascularity is temporarily enhanced, allowing for better morphological evaluation of lesions[51,55,56]. Kitano et al[55] reported that contrast-enhanced endoscopic sonography was a useful tool for characterising pancreatic tumors. Using contrast-enhanced EUS, Ohno et al[51] classified mural nodules of IPMN into four types: type I: low papillary nodule, type II: polypoid nodule, type III: papillary nodule, type IV: invasive nodule. The diagnosis of IPMNs with type III or IV mural nodule had a sensitivity of 60%, specificity of 92.9% and accuracy of 75.9% for predicting malignancy. Same authors, by using contrast-enhanced EUS, also showed that the existence of mural nodules and involvement of the main pancreatic duct at initial presentation of patients with BD-IPMNs were significant predictors of malignant transformation[56].
It is sometimes difficult to precisely evaluate the presence of mural nodules, as they can not be easily distinguised from mucous clots. Contrast-enhanced EUS discriminates mural nodules from mucous clots in IPMNs by evaluating the vascularity of the protrusions: nodules are vascular, whereas clots are not. A recent study showed that the sensitivity, specificity, positive predictive value, negative predictive value and accuracy of contrast-enhanced EUS for mural nodule detection were 100%, 80%, 92%, 100% and 94%, respectively[57].
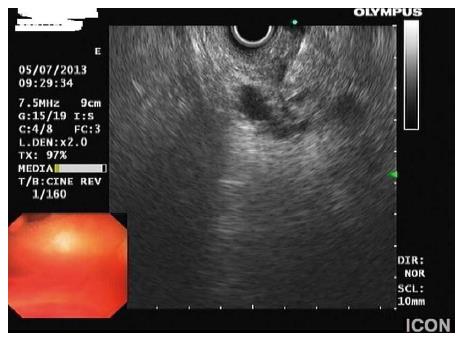
EUS has the advantage of allowing real-time guidance of FNA of cystic lesions. This is very important considering that EUS morphology alone has a diagnostic accuracy of just 50% in patients with pancreatic cysts[3]. The fluid obtained by EUS-FNA is mainly used for cytology and measurement of tumor markers. Cytological specimens in IPMNs may be characterized by abudant mucin, very little inflammatory cells, neoplastic cells either single, cohesive or forming papillae and mucinous epithelium[58]. Immunostaining can demonstrate cells positive for mucin 1, mucin 2 or mucin 5AC[7] (Figure 4).
The most important differential diagnosis achieved by EUS-FNA is the distinction between mucinous (including IPMNs) and non-mucinous cysts. A recent meta-analysis demonstrated that EUS with cyst fluid cytology could differentiate between mucinous and non-mucinous lesions with a sensitivity of 54% and specificity of 93%[59], while fluid’s CEA sensitivity was 63% and specificity 88%[59].
A cut-off of ≥ 192-200 ng/mL is approximately 80% accurate for the diagnosis of a mucinous cyst[31,60,61]. However, a low CEA level does not exclude a mucinous cyst. Cyst fluid amylase is not uniformly elevated in IPMN, and MCN may also exhibit elevated amylase levels[61]. Serous cysts typically have low levels of both CEA and amylase, while pseudocysts have amylase levels > 250 U/L, but low CEA levels[28]. In our experience it is difficult to differentiate between IPMNs and pseudocysts by cyst fluid markers alone, since there is an overlap between values of amylase and CEA levels in these two entities.
Fernández-Esparrach et al[62], demonstrated that EUS-FNA had a sensitivity of 82%, a specificity of 100%, positive predictive value of 100%, negative predictive value of 92% and accuracy of 94% in diagnosing IPMNs. Although cytology can be helpful in the diagnosis of IPMNs, its sensitivity is limited in many studies by the scant cellularity of the specimen[60,63,64]. In a recent study from Lim et al[65], 132 patients with cystic pancreatic lesions underwent EUS-FNA. Pseudocysts and IPMNs were the predominant lesions in the cohort and cytologic yield was 47%. However, when a solid component was present in the cyst, doing more than one pass increased the diagnostic yield from 44% to 78%.
