Published online Jun 28, 2014. doi: 10.3748/wjg.v20.i24.7767
Revised: February 23, 2014
Accepted: March 12, 2014
Published online: June 28, 2014
Processing time: 242 Days and 10 Hours
Endoscopic management of leakages and perforations of the upper gastrointestinal tract has gained great importance as it avoids the morbidity and mortality of surgical intervention. In the past years, covered self-expanding metal stents were the mainstay of endoscopic therapy. However, two new techniques are now available that enlarge the possibilities of defect closure: endoscopic vacuum therapy (EVT), and over-the-scope clip (OTSC). EVT is performed by mounting a polyurethane sponge on a gastric tube and placing it into the leakage. Continuous suction is applied via the tube resulting in effective drainage of the cavity and the induction of wound healing, comparable to the application of vacuum therapy in cutaneous wounds. The system is changed every 3-5 d. The overall success rate of EVT in the literature ranges from 84% to 100%, with a mean of 90%; only few complications have been reported. OTSCs are loaded on a transparent cap which is mounted on the tip of a standard endoscope. By bringing the edges of the perforation into the cap, by suction or by dedicated devices, such as anchor or twin grasper, the OTSC can be placed to close the perforation. For acute endoscopy associated perforations, the mean success rate is 90% (range: 70%-100%). For other types of perforations (postoperative, other chronic leaks and fistulas) success rates are somewhat lower (68%, and 59%, respectively). Only few complications have been reported. Although first reports are promising, further studies are needed to define the exact role of EVT and OTSC in treatment algorithms of upper gastrointestinal perforations.
Core tip: The novel technique of endoscopic vacuum therapy has recently been developed for the closure of upper gastrointestinal (GI) perforations. A sponge is connected to a gastric tube, and then endoscopically placed into the perforation or cavity. The first case series demonstrate excellent healing rates with very low procedure-related morbidity; it appears likely that this technique will become the new standard for upper GI perforations. A second novel endoscopic option is the over-the-scope clip (OTSC) which allows full thickness closure of smaller defects and fistulas. Both endoscopic vacuum therapy and OTSC are valuable contributions to endoscopic therapy of upper GI perforations.
- Citation: Mennigen R, Senninger N, Laukoetter MG. Novel treatment options for perforations of the upper gastrointestinal tract: Endoscopic vacuum therapy and over-the-scope clips. World J Gastroenterol 2014; 20(24): 7767-7776
- URL: https://www.wjgnet.com/1007-9327/full/v20/i24/7767.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i24.7767
Perforations and fistulas of the upper gastrointestinal (GI) tract occur as postoperative complications (anastomotic dehiscence or fistula)[1], during diagnostic or interventional endoscopy[2], iatrogenic as a consequence of other therapeutic measures (e.g., gastric tube placement, percutaneous endoscopic gastrostomy, transesophageal echocardiography)[3], or spontaneously (ulcers, tumors, Boerhaave syndrome, and others)[4]. These perforations often lead to severe septic conditions which are difficult to treat and give rise to a high morbidity and mortality, especially if leading to mediastinitis or peritonitis[1,4].
The management of upper GI perforations depends on the severity of the complication and on the condition of the patient. Small leakages or fistulas without septic complications (Clavien-Dindo classification[5] I and II) permit conservative management including the placement of a nasogastric tube and antibiotic therapy. Grade III perforations, defined by onset of fever or sepsis without organ failure, require endoscopic, surgical or radiological intervention. These grade III complications are in particular the domain of endoscopic treatment modalities. A typical example is the partial dehiscence of an esophagogastric anastomosis following esophagectomy, with a vital gastric tube and a mediastinal cavity developing from the leakage.
In recent years interventional endoscopy has evolved as an effective alternative to primary surgery in these cases. The placement of self-expanding fully or partially covered metal or plastic stents (SEMS or SEPS) has become the first line therapy in esophageal anastomotic leakages or perforations, if the patient is not in critical septic condition[6,7]. Due to the success of these techniques and their avoidance of the considerable risks of emergency surgery, the indications for endoscopic intervention are being widened to even encompass grade IV perforations, defined by critical states involving single or multiorgan failure. However, it must be carefully evaluated whether endoscopic intervention is sufficient to control the septic condition; surgical revision remains mandatory in cases of severe sepsis or life threatening conditions.
Although the placement of self-expanding stents has become the mainstay of endoscopic therapy of upper gastrointestinal perforations, failure of stent therapy still occurs in about 15%[6] of cases.
The well-established stent therapy is now being challenged by two relatively new endoscopic options, the first being endoscopic vacuum therapy (EVT). While it can already be considered as standard therapy for leakages of lower colorectal anastomoses, its use in the upper GI tract only evolved several years later. Yet soon after first reports of the technical feasibility of endoscopic vacuum therapy in the upper GI tract[8-10], several case series with good success rates were published. The technique appears to have potential as a first line therapy for postoperative upper GI leaks.
The second new option is a novel endoscopic clipping device, the over-the-scope clip (OTSC; Ovesco Endoscopy AG, Tübingen, Germany)[11]. This device has dramatically increased the possibilities of endoscopic defect closure, as compared to through-the-scope clips (TTSC). Much as for EVT, the number of promising case series being published for OTSC based management of upper GI leakage is ever increasing.
In this review, we describe both novel endoscopic options. We examine technical aspects, summarize the current literature with a focus on success rates for different indications, and discuss the role of the new techniques for management algorithms of upper GI perforations.
A review of the available literature on endoscopic vacuum therapy and over-the-scope clips was performed with the latest search date being October 15, 2013. References of all articles were searched to identify further relevant publications. For both novel techniques, the exact procedure is described in the section “Development and technical aspects”, based on published reports and the authors’ own experience. The success rates stated in the literature are summarized in the “Results” section for each technique. To this end, patient numbers of all available studies were added and pooled means were calculated. When a center published more than one paper on their patient series, only the newest publication was considered. For illustration purposes, a clinical case is presented from the authors’ institution demonstrating the use of both EVT and OTSC. Finally, in the section “Therapy algorithms of upper GI perforations including the novel techniques”, the potential roles of EVT and OTSC in clinical practice are critically discussed.
Over the last decade, endoscopic treatment has changed the approach to intrathoracic leakage after esophagectomy and esophageal perforation. The reported leak rates after esophagectomy vary widely from 1% to 30%[12,13]. Anastomotic leakage accounts for approximately 40% of all postoperative fatalities[14] and is highly challenging to treat: Control of the septic focus is essential, thus the already critically ill patient often requires intensive additional measures that themselves are associated with high morbidity, adding to the clinical burden. A number of competing treatment modalities ranging from conservative to surgical approaches are available for the management of this situation[15]. The surgical treatment options include revision of the anastomosis, closure of the defect and perifocal drainage or complete surgical deviation and creation of a cervical stoma[15,16]. These procedures are usually difficult and carry a high risk for severe complications associated with high morbidity and mortality rates. Therefore re-operation is not always a reasonable option.
In this context, numerous minimally invasive treatment options have more recently become available to treat a variety of secondary surgical complications. Conservative management may be advantageous if reliable endoscopic methods are available. Endoscopic clips, fibrin glue injection[17], absorbable plugs, and endoscopic suturing (EndoCinch)[18,19] have been used to close smaller defects. At present, the placement of completely covered metal or plastic stents is the favored conservative treatment option for esophageal leakage[7,20]. The implantation of these stents has been thoroughly studied and was proven to be effective[7,21,22]. However, stent implantation does not always lead to a sufficient sealing of the leakage, and dislocation rates of up to 40% have been reported[23]. Another important complication is failure of stent extraction due to ingrowth of granulation tissue and/or secondary strictures due to scarring[21,24]. While stents bridge the defect intraluminally and prevent further leakage, a continuous local drainage is necessary to prevent inflammatory fluids from remaining in the perianastomotic tissues and maintaining inflammation.
The vacuum-assisted closure (VAC) system device is an established treatment modality for extensive and infected cutaneous wounds[25]. Negative pressure is applied to the wound with a vacuum-sealed sponge, resulting in drainage of wound secretion, improved blood flow, reduction of edema, promotion of granulation, and consecutive wound closure. Since its introduction in the 1990s, the number of indications for the VAC system has steadily increased. Initial reports have shown good results for endoscopically placed VAC systems in the treatment of leakage of rectal anastomoses[26,27]. Encouraged by these results, EVT was then applied in the endoscopically accessible upper GI tract.
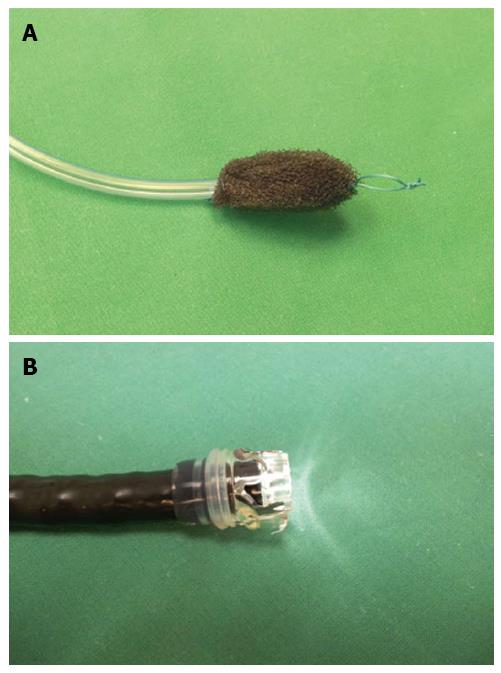
In brief, EVT is carried out by endoscopic insertion of polyurethane sponges into the leakage induced abscess cavity (Figures 1A and 2), followed by application of a controlled continuous negative pressure. Negative pressure is applied via a transnasal gastric tube that is connected to the sponge. Thus, the vital steps towards leak closure are achieved: drainage of inflammatory fluids and induction of tissue granulation. Initial placement and subsequent changing of sponges can be performed under sedation with midazolam and propofol and adequate monitoring of the patient.
Since its first description by Wedemeyer et al[10] and Loske et al[8,28], the above mentioned principle is used by all authors with only little variations in the procedure.
The polyurethane foam sponge (e.g., VAC® GranuFoamTM, pore size 400-600 μm; KCI® - KineticConcepts, Inc., TX, United States, and Wiesbaden, Germany) is cut into shape according to the particular wound size and geometry as estimated by the endoscopist. The sponge must be smaller than the wound cavity to promote collapse and subsequent closure. At each session, the size of the defect is assessed and treated with an individually prepared sponge, cut to fit the lesion’s dimensions. The sponge is fixed to the tip of a PVC (polyvinyl chloride) gastroduodenal tube [e.g., CovidienTM Salem SumpTM, 14 Fr/Ch (4.7 mm) × 114 cm; CovidienTM, MA, United States] with a suture at the proximal and distal ends of the sponge[10,28,29]. For successful drainage, it is vital that the side ports of the tube communicate with the sponge and that the tube be placed in the middle of the sponge. Some authors employ ready-to-use sets including an already connected drainage tube, omitting the need of manual attachment (e.g., Endo-Sponge system, B. Braun, Melsungen, Germany)[13,30].
When placing the sponge into the wound cavity, most authors grasp the sponge with a forceps, pull it close to the endoscope and place it in the wound cavity under direct endoscopic vision[10,28,29]. We prefer this method, and in our opinion it is further simplified by the placement of an additional suture loop at the tip of the sponge (Figure 1A). This loop serves as a purchase for the endoscopic forceps and facilitates manipulation of the sponge into difficult-to-access cavities and hollow spaces under direct vision using a regular orthograde video endoscope. The sponge drainage system is guided in parallel to the endoscope (“backpack-method”) and placed appropriately.
Other authors use the overtube-technique that has been frequently described for rectal applications[13,30]. In this case, the overtube is placed into the cavity, and, after the endoscope has been withdrawn from the overtube, the sponge is brought down by a pusher.