Cyst fluid analysis can also help in identifying malignancy in IPMNs. In a study from Pais et al[66], the sensitivity, specificity and accuracy of EUS-FNA for the diagnosis of malignancy in IPMNs were 75%, 91%, and 86%, respectively, while the level of CEA was of limited value. Similarly, a recent study from Kucera et al[67], showed that CEA level of cyst fluid is a poor predictor of malignancy within an IPMN. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of a cyst fluid CEA concentration greater than 200 ng/mL for the diagnosis of malignant IPMN was 52.4%, 42.3%, 42.3%, 52.4% and 46.8%, respectively, in the above study.
In cases of small sample size, DNA analysis may be possible and looks promising[68]. Recently, the presence of K-ras mutation was found helpful in the diagnosis of mucinous cysts with a specificity of 96%[69]. Detection of K-ras mutations supports a mucinous rather than malignant cyst. Recent studies indicated that Guanine nucleotide binding protein (G protein), alpha stimulating activity polypeptide 1 mutations (GNAS mutations) may be helpful in distinguishing mucinous cysts from indolent cysts that can be managed conservatively. GNAS mutations of codon 201 which are unique to IPMNs have been detected in more than 60% of IPMNs[70,71]. DNA analysis, therefore accurately identifies mucinous cysts, including IPMNs.
In summary, EUS-FNA has its limitations in identifying IPMNs, but by combining cytology, measurement of tumor markers and perhaps DNA analysis where possible, it can definitely aid in the diagnosis of these tumors. Cytology and measurement of CEA must always be performed in the fluid of a pancreatic cyst, while DNA analysis is still experimental and not widely available. Cystic fluid analysis alone is not usually adequate for identifying IPMNs. It’s results should always be interpreted in conjunction with clinical information and EUS morhologic findings.
Intraductal ultrasonography (IDUS) has higher resolution than EUS due to the higher ultrasound frequency. The probes that are used have a small size (5-10 Fr) and their scanning frequencies are between 12 and 30 MHz. After being inserted into the duct, either by free cannulation or over a guidewire, during standard ERCP, these probes give an image of the pancreatic duct with one or three layers[72]. IDUS has been reported as a reliable tool for a more detailed evaluation of pancreatic tumors, especially IPMNs[73,74]. Hara et al[52] reported that the combination of peroral pancreatoscopy and IDUS resulted in a considerably improved differential diagnosis between malignant and benign IPMN. Yamao et al[75] reported that the combination of EUS and intraductal ultrasonography showed great accuracy in the diagnosis of invasive IPMN. IDUS was also found to be useful in preoperative localization and prediction of extension of IPMN[72]. Kobayashi et al[76] performed IDUS in 24 patients with BD-IPMN and detected lateral spread of these tumors in 54% of cases. In this group of patients, the main pancreatic duct had a diameter of more than 6 mm. However, there is an inverse relationship between high ultrasound frequency and depth of penetration, which means that these probes have limited utility in the detection of lesions more than a few millimetres away from the pancreatic duct[24]. Preoperative IDUS may therefore be beneficial for the determination of resection line in IPMNs.
In summary, IDUS is helpful in differentiating malignant and invasive IPMNs from benign ones and also in determining the extent of surgical resection. However, it is a more invasive and less available procedure than standard EUS which can only be performed in tertiary centres and not in routine clinical practice.
Existing tumor markers in the cyst fluid have limited value in the diagnosis of IPMNs and more sensitive biomarkers need to be identified. Proteomics and molecular analysis are new techniques that may be helpful in the differential diagnosis of pancreatic cysts and thus, the identification of IPMNs[77].
In vivo real-time imaging can be performed using confocal laser endomicroscopy. This thechnology involves the EUS-guided placement of a miniprobe through a 22G needle inside lesions located near the digestive tract[78]. It is possible that EUS-guided confocal laser endomicroscopy might be practised soon and possibly aid in the diagnosis of IPMNs by giving in vivo histologic images of the pancreatic cysts.