If the defect entrance is not initially wide enough to accommodate the endoscope, the opening must be dilated. If however the cavity itself is too small to be accessed with the scope, the sponge may also be placed in the esophageal lumen. Over the course of the treatment and with diminishing defect size, sponge placement can be changed from its initial intracavitary position to intraluminal position at any time. Moreover, in large leakage cavities, up to two sponges can be placed separately to allow rapid and sufficient drainage. After sponge placement, the vacuum drainage tube is diverted through the nose. Some authors first insert the gastric tube nasally and extract it orally to connect the sponge and then proceed with the endoscopic placement[10,28].
Continuous suction of 100-125 mmHg generated by a vacuum pump (e.g., actiVAC® or VACulta® KCI®) is connected transnasally to the drainage tube under permanent direct endoscopic vision to ensure that the sponge stays in position. Vacuum containers can be used in lieu of a pump[13,30]; however, we prefer pumps as they ensure constant pressure which can be modified if necessary. Additionally, a transnasal enteral feeding tube can be placed in the same session to ensure full enteral nutrition (Figure 2C). In contrast to vacuum therapy for open wounds, EVT does not require sealing to obtain air tightness[10,28].
Suction must be discontinued for sponge removal. It is advisable to flush the tube with 0.9% saline solution to dissolve the granulation tissue from the pores of the sponge prior to removal. Subsequently, the tube should be grasped with the endoscopic forceps close to the distal end, retracted from the wound cavity with increasing force and withdrawn through the mouth. In some cases, the sponge can be removed without use of an endoscopic instrument, simply by removing the drainage tube. A subsequent endoscopic exploration of the cavity is obligatory. In accordance with the experience of VAC therapy for skin defects, the sponge should be replaced twice a week, until the cavity appears to be clean and firmly closed. The remaining wound cavity should be smaller than ca. 1 cm radius × 2 cm depth. After completion of EVT, patients should be followed up endoscopically once weekly until complete healing of the defect.
A synopsis of studies reporting endoscopic vacuum therapy for upper gastrointestinal perforations is shown in Table 1. All patients (n = 101) included in these studies did not suffer from any intervention related complications. The overall success of closing the leaks by EVT in these patients was 90% (84%-100%). One patient died during dilation of a stenosis after complete healing due to an aorto-esophageal fistula. The available studies and data indicate that EVT is feasible, safe and effective. The main complications associated with EVT are stenosis after completed therapy due to scarring. EVT is well-tolerated, effective and associated with good short-term and long-term clinical outcomes.
| Ref. | Year | Overall (n) | Postoperative (n) | Other perforations (n) | Overall success | Complications | Mortality | Stenosis after completed therapy |
| Ahrens et al[13] | 2010 | 5 | 5 | 0 | 5/5 (100) | 0/5 (0) | 1/5 (20)1 | 2/5 (40) |
| Brangewitz et al[55] | 2013 | 32 | 30 | 2 | 27/32 (84) | 0/32 (0) | 5/32 (16) | 3/32 (9) |
| Kuehn et al[29] | 2012 | 9 | 5 | 4 | 8/9 (89) | 0/9 (0) | 1/9 (11) | |
| Schorsch et al[3] | 2013 | 24 | 17 | 7 | 23/24 (96) | 1/24 (4) | 1/18 (6) | |
| Schniewind et al[54] | 2013 | 17 | 17 | 0 | 2/17 (12) | |||
| Wedemeyer et al[56] | 2010 | 8 | 8 | 0 | 7/8 (88) | 0/8 (0) | 0/8 (0) | 1/8 (13) |
| Weidenhagen et al[30] | 2010 | 6 | 6 | 0 | 6/6 (100) | 0/6 (0) | 1/6 (17) | 1/6 (17) |
| Overall | 101 | 88 | 13 | 76/84 (90) | 0/60 (0) | 11/101 (11) | 8/69 (12) |
In the past decades, endoscopic clip application has repeatedly been used as a minimally invasive treatment option for small leakages and fistulas of the upper GI tract. Most authors used through-the-scope clips (TTSC) that were designed for endoscopic bleeding control. However, the success of these attempts was limited to case reports[17,31], and endoscopic clipping of perforations did not reach widespread routine clinical use. The efficacy of TTSCs is limited by their little wing span and the low compression force that the clips can apply.
The novel over-the-scope clip (OTSC; Ovesco Endoscopy AG, Tübingen, Germany) has revolutionized the principle of endoscopic clipping and has overcome the above-mentioned limitations[11]. Instead of introducing the clip via the working channel, a nitinol clip with the shape of a “bear claw” is loaded on a transparent cap that is mounted on the tip of the endoscope (Figure 1B). Different sizes of caps and corresponding nitinol clips (11-14 mm) are available for different endoscope types and lesion characteristics. Clips are available with blunt (s or a type, atraumatic version) or with pointed teeth (t type, traumatic version). An additional clip type (gc type) has been added for the special purpose of closing gastric wall defects during natural orifice transluminal surgery.
The lesion (bleeding ulcer, fistula, or wall defect) is pulled into the cap; in the case of adequately mobile tissues, this can be accomplished by suction. A special “anchor” is supplied by Ovesco for the treatment of smaller fibrotic fistulas: the device is introduced into the fistula, and after opening of the anchor, the fistula as a whole is pulled into the cap. A further special device is the “twin grasper”. This instrument has two lateral branches that can be opened independently and close against a fixed central branch. This is especially helpful for adapting the edges of a larger defect and pulling them into the cap. However, some endoscopists prefer the use of “nondedicated” conventional endoscopic instruments (e.g., rat tooth, alligator forceps) being introduced via the working channel which is possible as well[32].
The method of deploying the clip is similar to that of devices used for rubber band ligation. A string that is attached to the clip is pulled through the working channel and is connected to a hand wheel; the clip is fired by turning the wheel.
The advantages of OTSC over TTSC are: (1) larger lesions can be closed by one clip (limited by cap diameter and flexibility of the tissue being pulled into the cap); and (2) greater compression force. The nitinol OTSC provides constant compression forces of ca. 8 to 9 Newton, which is necessary for successful permanent closure of defects[11].
A literature search in Pubmed was performed with the key words “over-the-scope clip” and “OTSC” (latest search date: 15 October 2013). Case reports, and series without gastrointestinal perforations (e.g., only reporting OTSC use in GI bleeding) were excluded from further analysis. Apart from perforations, gastrointestinal bleeding is an important indication for OTSCs, however, this was not the focus of this review. From the remaining 24 studies, indications for OTSC application, overall success, success by indication and leak site, and complications were extracted (Table 2)[2,11,32-53].
| Ref. | Year | n | Overall success | Postoperative | Acute endoscopic and interventional perforations | Other chronic leaks and fistulas | Upper GI | Colorectal | Complications |
| Albert et al[33] | 2011 | 12 | 8/12 (66) | 5/6 (83) | 2/2 (100) | 1/4 (25) | 7/9 (78) | 1/3 (33) | 0/12 (0) |
| Arezzo et al[34] | 2012 | 14 | 12/14 (86) | 12/14 (86) | 12/14 (86) | 0/14 (0) | |||
| Baron et al[35] | 2012 | 36 | 24/36 (67) | 10/14 (71) | 4/5 (80) | 10/17 (59) | 19/27 (70) | 5/9 (56) | 2/36 (6)3 |
| Jacobsen et al[36] | 2012 | 10 | 5/10 (50) | 5/10 (50) | 5/9 (56) | 0/1 (0) | 0/10 (0) | ||
| Disibeyaz et al[37] | 2012 | 9 | 5/9 (56) | 4/7 (57) | 1/1 (100) | 0/1 (0) | 4/8 (50) | 1/1 (100) | 0/9 (0) |
| Galizia et al[38] | 2012 | 3 | 3/3 (100) | 3/3 (100) | 3/3 (100) | 0/3 (0) | |||
| Gubler et al[39] | 2012 | 14 | 13/14 (93) | 13/14 (93)1 | 4/5 (80) | 9/9 (100)1 | 0/14 (0) | ||
| Hagel et al[40] | 2012 | 17 | 11/17 (65) | 2/3 (67) | 7/10 (70) | 2/4 (50) | 9/15 (60) | 2/2 (100)2 | 0/17 (0) |
| Jayaraman et al[32] | 2013 | 21 | 12/21 (57) | 0/21 (0) | |||||
| Kirschniak et al[11] | 2007 | 4 | 4/4 (100) | 4/4 (100) | 1/1 (100) | 3/3 (100) | 0/4 (0) | ||
| Kirschniak et al[41] | 2011 | 19 | 14/19 (74) | 1/2 (50) | 11/11 (100) | 2/6 (33) | 10/12 (83) | 4/7 (57) | 0/19 (0) |
| Manta et al[42] | 2011 | 12 | 11/12 (92) | 11/12 (92) | 7/8 (88) | 4/4 (100) | 0/12 (0) | ||
| Mennigen et al[43] | 2013 | 14 | 11/14 (79) | 10/12 (83) | 1/2 (50) | 8/9 (89) | 3/5 (60) | 0/14 (0) | |
| Mönkemüller et al[44] | 2013 | 7 | 3/7 (43) | 1/3 (33) | 2/4 (50) | 3/7 (43) | 0/7 (0) | ||
| Nishiyama et al[45] | 2013 | 13 | 11/13 (85) | 7/8 (88) | 4/5 (80) | 7/9 (78) | 4/4 (100) | 0/13 (0) | |
| Parodi et al[46] | 2010 | 10 | 8/10 (80) | 4/6 (67) | 1/1 (100) | 3/3 (100) | 4/4 (100) | 4/6 (67) | 0/10 (0) |
| Pohl et al[47] | 2010 | 2 | 1/2 (50) | 1/2 (50) | 1/2 (50) | 0/2 (0) | |||
| Repici et al[48] | 2009 | 2 | 2/2 (100) | 2/2 (100) | 2/2 (100) | 0/2 (0) | |||
| Sandmann et al[49] | 2011 | 10 | 9/10 (90) | 2/3 (67) | 3/3 (100) | 4/4 (100) | 7/8 (88) | 2/2 (100) | 0/10 (0) |
| Schlag et al[50] | 2013 | 6 | 6/6 (100) | 6/6 (100) | 6/6 (100) | 0/6 (0) | |||
| Seebach et al[51] | 2010 | 7 | 5/7 (71) | 2/3 (67) | 3/4 (75) | 1/2 (50) | 4/5 (80) | 0/7 (0) | |
| Surace et al[52] | 2011 | 19 | 8/19 (42) | 7/18 (39) | 1/1 (100) | 7/15 (47) | 1/4 (25) | 0/19 (0) | |
| Voermans et al[2] | 2012 | 36 | 32/36 (89) | 1/1 (100) | 31/35 (89) | 20/23 (87) | 12/13 (92) | 2/36 (6)4 | |
| Von Renteln et al[53] | 2010 | 4 | 2/4 (50) | 0/1 (0) | 2/3 (67) | 2/4 (50) | 0/4 (0) | ||
| Overall | 301 | 220/301 (73) | 81/120 (68) | 95/106 (90)b | 32/54 (59) | 135/186 (73) | 73/94 (78) | 4/301 (1.3) |
Early reports stem from Europe, where the OTSC device was available first[11], however, after introduction of the OTSC to the United States and Japanese market, several case series signify an increasing use in these countries as well[35,44,45]. The indications for OTSC use and the obtained results are similar to the early experiences from Europe.
There are no randomized trials on OTSC application, and most authors provide retrospective case series with heterogeneous indications and applications. Most studies included less than 20 patients with gastrointestinal perforations; cases of gastrointestinal bleeding were excluded from the present analysis. Several publications report the pooled results of 2 or 3 centers[35,44,52]. The largest numbers of patients with gastrointestinal perforations treated by OTSC are provided by two multicenter studies. The first was a prospective European multicenter study on OTSC application (“the CLIPPER study group”)[2], the second a retrospective study carried out by 3 North American tertiary-care referral medical centers[35], both reporting on 36 patients with gastrointestinal perforations.