Surgical resection is the treatment of choice when IPMNs meet the criteria mentioned earlier. However, recent studies showed that pancreatic cyst ablation with ethanol or ethanol followed by paclitaxel is feasible[79-81]. Studies included patients with IPMNs, but they generally had a short follow-up of patients for documentaion of cyst resolution. Therefore, EUS-guided ablation is currently experimental and should be used only in patients who refuse surgery or are high-risk surgical candidates[82]. Further prospective studies with longer follow-up are necessary.
IPMNs represent an increasingly common diagnostic and therapeutic challenge. Once a pancreatic cyst is detected, usually by other imaging modalities, EUS will greatly help in the differential diagnosis. It can accurately classify IPMNs into MD-IPMNs, BD-IPMNs or mixed-type tumors and also identify features highly indicative of malignancy. The use of contrast can also point out malignant features, such as mural nodules, and EUS-FNA can give further information by providing fluid for cytology, tumor markers and possibly DNA analysis. IDUS, if available, is useful in the evaluation of IPMNs, allowing accurate localization and prediction of extension. Therefore, EUS is a valuable tool in the diagnosis and further management of these intriguing cystic tumors of the pancreas.
P- Reviewers: Frider B, Fölsch UR, Fujino Y S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Ohashi K, Murakami Y, Takekoshi T. Four cases of mucin producing cancer of the pancreas on specific findings of the papilla of Vater. Prof Dig Endosc. 1982;20:348-351. |
| 2. | Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. World Health Organization international histologic typing of tumors of the exocrine pancreas. Berlin, Germany: Springer 1996; 11-20. |
| 3. | Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 490] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 4. | Yoon WJ, Lee JK, Lee KH, Ryu JK, Kim YT, Yoon YB. Cystic neoplasms of the exocrine pancreas: an update of a nationwide survey in Korea. Pancreas. 2008;37:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Pedrosa I, Boparai D. Imaging considerations in intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:324-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Salvia R, Crippa S, Partelli S, Armatura G, Malleo G, Paini M, Pea A, Bassi C. Differences between main-duct and branch-duct intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:342-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Grützmann R, Niedergethmann M, Pilarsky C, Klöppel G, Saeger HD. Intraductal papillary mucinous tumors of the pancreas: biology, diagnosis, and treatment. Oncologist. 2010;15:1294-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Brugge WR. The role of EUS in the diagnosis of cystic lesions of the pancreas. Gastrointest Endosc. 2000;52:S18-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B, Klöppel G. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 253] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Allen PJ, D’Angelica M, Gonen M, Jaques DP, Coit DG, Jarnagin WR, DeMatteo R, Fong Y, Blumgart LH, Brennan MF. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244:572-582. [PubMed] |
| 11. | Lee CJ, Scheiman J, Anderson MA, Hines OJ, Reber HA, Farrell J, Kochman ML, Foley PJ, Drebin J, Oh YS. Risk of malignancy in resected cystic tumors of the pancreas & lt; or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008;12:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 658] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 13. | Farrell JJ, Brugge WR. Intraductal papillary mucinous tumor of the pancreas. Gastrointest Endosc. 2002;55:701-714. [PubMed] |
| 14. | Azar C, Van de Stadt J, Rickaert F, Devière M, Baize M, Klöppel G, Gelin M, Cremer M. Intraductal papillary mucinous tumours of the pancreas. Clinical and therapeutic issues in 32 patients. Gut. 1996;39:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 136] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788-97; discussion 797-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 621] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 16. | Salvia R, Fernández-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678-85; discussion 685-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 545] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 17. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1442] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 18. | Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, Qin R, Chari ST, Farnell MB. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639-46; discussion 646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 19. | Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, Howard TJ, Zyromski NJ, Nakeeb A, DeWitt JM. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644-51; discussion 651-4. [PubMed] |