Overall, 301 patients with gastrointestinal perforations were included in 24 publications. The etiology of perforations was highly heterogeneous across all studies; this must be taken into account when comparing the actual success rates. Most studies report initial technical success, i.e., immediate closure of the perforation, proven by imaging using a contrasting agent. Long term clinical success was termed to mean lasting closure of the perforation without need of further endoscopic or surgical treatment. The reported long term overall success rates for the closure of gastrointestinal perforations, leakages and fistulas range from 42% to 100% (Table 2). The pooled overall success rate was 73% (220/301). It is important to note, however, that follow-up times vary significantly between the studies, and some authors only provide short follow-up times.
There was considerable heterogeneity regarding the classification of gastrointestinal perforations throughout the investigated studies. For more valid comparison of success rates, we propose the following classification: (1) Postoperative leaks and fistulas, including acute anastomotic dehiscence as well as chronic fistulas. A further stratification into acute vs chronic leaks should be mandatory for future studies, but this information could not be satisfactorily retrieved from the presently investigated publications; (2) Acute iatrogenic perforations, i.e., perforations during diagnostic/therapeutic endoscopy and interventional procedures; and (3) Other chronic leaks and fistulas. Many authors include chronic leaks and fistulas in their series that do not meet the definitions of categories 1 and 2, e.g., enterocutaneous fistulas, perforated ulcers, or persistent gastrocutaneous fistulas following the extraction of a gastrostomy tube.
We classified all reported patients - for which the necessary information was available in the respective publication - into these 3 categories and calculated the success rates for each category as a whole (pooled overall estimate) and per single study. Finally, we analyzed success rates for upper GI perforations vs colorectal perforations.
The overall success rate for acute endoscopic and interventional perforations (category 2) was as high as 90% (95/106), which is significantly higher than the values for postoperative perforations (category 1) (68%, 81/120), and other chronic leaks and fistulas (category 3) (59%, 32/54) (χ2; P < 0.001). From the technical point of view, the setting of acute endoscopic perforation is optimal for OTSC use: the lesion is fresh and without fibrotic alterations or inflammation and usually free from foreign bodies in the leakage area, e.g., food. Further, due to the iatrogenic nature of these perforations, the patient mostly already is in a specialized endoscopy unit, thus enabling a rapid OTSC closure. In cases of sufficient closure, the otherwise mandatory surgical intervention can be avoided; some authors have already coined the phrase “sparing the surgeon” for this process[51]. However, some concerns remain. Endoscopic therapies with a high risk of perforation should be performed with CO2 insufflation, as the resulting pneumoperitoneum can resolve much quicker once the perforation has been closed by OTSC. Massive pneumoperitoneum and consecutive abdominal pain can lead to surgical exploration, although the perforation was sufficiently closed by an OTSC. Gubler et al[39] reported successful closure of acute endoscopic perforations in 13/14 patients. Of note: 3 of the patients with successful OTSC closure were still taken to surgery after OTSC application. Sufficient closure of the perforations was then, however, documented in the OR; all of these patients had colorectal perforations. Hagel et al[40] reported a success rate of 7/10 patients for acute perforations; however, of the 2 patients with colorectal perforations, 1 underwent surgery which again showed successful OTSC application. None of the patients with upper GI perforations treated by OTSC was taken to surgery unnecessarily. It appears that in clinical practice colorectal perforations tend to prompt a surgical exploration, as a persistent colorectal leak can lead to a dismal clinical course. Among the many encouraging reports, one lethal case from the European multicenter study by Voermans et al[2] deserves special attention. A patient with initially successful closure of a colonic perforation deteriorated several hours later and was taken to the OR. During laparotomy, the OTSC was found to be dislocated and the perforation reopened. Despite surgical therapy, the patient died. This case illustrates that the successful application of the OTSC should be proven by contrast studies, patients must be closely monitored, and necessary surgical therapies must not be delayed.
The overall success rate for postoperative leakages and fistulas is 68% (81/120). There is a wide span of indications that are summarized in this category; besides chronic fistulas of gastric sleeve resection, chronic fistulas of esophagogastric or esophagojejunal anastomoses, chronic fistulas of colorectal anastomoses, some cases of acute anastomotic dehiscence are included. The numbers are too small to clarify which indications profit the most from OTSC closure. However, as most leakages and fistulas were chronic, this likely is the cause for the lower success rates vs acute endoscopic perforations. The most frequently mentioned reasons for failure of OTSC closure are fibrotic alterations of the lesion and active inflammation - both common features of chronic anastomotic fistulas.
The same is true for the variety of chronic fistulas of different etiology summarized in category 3. The success rate in this category (59%, 32/54) is similar to that of category 1, comprised of postoperative leakages.
Regarding the site of OTSC application, there were no significant differences between the outcomes of upper GI (135/186, 73%) and colorectal (73/94, 78%) placement. Both sites seem suitable for OTSC applications.
Complications are infrequent, indeed most authors did not observe any at all. Only two multicenter studies[2,35] report complications: in the European multicenter study[2], one OTSC detached from a colorectal perforation leading to a delayed operation (as discussed above), and one esophageal perforation occurred during the introduction of the OTSC device. In the US study[35], in 2 patients the lumen of the small bowel was occluded by a misplaced OTSC, leading to surgical management. Taken together, the complication rate in all reported patients was 4/301 (1.3%).
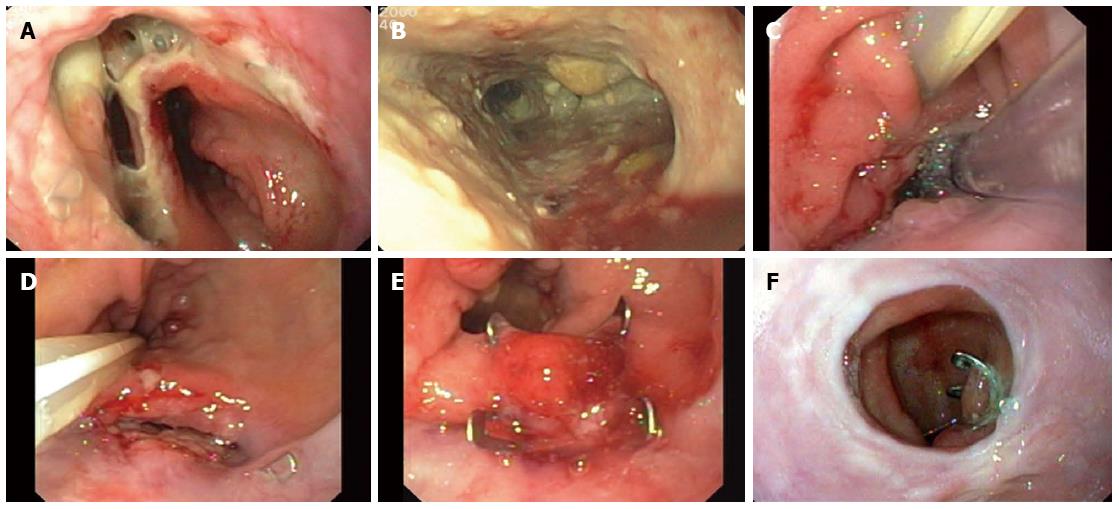
In a 65-year-old woman, we performed esophagectomy with esophagogastric anastomosis using a circular stapler (Figure 2). The patient developed fever and blood work showed persisting signs of inflammation, triggering endoscopy and diagnosis of anastomotic dehiscence on day 5. The anastomosis showed a rather small dehiscence and the patient was in a stable condition without sepsis. Therefore we placed a covered stent which remained in place for 6 wk. Surprisingly, after planned extraction of the stent, the dehiscence had grown in size (Figure 2A), and endoscopy revealed a large mediastinal cavity (Figure 2B) with a fistula to the right bronchial system (demonstrated by contrast study). We initiated EVT of the cavity, with changes of the sponge every 3-5 d (Figure 2C). During EVT, enteral nutrition was applied via a second gastric tube which was placed in the duodenum. After 15 d, the former cavity had shrunk to a fistula of approximately 3 cm in length and 1 cm in width (Figure 2D). Further closure could not be obtained because of the bronchial fistula. Finally, an OTSC was placed on the internal esophageal fistula opening which led to permanent closure (Figure 2E). Endoscopic control at last follow-up (11 mo) demonstrated that the OTSC was still in situ (Figure 2F). This case demonstrates how patients with stent failure can be successfully managed using the discussed novel endoscopic treatment options.
Many centers have established algorithms for upper GI perforations that include endoscopic stent therapy. This therapy is reported as effective and safe by a large number of publications, and it is available at most hospitals. However, the novel techniques have the potential to further enlarge the portion of patients that can be managed by endoscopy; stent failure rates of about 15%[6] require the development of a “plan B” beyond stent-placement.
Although the existing reports on both EVT and OTSC are enthusiastic, the present review of their use for gastrointestinal perforations has several limitations. First of all, both novel technical developments were used as individual, non-standardized therapies in very different settings. Virtually all publications on these novel techniques include a variety of perforation types, different localizations, and different underlying diseases. Up to now, there is no controlled trial to evaluate the value of these methods in comparison to existing standard therapies, such as stent placement.
Only a few studies are focused on a special type of perforation, the vast majority of studies lack this standardization. As shown in Table 2 for application of OTSC, most studies are very small, with patient numbers below n = 20, and include very heterogeneous types of perforations.
In an effort to organize this heterogeneous data pool on OTSCs, we propose the classification into the three types of perforation “postoperative”, “acute endoscopic and interventional perforations”, and “other chronic leaks and fistulas”, as we are certain that at the very least, these categories must be separately examined due to the completely different settings in which they occur. The same is true for EVT (Table 1): the number of available studies is even lower, patient numbers are small, and the largest studies again include postoperative as well as other perforations. Keeping these limitations in mind, suitable indications for EVT and OTSC are as follows.
In the setting of acute endoscopic perforations, the OTSC obviously is a very effective treatment option, and in our opinion it is clearly superior to the placement of covered stents. However, comparative studies are not available, and in regard of the excellent results obtained by OTSC, it seems unethical to plan such a study. We propose that OTSC should be the first line therapy for acute endoscopic or interventional perforations of the upper gastrointestinal tract.
Endoscopic treatment of chronic fistulas and leaks can be challenging. Stent therapy, application of fibrin glue, or closure with TTS clips have shown disappointing results in the past. In our opinion, OTSCs are a very promising option in these situations. The risk of OTSC application is very low, it is easy to perform, and success rates of about 60% are satisfactory, especially as OTSCs often are used in cases refractory to various other treatment modalities. Furthermore, in case of failure, the OTSC procedure does not impair further treatment, such as EVT, or surgery[43].
We believe that EVT will become the new gold standard in the endoscopic therapy of acute anastomotic dehiscence, especially following esophagectomy and gastrectomy. Usually, these leakages are associated with mediastinal cavities. After closure of the leak by stent placement, the drainage of these cavities can be insufficient. This accounts for many cases of stent failure, as in our presented case. In contrast to stent therapy, EVT allows optimal drainage of the cavity and additionally the lesion can be inspected regularly, allowing early detection of any deterioration. Finally, vacuum therapy leads to a remarkable debridement of the cavity with ensuing granulation.
The success rates of defect closure by EVT are excellent and seem to be higher than those reported for stent therapy. However, there is a paucity of data comparing EVT to stent therapy: up to date, only two retrospective studies exist[54,55]. Schniewind et al[54] analyzed the outcomes of 62 patients with anastomotic leaks following esophagectomy. After matching for APACHE-Scores at the beginning of complication therapy, EVT resulted in a significantly lower mortality (12%) compared to surgically treated patients (50%) and patients treated by stent placement (83%). Brangewitz et al[55] compared 39 patients managed by stent placement and 32 patients managed by EVT. The rate of leakage closure was significantly higher in the EVT group (84% vs 54%). However, in contrast to Schniewind’s study[54], no difference was found for hospital mortality.
Although these first data indicate advantages for EVT, further studies comparing this new technique to stent therapy are necessary. In the above mentioned studies[54,55], both mortality (83% and 25%, respectively) of patients treated by stent placement, and defect closure rate (54%) are dramatically inferior to values published in current dedicated studies on stent therapy[6]. This might in part be explained by early discontinuation of stent therapy in favor of EVT; a bias in patient selection in these retrospective studies could be another explanation. Taken together, the advantage of EVT will probably be lower than these two publications suggest.