| 20. | Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, Pederzoli P. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56:1086-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Thayer SP, Lauwers GY, Capelli P, Mino-Kenudson M. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72-9; quiz 309-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 22. | Tanaka M. Intraductal papillary mucinous neoplasm of the pancreas: diagnosis and treatment. Pancreas. 2004;28:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Sugiyama M, Atomi Y. Extrapancreatic neoplasms occur with unusual frequency in patients with intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 1999;94:470-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Petrone MC, Arcidiacono PG. Role of endosocopic ultrasound in the diagnosis of cystic tumours of the pancreas. Dig Liver Dis. 2008;40:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Berland LL. The American College of Radiology strategy for managing incidental findings on abdominal computed tomography. Radiol Clin North Am. 2011;49:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 436] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 27. | Girometti R, Intini S, Brondani G, Como G, Londero F, Bresadola F, Zuiani C, Bazzocchi M. Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopancreatography: prevalence and relation with clinical and imaging features. Abdom Imaging. 2011;36:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, Friess H, Manfredi R, Van Cutsem E, Löhr M. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 29. | Lim LG, Itoi T, Lim WC, Mesenas SJ, Seo DW, Tan J, Wang HP, Akaraviputh T, Lakhtakia S, Omar S. Current status on the diagnosis and management of pancreatic cysts in the Asia-Pacific region: role of endoscopic ultrasound. J Gastroenterol Hepatol. 2011;26:1702-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Sarr MG, Carpenter HA, Prabhakar LP, Orchard TF, Hughes S, van Heerden JA, DiMagno EP. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 233] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 32. | Crippa S, Fernández-Del Castillo C, Salvia R, Finkelstein D, Bassi C, Domínguez I, Muzikansky A, Thayer SP, Falconi M, Mino-Kenudson M. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (2)] |
| 33. | Kim SC, Park KT, Lee YJ, Lee SS, Seo DW, Lee SK, Kim MH, Jang SJ, Byun JH, Han DJ. Intraductal papillary mucinous neoplasm of the pancreas: clinical characteristics and treatment outcomes of 118 consecutive patients from a single center. J Hepatobiliary Pancreat Surg. 2008;15:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Crippa S, Partelli S, Falconi M. Extent of surgical resections for intraductal papillary mucinous neoplasms. World J Gastrointest Surg. 2010;2:347-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 35. | Kanno A, Satoh K, Hirota M, Hamada S, Umino J, Itoh H, Masamune A, Asakura T, Shimosegawa T. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol. 2010;45:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Akita H, Takeda Y, Hoshino H, Wada H, Kobayashi S, Marubashi S, Eguchi H, Tanemura M, Mori M, Doki Y. Mural nodule in branch duct-type intraductal papillary mucinous neoplasms of the pancreas is a marker of malignant transformation and indication for surgery. Am J Surg. 2011;202:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Maguchi H, Tanno S, Mizuno N, Hanada K, Kobayashi G, Hatori T, Sadakari Y, Yamaguchi T, Tobita K, Doi R. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas. 2011;40:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 38. | Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, Pearson RK, Petersen BT, Topazian MD, Vege SS. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Shin SH, Han DJ, Park KT, Kim YH, Park JB, Kim SC. Validating a simple scoring system to predict malignancy and invasiveness of intraductal papillary mucinous neoplasms of the pancreas. World J Surg. 2010;34:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Kang MJ, Jang JY, Kim SJ, Lee KB, Ryu JK, Kim YT, Yoon YB, Kim SW. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2011;9:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Rautou PE, Lévy P, Vullierme MP, O’Toole D, Couvelard A, Cazals-Hatem D, Palazzo L, Aubert A, Sauvanet A, Hammel P. Morphologic changes in branch duct intraductal papillary mucinous neoplasms of the pancreas: a midterm follow-up study. Clin Gastroenterol Hepatol. 2008;6:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Matthaei H, Norris AL, Tsiatis AC, Olino K, Hong SM, dal Molin M, Goggins MG, Canto M, Horton KM, Jackson KD. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Walsh RM, Vogt DP, Henderson JM, Hirose K, Mason T, Bencsath K, Hammel J, Brown N. Management of suspected pancreatic cystic neoplasms based on cyst size. Surgery. 2008;144:677-84; discussion 684-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Weinberg BM, Spiegel BM, Tomlinson JS, Farrell JJ. Asymptomatic pancreatic cystic neoplasms: maximizing survival and quality of life using Markov-based clinical nomograms. Gastroenterology. 2010;138:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Tanno S, Nakano Y, Nishikawa T, Nakamura K, Sasajima J, Minoguchi M, Mizukami Y, Yanagawa N, Fujii T, Obara T. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long-term follow-up results. Gut. 2008;57:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 46. | Kubo H, Chijiiwa Y, Akahoshi K, Hamada S, Harada N, Sumii T, Takashima M, Nawata H. Intraductal papillary-mucinous tumors of the pancreas: differential diagnosis between benign and malignant tumors by endoscopic ultrasonography. Am J Gastroenterol. 2001;96:1429-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 308] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 48. | Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg. 2012;397:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Kobayashi G, Fujita N, Noda Y, Ito K, Horaguchi J, Takasawa O, Akaishi S, Tsuchiya T, Kobari M. Mode of progression of intraductal papillary-mucinous tumor of the pancreas: analysis of patients with follow-up by EUS. J Gastroenterol. 2005;40:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Baba T, Yamaguchi T, Ishihara T, Kobayashi A, Oshima T, Sakaue N, Kato K, Ebara M, Saisho H. Distinguishing benign from malignant intraductal papillary mucinous tumors of the pancreas by imaging techniques. Pancreas. 2004;29:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Ohno E, Hirooka Y, Itoh A, Ishigami M, Katano Y, Ohmiya N, Niwa Y, Goto H. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg. 2009;249:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 52. | Hara T, Yamaguchi T, Ishihara T, Tsuyuguchi T, Kondo F, Kato K, Asano T, Saisho H. Diagnosis and patient management of intraductal papillary-mucinous tumor of the pancreas by using peroral pancreatoscopy and intraductal ultrasonography. Gastroenterology. 2002;122:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 53. | Kim YI, Woo SM, Lee WJ, Han SS, Park SJ, Kim TH, Koh YH, Hong EK. Appropriate indications of initial endoscopic ultrasound evaluation for detecting mural nodules in branch duct intraductal papillary mucinous neoplasms of the pancreas. Scand J Gastroenterol. 2013;48:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Kobayashi N, Sugimori K, Shimamura T, Hosono K, Watanabe S, Kato S, Ueda M, Endo I, Inayama Y, Maeda S. Endoscopic ultrasonographic findings predict the risk of carcinoma in branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2012;12:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Kitano M, Sakamoto H, Matsui U, Ito Y, Maekawa K, von Schrenck T, Kudo M. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video). Gastrointest Endosc. 2008;67:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 56. | Ohno E, Itoh A, Kawashima H, Ishikawa T, Matsubara H, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M, Miyahara R. Malignant transformation of branch duct-type intraductal papillary mucinous neoplasms of the pancreas based on contrast-enhanced endoscopic ultrasonography morphological changes: focus on malignant transformation of intraductal papillary mucinous neoplasm itself. Pancreas. 2012;41:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 57. | Yamashita Y, Ueda K, Itonaga M, Yoshida T, Maeda H, Maekita T, Iguchi M, Tamai H, Ichinose M, Kato J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: a single-center prospective study. J Ultrasound Med. 2013;32:61-68. [PubMed] |
| 58. | Salla C, Chatzipantelis P, Konstantinou P, Karoumpalis I, Sakellariou S, Pantazopoulou A, Manika Z. Endoscopic ultrasound-guided fine-needle aspiration cytology in the diagnosis of intraductal papillary mucinous neoplasms of the pancreas. A study of 8 cases. JOP. 2007;8:715-724. [PubMed] |
| 59. | Thornton GD, McPhail MJ, Nayagam S, Hewitt MJ, Vlavianos P, Monahan KJ. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology. 2013;13:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 60. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 901] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 61. | Park WG, Mascarenhas R, Palaez-Luna M, Smyrk TC, O’Kane D, Clain JE, Levy MJ, Pearson RK, Petersen BT, Topazian MD. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas. 2011;40:42-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 62. | Fernández-Esparrach G, Pellisé M, Solé M, Soria MT, Miquel R, Mata A, Llach J, Bordas JM, Ginès A. EUS FNA in intraductal papillary mucinous tumors of the pancreas. Hepatogastroenterology. 2007;54:260-264. [PubMed] |