Both novel techniques, EVT and OTSC application, are comparatively easy to use, and are safe and effective in the treatment of upper GI perforations. Anastomotic dehiscence with mediastinal cavity appears to profit intensively from EVT, and EVT has the potential to become the new gold standard for this indication. OTSC closure is very effective in acute endoscopic perforations and is a viable option for small chronic fistulas. However, data on both techniques are limited, and no controlled studies exist. Further studies are needed to compare the novel techniques to other treatment modalities, and to define their exact place in treatment algorithms of upper GI perforations.
P- Reviewers: Sajid MS, Yokoyama S S- Editor: Zhai HH L- Editor: O’Neill M E- Editor: Zhang DN
| 1. | Junemann-Ramirez M, Awan MY, Khan ZM, Rahamim JS. Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg. 2005;27:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Voermans RP, Le Moine O, von Renteln D, Ponchon T, Giovannini M, Bruno M, Weusten B, Seewald S, Costamagna G, Deprez P. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol. 2012;10:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Schorsch T, Müller C, Loske G. Endoscopic vacuum therapy of anastomotic leakage and iatrogenic perforation in the esophagus. Surg Endosc. 2013;27:2040-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Biancari F, D’Andrea V, Paone R, Di Marco C, Savino G, Koivukangas V, Saarnio J, Lucenteforte E. Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studies. World J Surg. 2013;37:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8516] [Article Influence: 532.3] [Reference Citation Analysis (0)] |
| 6. | van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther. 2011;33:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Tuebergen D, Rijcken E, Mennigen R, Hopkins AM, Senninger N, Bruewer M. Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: efficacy and current limitations. J Gastrointest Surg. 2008;12:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Loske G, Müller C. [Vacuum therapy of an esophageal anastomotic leakage--a case report]. Zentralbl Chir. 2009;134:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Loske G, Schorsch T, Müller C. Endoscopic vacuum sponge therapy for esophageal defects. Surg Endosc. 2010;24:2531-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Wedemeyer J, Schneider A, Manns MP, Jackobs S. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc. 2008;67:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Kirschniak A, Kratt T, Stüker D, Braun A, Schurr MO, Königsrainer A. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc. 2007;66:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Whooley BP, Law S, Murthy SC, Alexandrou A, Wong J. Analysis of reduced death and complication rates after esophageal resection. Ann Surg. 2001;233:338-344. [PubMed] |
| 13. | Ahrens M, Schulte T, Egberts J, Schafmayer C, Hampe J, Fritscher-Ravens A, Broering DC, Schniewind B. Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy. 2010;42:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Pross M, Manger T, Reinheckel T, Mirow L, Kunz D, Lippert H. Endoscopic treatment of clinically symptomatic leaks of thoracic esophageal anastomoses. Gastrointest Endosc. 2000;51:73-76. [PubMed] |
| 15. | Crestanello JA, Deschamps C, Cassivi SD, Nichols FC, Allen MS, Schleck C, Pairolero PC. Selective management of intrathoracic anastomotic leak after esophagectomy. J Thorac Cardiovasc Surg. 2005;129:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Page RD, Shackcloth MJ, Russell GN, Pennefather SH. Surgical treatment of anastomotic leaks after oesophagectomy. Eur J Cardiothorac Surg. 2005;27:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Rodella L, Laterza E, De Manzoni G, Kind R, Lombardo F, Catalano F, Ricci F, Cordiano C. Endoscopic clipping of anastomotic leakages in esophagogastric surgery. Endoscopy. 1998;30:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Adler DG, McAfee M, Gostout CJ. Closure of an esophagopleural fistula by using fistula tract coagulation and an endoscopic suturing device. Gastrointest Endosc. 2001;54:652-653. [PubMed] |
| 19. | Fritscher-Ravens A, Cuming T, Eisenberger CF, Ghadimi M, Nilges A, Meybohm P, Schiffmann S, Jacobsen B, Seehusen F, Niemann H. Randomized comparative long-term survival study of endoscopic and thoracoscopic esophageal wall repair after NOTES mediastinoscopy in healthy and compromised animals. Endoscopy. 2010;42:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Salminen P, Gullichsen R, Laine S. Use of self-expandable metal stents for the treatment of esophageal perforations and anastomotic leaks. Surg Endosc. 2009;23:1526-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Doniec JM, Schniewind B, Kahlke V, Kremer B, Grimm H. Therapy of anastomotic leaks by means of covered self-expanding metallic stents after esophagogastrectomy. Endoscopy. 2003;35:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Hünerbein M, Stroszczynski C, Moesta KT, Schlag PM. Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents. Ann Surg. 2004;240:801-807. [PubMed] |
| 23. | Kauer WK, Stein HJ, Dittler HJ, Siewert JR. Stent implantation as a treatment option in patients with thoracic anastomotic leaks after esophagectomy. Surg Endosc. 2008;22:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Schubert D, Scheidbach H, Kuhn R, Wex C, Weiss G, Eder F, Lippert H, Pross M. Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self-expanding polyester stents. Gastrointest Endosc. 2005;61:891-896. [PubMed] |
| 25. | Vikatmaa P, Juutilainen V, Kuukasjärvi P, Malmivaara A. Negative pressure wound therapy: a systematic review on effectiveness and safety. Eur J Vasc Endovasc Surg. 2008;36:438-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Glitsch A, von Bernstorff W, Seltrecht U, Partecke I, Paul H, Heidecke CD. Endoscopic transanal vacuum-assisted rectal drainage (ETVARD): an optimized therapy for major leaks from extraperitoneal rectal anastomoses. Endoscopy. 2008;40:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc. 2008;22:1818-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 28. | Loske G, Müller C. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc. 2009;69:601-602; author reply 602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Kuehn F, Schiffmann L, Rau BM, Klar E. Surgical endoscopic vacuum therapy for anastomotic leakage and perforation of the upper gastrointestinal tract. J Gastrointest Surg. 2012;16:2145-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Weidenhagen R, Hartl WH, Gruetzner KU, Eichhorn ME, Spelsberg F, Jauch KW. Anastomotic leakage after esophageal resection: new treatment options by endoluminal vacuum therapy. Ann Thorac Surg. 2010;90:1674-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Yoshikane H, Hidano H, Sakakibara A, Ayakawa T, Mori S, Kawashima H, Goto H, Niwa Y. Endoscopic repair by clipping of iatrogenic colonic perforation. Gastrointest Endosc. 1997;46:464-466. [PubMed] |