| 63. | Centeno BA, Warshaw AL, Mayo-Smith W, Southern JF, Lewandrowski K. Cytologic diagnosis of pancreatic cystic lesions. A prospective study of 28 percutaneous aspirates. Acta Cytol. 1997;41:972-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | de Jong K, Bruno MJ, Fockens P. Epidemiology, diagnosis, and management of cystic lesions of the pancreas. Gastroenterol Res Pract. 2012;2012:147465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Lim LG, Lakhtakia S, Ang TL, Vu CK, Dy F, Chong VH, Khor CJ, Lim WC, Doshi BK, Varadarajulu S. Factors determining diagnostic yield of endoscopic ultrasound guided fine-needle aspiration for pancreatic cystic lesions: a multicentre Asian study. Dig Dis Sci. 2013;58:1751-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Pais SA, Attasaranya S, Leblanc JK, Sherman S, Schmidt CM, DeWitt J. Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: correlation with surgical histopathology. Clin Gastroenterol Hepatol. 2007;5:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Kucera S, Centeno BA, Springett G, Malafa MP, Chen YA, Weber J, Klapman J. Cyst fluid carcinoembryonic antigen level is not predictive of invasive cancer in patients with intraductal papillary mucinous neoplasm of the pancreas. JOP. 2012;13:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 68. | Shen J, Brugge WR, Dimaio CJ, Pitman MB. Molecular analysis of pancreatic cyst fluid: a comparative analysis with current practice of diagnosis. Cancer. 2009;117:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, Brugge WR, Edmundowicz SA, Hawes RH, McGrath KM. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 70. | Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci USA. 2011;108:21188-21193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 482] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 71. | Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 597] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 72. | Soweid A, Azar C, Labban B. Endosonographic evaluation of intraductal papillary mucinous tumors of the pancreas. JOP. 2004;5:258-265. [PubMed] |
| 73. | Furukawa T, Oohashi K, Yamao K, Naitoh Y, Hirooka Y, Taki T, Itoh A, Hayakawa S, Watanabe Y, Goto H. Intraductal ultrasonography of the pancreas: development and clinical potential. Endoscopy. 1997;29:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Yamao K, Ohashi K, Furukawa T, Nakamura T, Suzuki T, Kanemaki N, Nakamura Y, Teramoto S. [Progress in the instrument used for diagnosis of obstructive jaundice. 1) Ultrasonic endoscope]. Nihon Naika Gakkai Zasshi. 1997;86:582-587. [PubMed] |
| 75. | Yamao K, Ohashi K, Nakamura T, Suzuki T, Watanabe Y, Shimizu Y, Nakamura Y, Ozden I. Evaluation of various imaging methods in the differential diagnosis of intraductal papillary-mucinous tumor (IPMT) of the pancreas. Hepatogastroenterology. 2001;48:962-966. [PubMed] |
| 76. | Kobayashi G, Fujita N, Noda Y, Ito K, Horaguchi J, Obana T, Koshida S, Kanno Y, Yamashita Y, Kato Y. Lateral spread along the main pancreatic duct in branch-duct intraductal papillary-mucinous neoplasms of the pancreas: usefulness of intraductal ultrasonography for its evaluation. Dig Endosc. 2011;23:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Ke E, Patel BB, Liu T, Li XM, Haluszka O, Hoffman JP, Ehya H, Young NA, Watson JC, Weinberg DS. Proteomic analyses of pancreatic cyst fluids. Pancreas. 2009;38:e33-e42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Becker V, Wallace MB, Fockens P, von Delius S, Woodward TA, Raimondo M, Voermans RP, Meining A. Needle-based confocal endomicroscopy for in vivo histology of intra-abdominal organs: first results in a porcine model (with videos). Gastrointest Endosc. 2010;71:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | DeWitt J, DiMaio CJ, Brugge WR. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc. 2010;72:862-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | DeWitt J, McGreevy K, Schmidt CM, Brugge WR. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc. 2009;70:710-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 81. | DiMaio CJ, DeWitt JM, Brugge WR. Ablation of pancreatic cystic lesions: the use of multiple endoscopic ultrasound-guided ethanol lavage sessions. Pancreas. 2011;40:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 82. | Zhang WY, Li ZS, Jin ZD. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World J Gastroenterol. 2013;19:3397-3403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (1)] |