| 32. | Jayaraman V, Hammerle C, Lo SK, Jamil L, Gupta K. Clinical Application and Outcomes of Over the Scope Clip Device: Initial US Experience in Humans. Diagn Ther Endosc. 2013;2013:381873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Albert JG, Friedrich-Rust M, Woeste G, Strey C, Bechstein WO, Zeuzem S, Sarrazin C. Benefit of a clipping device in use in intestinal bleeding and intestinal leakage. Gastrointest Endosc. 2011;74:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Arezzo A, Verra M, Reddavid R, Cravero F, Bonino MA, Morino M. Efficacy of the over-the-scope clip (OTSC) for treatment of colorectal postsurgical leaks and fistulas. Surg Endosc. 2012;26:3330-3333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA. Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos). Gastrointest Endosc. 2012;76:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 36. | Jacobsen GR, Coker AM, Acosta G, Talamini MA, Savides TJ, Horgan S. Initial experience with an innovative endoscopic clipping system. Surg Technol Int. 2012;22:39-43. [PubMed] |
| 37. | Dişibeyaz S, Köksal AŞ, Parlak E, Torun S, Şaşmaz N. Endoscopic closure of gastrointestinal defects with an over-the-scope clip device. A case series and review of the literature. Clin Res Hepatol Gastroenterol. 2012;36:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Galizia G, Napolitano V, Castellano P, Pinto M, Zamboli A, Schettino P, Orditura M, De Vita F, Auricchio A, Mabilia A. The Over-The-Scope-Clip (OTSC) system is effective in the treatment of chronic esophagojejunal anastomotic leakage. J Gastrointest Surg. 2012;16:1585-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Gubler C, Bauerfeind P. Endoscopic closure of iatrogenic gastrointestinal tract perforations with the over-the-scope clip. Digestion. 2012;85:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Hagel AF, Naegel A, Lindner AS, Kessler H, Matzel K, Dauth W, Neurath MF, Raithel M. Over-the-scope clip application yields a high rate of closure in gastrointestinal perforations and may reduce emergency surgery. J Gastrointest Surg. 2012;16:2132-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901-2905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 42. | Manta R, Manno M, Bertani H, Barbera C, Pigò F, Mirante V, Longinotti E, Bassotti G, Conigliaro R. Endoscopic treatment of gastrointestinal fistulas using an over-the-scope clip (OTSC) device: case series from a tertiary referral center. Endoscopy. 2011;43:545-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 43. | Mennigen R, Colombo-Benkmann M, Senninger N, Laukoetter M. Endoscopic closure of postoperative gastrointestinal leakages and fistulas with the Over-the-Scope Clip (OTSC). J Gastrointest Surg. 2013;17:1058-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Mönkemüller K, Peter S, Toshniwal J, Popa D, Zabielski M, Stahl RD, Ramesh J, Wilcox CM. Multipurpose use of the ‘bear claw’ (over-the-scope-clip system) to treat endoluminal gastrointestinal disorders. Dig Endosc. 2014;26:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Nishiyama N, Mori H, Kobara H, Rafiq K, Fujihara S, Kobayashi M, Oryu M, Masaki T. Efficacy and safety of over-the-scope clip: including complications after endoscopic submucosal dissection. World J Gastroenterol. 2013;19:2752-2760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Parodi A, Repici A, Pedroni A, Blanchi S, Conio M. Endoscopic management of GI perforations with a new over-the-scope clip device (with videos). Gastrointest Endosc. 2010;72:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 47. | Pohl J, Borgulya M, Lorenz D, Ell C. Endoscopic closure of postoperative esophageal leaks with a novel over-the-scope clip system. Endoscopy. 2010;42:757-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Repici A, Arezzo A, De Caro G, Morino M, Pagano N, Rando G, Romeo F, Del Conte G, Danese S, Malesci A. Clinical experience with a new endoscopic over-the-scope clip system for use in the GI tract. Dig Liver Dis. 2009;41:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Sandmann M, Heike M, Faehndrich M. Application of the OTSC system for the closure of fistulas, anastomosal leakages and perforations within the gastrointestinal tract. Z Gastroenterol. 2011;49:981-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Schlag C, Wilhelm D, von Delius S, Feussner H, Meining A. EndoResect study: endoscopic full-thickness resection of gastric subepithelial tumors. Endoscopy. 2013;45:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Seebach L, Bauerfeind P, Gubler C. “Sparing the surgeon”: clinical experience with over-the-scope clips for gastrointestinal perforation. Endoscopy. 2010;42:1108-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Surace M, Mercky P, Demarquay JF, Gonzalez JM, Dumas R, Ah-Soune P, Vitton V, Grimaud J, Barthet M. Endoscopic management of GI fistulae with the over-the-scope clip system (with video). Gastrointest Endosc. 2011;74:1416-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 53. | von Renteln D, Denzer UW, Schachschal G, Anders M, Groth S, Rösch T. Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos). Gastrointest Endosc. 2010;72:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose T, Kurdow R, Arlt A, Ellrichmann M, Jürgensen C. Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc. 2013;27:3883-3890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 55. | Brangewitz M, Voigtländer T, Helfritz FA, Lankisch TO, Winkler M, Klempnauer J, Manns MP, Schneider AS, Wedemeyer J. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy. 2013;45:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 56. | Wedemeyer J, Brangewitz M, Kubicka S, Jackobs S, Winkler M, Neipp M, Klempnauer J, Manns MP, Schneider AS. Management of major postsurgical gastroesophageal intrathoracic leaks with an endoscopic vacuum-assisted closure system. Gastrointest Endosc. 2010;71:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |