Published online Jun 28, 2014. doi: 10.3748/wjg.v20.i24.7730
Revised: December 14, 2013
Accepted: March 6, 2014
Published online: June 28, 2014
Processing time: 326 Days and 3.9 Hours
One potential option for the management of refractory gastro-esophageal reflux disease (GERD) is the delivery of radiofrequency energy to the gastro-esophageal junction (Stretta). This endoscopic therapy is safe, effective, durable, and repeatable if necessary and serves an unmet need for many GERD sufferers. Stretta could be effective in decreasing esophageal sensitivity to acid and in decreasing the gastro-esophageal junction compliance, which in turn contributes to symptomatic benefit by decreasing refluxate volume. Therefore, Stretta may serve as an endoscopic pain modulator and should be considered in patients with refractory symptoms despite proton pump inhibitors, as well as in patients with functional heartburn.
Core tip: Stretta may serve as an endoscopic pain modulator and should be considered in patients with refractory symptoms despite proton pump inhibitors, as well as in patients with functional heartburn.
- Citation: Triadafilopoulos G. Stretta: A valuable endoscopic treatment modality for gastroesophageal reflux disease. World J Gastroenterol 2014; 20(24): 7730-7738
- URL: https://www.wjgnet.com/1007-9327/full/v20/i24/7730.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i24.7730
Gastroesophageal reflux disease (GERD) is the most frequent outpatient diagnosis in the United States, with almost 9 million visits in 2009[1]. The typical symptoms of GERD, heartburn and regurgitation, impair quality of life, activity, and overall work productivity. Although effective, proton pump inhibitors (PPI) provide incomplete control of reflux symptoms in up to 40% of patients. A partial response can occur because these drugs do not address an incompetent sphincter or prevent reflux. Consequently, some patients seek alternative treatment if their quality of life is compromised[2]. Failure of the PPI treatment to resolve acid reflux symptoms has become the most common presentation of GERD among gastroenterologists[3]. In patients with non-erosive disease the pooled symptomatic response rate to PPI once daily is 37%. In patients with erosive esophagitis, which accounts for 30%-40% of the GERD population, the pooled symptomatic response rate is 56%. Refractory GERD implies clinically significant impairment of quality of life due to episodes of reflux while on PPI therapy. It is important to emphasize that such refractory GERD symptoms may not always reflect the acidity of the refluxate but may be due to increased refluxate volume, esophageal compliance and individual sensitivity to acid[4,5].
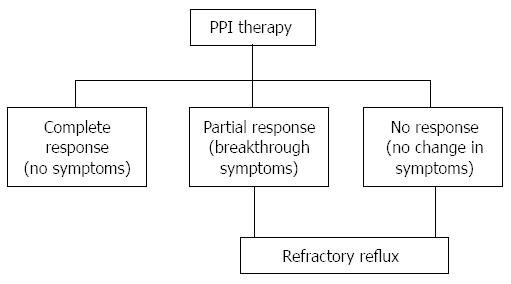
Radiofrequency (RF) energy application to the lower esophageal sphincter (LES) (Stretta procedure) is a valuable option for such refractory patients who are not willing to undergo surgery (fundoplication). Figure 1 depicts the possible outcomes of PPI therapy in the primary care management of GERD. Potential candidates for Stretta would be those with GERD who have breakthrough symptoms, such as, persistent heartburn and/or regurgitation despite escalating doses of PPI (refractory GERD), patients with GERD who are symptomatic because they cannot tolerate PPIs (2% of PPI users), those who desire to stop drug therapy and those who do not wish to undergo anti-reflux surgery (ARS) or are poor surgical candidates. In contrast, patients with refractory GERD who have large sliding hiatal hernia (> 3 cm long), very low LES pressure (LESP < 5 mmHg), no response or change of symptoms with PPI use, or those with negative pH/impedance studies and no symptom correlation with acid events, are not appropriate candidates for either Stretta or ARS and alternate diagnoses should be pursued[6]. In general, patients who exhibit complete response to PPI therapy should not be considered as candidates for Stretta unless there are concerns about the long-term adverse events with PPI use.
Based on several pivotal animal and human studies, Stretta was approved by the United States Food and Drug Administration (FDA) in 2000. This endoscopic technology was originally produced and marketed by Curon Medical, Inc., but the company filed for bankruptcy in 2006. In 2008 Mederi Therapeutics, Inc. (Greenwich, CT) acquired Curon Medical’s assets and Stretta became commercially available again in 2010 but concerns about the procedure’s efficacy, safety and durability have limited its widespread adoption and clinical use. Herein we address seven key questions that provide a framework for a reassessment of the role of Stretta for PPI-dependent, symptomatic GERD sufferers, as a safe and effective endoscopic alternative or adjunct to medical or surgical therapies.
Stretta involves an upper gastrointestinal endoscopy with delivery of thermal energy to the muscle of the lower esophageal sphincter and gastric cardia for the treatment of GERD (CPT code 43257). The Society of American Gastrointestinal and Endoscopic Surgeons has concluded that Stretta is an appropriate therapy for patients with GERD who are18 years of age or older with symptoms of heartburn and/or regurgitation for 6 mo or more, who have been completely or partially responsive to anti-secretory pharmacologic therapy, and who have declined laparoscopic fundoplication[7]. Stretta’s four-channel RF generator and catheter system delivers pure sine-wave energy (465 kHz, 2 to 5 watts per channel, 80 volts maximum at 100 to 800 ohms). Each needle tip incorporates a thermocouple that automatically adjusts the power output to a desired target temperature in the muscle layer. Maintaining target temperatures below 100 °C minimizes any adjacent tissue damage due to vaporization and high impedance values. Temperature is similarly monitored with a thermocouple at each needle base abutting the mucosa and the power delivery ceases if such mucosal temperature exceeds 47 °C.
Patients are prepared for an upper gastrointestinal endoscopy and they typically require high doses of midazolam and either fentanyl or meperidine, or preferably, intravenous propofol. Depending on the level of sedation, there may be mild discomfort due to catheter passage and mild-to-moderate discomfort with RF delivery. An upper gastrointestinal endoscopy is first performed, and the distance from the incisors to the squamo-columnar junction (Z-line) is measured. The endoscope is removed, and the RF catheter is passed through the mouth and positioned 1 cm above the z-line according to the distance previously determined. The four needle electrodes are deployed to a preset length of 5.5 mm and RF delivery is initiated. Each electrode delivers RF energy for 60 s to achieve a target temperature of 85 °C. Additional treatment sites are created by rotating and changing the linear position of the catheter so as to create several rings over a span of 2 cm above and below cardia. The catheter is then removed and the endoscopy repeated. Overall, patients receive RF energy at 56 treatment sites over a period of 35 min (Figure 2). Stretta can be used in challenging anatomic situations since it requires minimal working space and can used to treat the lower esophageal sphincter of patients who have undergone prior gastric bypass or subtotal gastrectomy[8].
The primary outcome of GERD treatment should be rapid and sustained achievement of comprehensive symptom resolution, because this is associated with marked improvement - often normalization - in health-related quality of life[9]. Other desired outcomes are to heal esophageal mucosal damage if it is present and to prevent relapse of erosive esophagitis in the hope that this will reduce the development of complications. Adequate treatment of GERD should either prevent repeated reflux of gastric contents into the esophagus or reduce the damaging effect of gastric acid. The generally accepted outcome measure of efficacy for GERD medical therapy, typically based upon the role of PPI, is symptom control and/or GERD-related quality of life[10]. Stretta has demonstrated consistent attainment of this goal in patients who are well-controlled by PPI but do not wish to take lifelong medications as well as in patients who have partially responded to PPI therapy and do not have a specific surgical indication (i.e., large hiatal hernia).
In a cross-over, randomized trial of 64 patients who were assigned to RF treatment or a sham procedure, those who had undergone the RF procedure were significantly more likely to experience a > 50% improvement in GERD-related quality-of-life scores at six months compared with sham-treated patients (61% vs 30%) and were more likely to be without daily heartburn symptoms (61% vs 33%). The groups had similar median acid exposure times, though acid exposure time was significantly improved when responders (> 30% decrease in heartburn score) were compared with non-responders[11].
In another controlled trial, 36 patients were assigned to a single session Stretta (12 patients), a sham procedure (12 patients), or Streta repeated once if GERD health-related quality of life (HRQL) was not 75% improved after four months (12 patients, 10 of whom underwent a second RF procedure)[12]. Patients who underwent Stretta had greater improvements in quality of life scores at 12 mo than patients who underwent sham therapy, and those treated twice showed a greater improvement than patients who underwent a single treatment. In the single Stretta group, two patients (17%) normalized their quality of life scores, in the double Stretta group seven patients (58%) normalized, and in the sham group no patients normalized. Similar results were seen in the two Stretta treatment groups with regard to the number of patients who were no longer requiring antisecretory drugs.
A nonrandomized, prospective, multicenter study included 118 patients (72 men and 46 women) with chronic heartburn and/or regurgitation who required daily antisecretory medication and exhibited pathologic esophageal acid exposure, a sliding hiatal hernia (≤ 2 cm), and esophagitis (≤ grade 2)[13]. GERD symptom scores, quality of life (short form-36, SF-36), and medication use were assessed at 0, 1, 4, 6, and 12 mo; esophageal acid exposure, motility, and endoscopy were assessed at 0 and 6 mo. At 12 mo, 94 patients were available for follow-up. There were improvements after 12 mo in the median heartburn score (4 to 1, P = 0.0001), GERD score (27 to 9, P = 0.0001), satisfaction (1 vs 4, P = 0.0001), mental SF-36 (46.3 vs 55.4, P < 0.0001), and physical SF-36 (40.9 vs 53.1, P = 0.0001); proton pump inhibitor requirement fell from 88.1% to 30% of patients. Esophageal acid exposure improved significantly (10.2% vs 6.4%, P = 0.0001). There were 10 (8.6%) complications, none of which required therapeutic intervention. The authors concluded that Stretta significantly improves GERD symptoms, quality of life, and esophageal acid exposure and eliminates the need for antisecretory medication in the majority of patients at 12 mo. Follow-up information was available for 94 patients (80%) at 12 mo. Significant improvements were observed in the median heartburn, GERD, and satisfaction scores, and on the mental and physical components of the Medical Outcomes Study SF-36. The proportion of patients requiring proton pump inhibitors fell from 30% to 88%. Esophageal acid exposure improved significantly (from 6% to 10%). The authors noted that the degree of improvement in quality of life was similar to the improvement described following fundoplication. The 24 patients who did not return their questionnaires were counted as treatment failures in the analysis. However, all of the patients were contacted and none had experienced complications. Eighteen of the patients agreed to undergo additional testing that included esophageal manometry. No significant change in any esophageal motility parameter was observed, although there was a trend toward a reduction in the number of transient lower esophageal sphincter relaxations.
In another open trial of 90 patients with non-erosive or mildly erosive disease, the onset of GERD symptom relief after Stretta was less than two mo in 70% or two to six months in 16.7%. The mean GERD-HRQL score was 25.6 (baseline), 7.3 (six months, P < 0.01), and 8.1 (12 mo, P < 0.01). The mean heartburn score was 3.3 (baseline), and 1.2 (12 mo, P < 0.05). The percentage of patients with satisfactory GERD control improved from 31.1% at baseline to 86.7% after treatment, and patient satisfaction improved from 1.4 at baseline to 4.0 at 12 mo (P < 0.01). Medication usage decreased significantly from 100% of patients on PPI therapy at baseline to 76.7% of patients showing elimination of medications or only as-needed use of antacids/H2-receptor antagonists at 12 mo[14].
A recent meta-analysis[15] of 18 studies and 1441 patients concluded that: (1) Stretta is very effective in GERD symptom relief; (2) Is safe and well-tolerated; and (3) Stretta significantly reduces acid exposure to the esophagus, but does not consistently normalize pH. On this last point it is important to note that even PPIs do not normalize pH in up to 50% of symptomatically controlled GERD patients treated with PPIs[16]. Hence, pH normalization is not necessarily an important clinical endpoint to be applied to Stretta.
Overall, Stretta has been shown to be effective in 32 separate clinical studies and a meta-analysis. The primary endpoint of GERD therapy has been consistently achieved, that is, a high-rate of symptom control over and above PPI therapy. Secondarily, a dramatic decrease or elimination of GERD medication use has also been consistently shown.
Thirty-two clinical studies have demonstrated that Stretta is a safe and well-tolerated treatment for GERD. The total number of patients (2774) treated in these studies and the exceedingly low complication rates noted in each, as well as the fact that any noted complications were minor and transient, mirrors the generally good post-marketing experience and safety profile as recorded on the FDA MAUDE website (< 1%). Both numbers are relevant as clinical studies are typically conducted by more experienced users, but FDA reported complications would include non-expert users. To date, more than 15000 Stretta procedures have been performed globally without serious sequela attributable to the procedure[15]. In the study of 118 patients described above, 10 complications were observed (9%), none of which required therapeutic intervention. These included fever (two patients), superficial mucosal injury (three patients), chest pain requiring opioid analgesic use (two patients), transient dysphagia (one patient), sedation-related hypotension (one patient), and submental swelling related to topical analgesia allergy (one patient)[13]. However, serious complications have been described, including esophageal perforation in three patients and two deaths due to aspiration pneumonia[17]. The perforations were attributed to either poor patient selection or operator error.
The effective use of RF therapy in medicine is extremely broad and yet often misunderstood. Different frequencies, power outputs and temperatures create a broad array of therapeutic outcomes. At high frequencies and power outputs, RF is destructive, and typically used to ablate aberrant tissue. At low frequencies and power settings and temperatures, RF can be non-ablative. Stretta therapy operates at 465 mHz and 5 W of power output. The treatment range is 65 °C-85 °C in the muscularis propria, while the maximum mucosal temperature is 49 °C due to constant chilled irrigation of the esophageal and cardiac mucosa. This chilled irrigation typically keeps mucosal temperature below 35 °C. As the muscularis has heat sink properties and the treatment temperature is below the accepted level of tissue ablation (100 °C) and the mucosa is untreated by design, the hypothesis of tissue destruction, followed by formation of fibrosis is completely unfounded by any published clinical studies. The neuromuscular control of the LES and the mechanisms for acid clearance are a complex and incompletely understood set of physiologic functions. Clinical data from several Stretta trials have defined a number of potential improvements in the physiologic function of the LES and distal esophagus (Table 1).
| Increased gastric yield pressure |
| Increased thickness of the lower esophageal sphincter muscle |
| Decreased gastro-esophageal junction compliance without fibrosis |
| Decreased transient lower esophageal sphincter relaxations (tLESRs) |
One animal study explored the effect of Stretta to the porcine gastroesophageal junction and its effect on LES pressure and gastric yield pressure. Twenty pigs underwent esophageal manometry and endoscopic injection of botulinum toxin (100 units) into the lower esophageal sphincter. After 1 wk, animals were randomized to either Stretta (RF, n = 13) or no further intervention (control, n = 7). At 9 wk, animals underwent endoscopy, manometry, and gastric yield pressure determination. The mean LES pressure declined by 3.7 ± 2.6 mmHg (control, P = 0.03) vs 0.97 ± 5.8 mmHg (RF, P = 0.29) after 9 wk. Mean gastric yield pressure was 24.9 ± 8.2 mmHg (control), compared with 43.4 ± 10.7 mmHg (RF) (P = 0.0007). The authors concluded that Stretta reversed much of the LES pressure reduction achieved with botulinum toxin injection and augmented gastric yield pressure by 75% compared with controls[18].
Another animal study assessed Stretta’s effect to the gastric cardia on the triggering of transient LES relaxations and GERD in 13 dogs. Esophageal motility and pH were measured for 1 hour after a standard liquid meal and air infusion, as well as before and 3 mo after radiofrequency energy treatment. At 7 mo, histologic evaluation of the gastroesophageal junction was performed. Stretta reduced the frequency of transient LES relaxations from 4.0 (3.0-6.75) [median (interquartile range)] per hour to 3.0 (2.0-3.0) per hour (P < 0.05). This was accompanied by a significant reduction in acid reflux episodes and esophageal acid exposure. Basal LES pressure and relaxation during swallowing were unchanged. There was a 63% increase in wall thickness at the gastric cardia compared with that in 2 control dogs, but no gross or histopathologic abnormalities of the esophageal or gastric mucosa were seen. The authors concluded that RF delivery to the gastric cardia in dogs inhibits the triggering of transient lower esophageal sphincter relaxations and thereby reduces gastroesophageal reflux[19].
In a double-blind randomized crossover study of Stretta and sham treatment in patients with GERD, Arts et al[20] tested the hypothesis that Stretta alters gastro-esophageal junction (GEJ) resistance. Patients underwent two upper gastrointestinal endoscopies with 3 mo interval, during which active or sham Stretta treatment was performed in a randomized double-blind manner. Symptom assessment, endoscopy, manometry, 24-h esophageal pH monitoring, and a distensibility test of the GEJ using Barostat were done before the start of the study and after 3 mo. Their main outcome measure was Barostat distensibility test of the GEJ before and after administration of sildenafil. In all, 22 GERD patients (17 females, mean age 47 ± 12 years) participated in the study; 11 in each group. Initial sham treatment did not affect any of the parameters studied. Three mo after initial Stretta procedure, no changes were observed in esophageal acid exposure and LES pressure. In contrast, symptom score was significantly improved and GEJ compliance was significantly decreased. Administration of sildenafil, an esophageal smooth muscle relaxant, normalized GEJ compliance again to pre-Stretta level, arguing against GEJ fibrosis as the underlying mechanism. The authors concluded that decreased GEJ compliance, which reflects altered LES neuromuscular function, might contribute to symptomatic benefit by decreasing refluxate volume.
Tam et al[21] investigated the effects of Stretta on mechanisms of spontaneous reflux in patients with GERD. Twenty patients with GERD underwent endoscopy, symptom evaluation, and combined postprandial esophageal manometry and pH monitoring before and six mo after Stretta, and 24 h ambulatory pH monitoring before and at 6 and 12 mo after treatment. They found that Stretta reduced the rate of postprandial transient LES relaxations from 6.8 (5.7-8.1) per hour to 5.2 (4.2-5.8) per hour (P < 0.01), and increased mean basal LES pressure from 5.2 (SE = 0.3) mmHg to 8.0 (SE = 0.4) mmHg (P < 0.01). The number of reflux events was reduced from 10 (2-15.3)/3 h to 5 (3.5-8.5)/3 h (P < 0.05) and there was an associated significant reduction in acid exposure time from 5.4% (0.4-14.7) to 3.9% (0.4-6.6) (P < 0.05). Stretta significantly reduced ambulatory esophageal acid exposure from 10.6% (7.8%-13.0%) to 6.8% (3.1%-9.1%) (P < 0.01) at six mo and 6.3% (4.7%-10.9%) (P < 0.05) at 12 mo. All patients required acid suppressant medication for symptom control before Stretta. Six months after treatment, 15 patients (75%) were in symptomatic remission and 13 (65%) at 12 mo. The authors concluded that Stretta has significant effects on LES function that are associated with improvement in the antireflux barrier[21].
In view of the role of esophageal sensitivity in the symptomatic expression of GERD, decreased esophageal sensitivity could potentially contribute to symptom improvement. Esophageal acid sensitivity depends on multiple factors, including esophageal exposure to hydrogen ions, mucosal permeability, number and activation state of acid-sensitive nerve endings, and central processing of incoming sensory information. Esophageal inflammation is generally considered a factor that contributes to increased sensitivity to acid. This last point demands attention, as there is strong evidence that Stretta heals erosions. A study by Liu et al[14] showed medication usage decrease from 100% of patients on PPI therapy at baseline to 76.7% of patients not using medications or using them only as needed at 12 mo. Also noted was a corresponding improvement in endoscopic grade of esophagitis in 33 of the 41 patients. All patients either had no erosions or only mild erosive disease (grade A) at 6 mo.
Several studies have indicated that esophageal acid sensitivity and hypersensitivity are important contributors to the symptomatic manifestations of GERD. Indeed, visceral analgesics, such as a tricyclic antidepressants, selective serotonin uptake inhibitors, or trazodone, are used as adjunctive tools in the management of PPI-refractory GERD patients. On the other hand, decreased esophageal sensitivity might be disadvantageous in severe, symptomatically silent, esophageal erosive disease but this has not been observed. Therapeutic treatment with Radiofrequency energy in other diseases/conditions has the potential to induce nerve ablation, for example this is the basis for its therapeutic application in chronic pain or cardiac arrhythmias. One criticism of Stretta has been the theoretical concern that it induces partial desensitization of the esophageal body through ablation of sensory nerve endings rather than a reduction in esophageal acid exposure. Esophageal desensitization is another completely unsubstantiated conjecture, again based upon the false assumption that Stretta relies upon tissue destruction for it treatment effect. In fact, the available clinical data completely refutes this conclusion. Further, it is ironic that on one hand, visceral analgesics are accepted in the management of refractory GERD without concerns about desensitization and on the other Stretta is criticized as potentially harmful. The following studies provide strong evidence that there is no credible evidence of desensitization, other than the normal desensitization that occurs in non-inflamed tissue.
Scholten[10] found that a “normal” esophagus is less sensitive to acid. These authors state that the exact pathologic process by which this occurs is complex and yet to be fully characterized. There are two requirements for heartburn, regardless of erosive or non-erosive disease: high acid concentrations within the esophageal lumen (reflux) and a damaged esophageal epithelium. When these situations co-exist, luminal acid enters the tissue where stimulation of nociceptors results in the symptom of heartburn[22]. It has already been demonstrated in numerous studies that Stretta produces significant improvement in esophageal erosion grade. To suggest that any decrease in sensitivity is due to a direct effect of Stretta other than normalizing esophageal tissue is conjecture in the existence of contradictory fact.
Arts et al[23] aimed to evaluate the influence of Stretta on symptoms, acid exposure, and sensitivity to esophageal acid perfusion in GERD. Thirteen patients with established PPI-dependent GERD (three males; mean age, 51 ± 10 years) participated in the study. Before and 6 mo after the procedure, symptom scores, pH monitoring and Bernstein acid perfusion test were performed. The latter was done by infusing hydrochloric acid (pH 0.1) at a rate of 6 mL/min 15 cm proximal to the gastro-esophageal junction for a maximum of 30 min or until the patients experienced heartburn. Six months after Stretta, the symptom scores were significantly improved (12.5 ± 2.0 to 7.5 ± 2.1, P < 0.05), seven patients no longer needed daily PPI, and acid exposure was significantly decreased (11.6% ± 1.6% to 8.5% ± 1.8% of time pH < 4, P < 0.05). The time needed to induce heartburn during acid perfusion (as a measure of esophageal sensitivity) decreased from 9.5 ± 2.3 to 18.1 ± 3.4 min (P = 0.01), and five patients became insensitive to 30-min acid perfusion, vs none at baseline (P = 0.04). However, esophageal acid perfusion occurred through a mid-esophageal infusion port, whereas radiofrequency energy delivery involved only a narrow area around the LES. As esophageal acid sensitivity is not limited to this small region, it seems less likely that sensory nerve ablation sufficiently explains the changes. Although the underlying pathways are poorly understood, acid exposure and esophageal inflammation have also been implicated in esophageal sensitivity and sensitization. Esophageal acid exposure has been shown to induce central sensitization of esophageal perceptive pathways.
Esophageal inflammation is generally considered a factor that contributes to increased sensitivity to acid, through disruption of the antireflux and luminal clearance mechanisms. In the Arts study, pH monitoring was significantly improved 6 mo after Stretta, potentially leading to decreased esophageal inflammation and decreased esophageal acid sensitivity and, thereby, contributing to decreased sensitivity to esophageal acid perfusion. Further, Arts found no direct correlation between (changes in) symptom scores and (changes in) esophageal sensitivity to acid perfusion. In fact, improvement of symptom scores was related to improvement of esophageal acid exposure, suggesting that the evolution of symptoms in this cohort of patients was mainly driven by the evolution of reflux control, and not by esophageal sensitivity.
Improvements of symptom scores were directly related to improvement of esophageal acid exposure and not to desensitization in two other clinical studies. In the first, Triadafilopoulos sought to determine if there was a correlation between the improvement in GERD outcomes and esophageal acid exposure after Stretta. He performed subgroup analyses between “responder” and “non-responder” groups from the US Stretta open label trial (n = 118), on the basis of post-treatment responses for GERD-HRQL heartburn, satisfaction, and proton pump inhibitor use. Outcomes were analyzed within and between subgroups. Pearson correlation coefficient analysis was performed comparing distal esophageal acid exposure with each of the continuous outcomes (GERD-HRQL, heartburn, satisfaction). Responder subgroups had significant improvements in esophageal acid exposure, whereas non-responders had no change or less improvement in the same. Changes in GERD-HRQL and heartburn severity were correlated with changes in acid exposure (r = 0.16, P = 0.12 and r = 0.26, P = 0.01, respectively). Changes in satisfaction were negatively correlated with changes in esophageal acid exposure (r = 0.23, P = 0.02) because satisfaction, as expected, increased as acid exposure decreased[24].
In another similar study to address the same concern, Richards et al[25] analyzed their postoperative pH data and showed that all of patients who were failures of the procedure and were continued on maximal PPI therapy had pathologic distal esophageal acid exposure. Eighty percent of the patients who had a complete response to Stretta and were no longer taking PPIs, had normalized their pH scores. There were no patients who returned after Stretta with evidence of esophagitis while they were improving their GERD symptom scores.
A study by DiBaise et al[26] examined Stretta’s efficacy and potential mechanism of action. They followed 18 patients for 6 mo after Stretta and did find a trend toward reduction in the number of tLESRs, but found no adverse effect on abdominal vagal function and no significant change in any esophageal motility parameter. They concluded that there was no evidence of adverse effects on either swallow-induced LES relaxation or esophageal peristalsis. None of their patients demonstrated an abnormal pancreatic polypeptide response to sham feeding after treatment, suggesting integrity of abdominal efferent vagal function.
In summary, strong evidence exists to support that symptomatic improvement after Stretta is attributable to a decrease in esophageal acid exposure and not to esophageal desensitization or nerve damage. In fact, studies have also noted an improvement in esophagitis, which would not be possible in a densensitized lower esophagus that was still exposed to acid.
There have been no Stretta clinical trials that used pH normalization (or even % reduction) as a primary endpoint. Therefore, all the pH data acquired have not been powered to show statistically significant differences. Nevertheless, the available evidence from multiple studies shows that Stretta reduces esophageal acid exposure and normalizes pH in many patients.
Normalization of esophageal acid exposure has been a controversial outcome in patients with GERD. Despite effective control of GERD-related symptoms during PPI treatment, a sizeable percentage of patients have been shown not to have achieve normalized intra-esophageal acid exposure[16]. This has raised questions about whether normalized intra-esophageal pH is a necessary therapeutic goal and how much acid control is necessary to achieve a good clinical response. Although some studies have shown a correlation between increased healing of erosive esophagitis and maintaining intra-esophageal pH > 4, no studies to date have shown that complete normalization of intra-esophageal pH is necessary for esophagitis healing or to achieve effective symptom control. Yet, criticism of the use of endoscopic techniques for GERD (including Stretta) has focused on the fact that only 30% to 37% of patients achieve normalization of intra-esophageal acid exposure. Despite these caveats and despite earlier reports of higher normalization rates and far more effective (albeit, not absolute) acid control with PPIs, the results of Milkes’s study show a correlation between intra-gastric pH and pathologic intra-esophageal reflux, challenge the appropriateness of targeted normalization of intra-esophageal acid exposure, and leave us wondering whether intra-esophageal normalization is necessary for symptom control, healing and for prevention of complications.
Several studies provide strong evidence of significant reduction of esophageal acid exposure but not consistent normalization esophageal acid exposure after Stretta. Both DeMeester scores and acid exposure have been reported to improve significantly in most studies with a few exceptions. DiBaise et al[26] reported a 72% improvement in the distal esophageal acid exposure with normalization of in 4 of 18 patients at 6 mo after Stretta. However, these results failed to reach statistical significance. Corley et al[11] reported absence of significant decrease in the distal esophageal acid exposure in their study population at 6 mo as well. However, upon stratifying the patients on the basis of responders and non-responders, they observed significant improvement in the acid exposure of the former group after the procedure. This was also observed by Triadafilopoulos.
In the Stretta meta-analysis[15], pre-procedure and post-procedure DeMeester scores improved from 44.37 ± 9.3 pre-Stretta to 28.53 ± 33.4 post-Stretta over an average period of 13.1 mo in 267 patients across 7 studies (P = 0.0074). Esophageal acid exposure was reported in 11 studies comprising of 364 patients over a mean follow-up period of 11.9 mo. Esophageal acid exposure decreased from a mean of 10.29% ± 17.8% to 6.51% ± 12.5% (P = 0.0003). In the previously mentioned study by Richards et al[25], the authors also looked at acid exposure post Stretta, and found that 80% of the patients who had a complete response to Stretta (no longer taking PPIs) normalized their pH scores.
There is strong evidence for the durability of Stretta in the long-term treatment of GERD. At 48-mo follow up, Reymunde et al[27] observed the mean GERD-QOL score to be 2.4 (baseline), 4.6 (36 mo), and 4.3 (48 mo, P < 0.001). The mean GERD symptom score was 2.7 (baseline), 0.3 (36 mo), and 0.6 (48 mo, P < 0.001). Daily medication usage was 100% (baseline) and 13.6% (48 mo, P < 0.001). Another uncontrolled, nonrandomized case series of 109 drug-refractory patients with GERD found Stretta to be a safe, effective, and durable treatment that produced significant improvements in heartburn and quality of life and decreased medication usage during a 4-year period of follow-up. Complete long-term follow-up assessment was available in matched data for 109 patients at 12 mo, 108 patients at 24 mo, 102 patients at 36 mo, and 96 patients at 48 mo. A second procedure was performed in 13 patients. Heartburn scores decreased from 3.6 to 1.18 (P < 0.001), total heartburn score (GERD health-related quality-of-life questionnaire) decreased from 27.8 to 7.1 (P < 0.001), and patient satisfaction improved from 1.4 to 3.8 (P < 0.001). Medication usage decreased significantly from 100% of patients on twice-daily PPI therapy at baseline to 75% of patients showing elimination of medications or only as-needed use of antacids/over-the-counter PPIs at 48 mo (P < 0.005). There were no long-term complications of the procedure[28]. In Dughera et al[29], the following outcomes were observed at 48 mo follow up: Stretta significantly improved heartburn scores, GERD-specific quality of life scores, and general quality of life scores at 24 and 48 mo in 52 out of 56 patients (92.8%) At each control time both mean heartburn and GERD HRQL scores decreased (P = 0.001 and P = 0.003, respectively) and both mental SF-36 and physical SF-36 ameliorated (P = 0.001 and 0.05, respectively). At 48 mo, 41 out of 56 patients (72.3%) were completely off PPIs and some using only occasionally oral antacids.
In a 10-year, open, single center, prospective assessment of Stretta for medically refractory GERD in 99 evaluable patients, a significant and sustained improvement of GERD-specific quality of life scores, patient satisfaction, and improved PPI use (P < 0.0001 for all outcomes) validated the long-term usefulness of the procedure. There was improvement in Barrett’s esophagus and in some cases disappearance of metaplasia and dysplasia noted following Stretta. There were no cases of esophageal cancer that developed during this 10 year time period; however, direct endoscopic confirmation was only possible in 51 patients[30].
Overall, GERD is a complex disease caused by any number of types of reflux (acidic, weakly acidic or non-acidic) into the esophagus. Since Stretta effectively addresses several underlying mechanisms of GERD (such as tLESRs), it does not discriminate to the type of reflux, and it may be utilized in those refractory patients who are not interested in pursuing anti-reflux surgery (Table 2). Since its introduction in 2000, multiple studies have demonstrated the safety and efficacy of Stretta for GERD but very few were company-sponsored and most were investigator-initiated at both academic and community sites, worldwide. Therefore, data on the procedure’s effectiveness and durability have at times produced mixed results. Definitive conclusions were problematic because of the heterogeneity of measured variables in different studies of variable patient populations.
| An outpatient endoscopic option with unique mechanisms of action |
| Effective, safe and durable |
| Distinct from medical therapy (pH control, refractoriness) |
| Distinct from anti-reflux surgery (acid and volume reflux control, side effects) |
| Not precluding anti-reflux surgery |
| Repeatable |
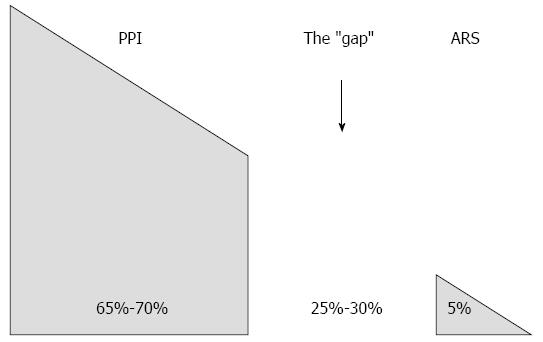
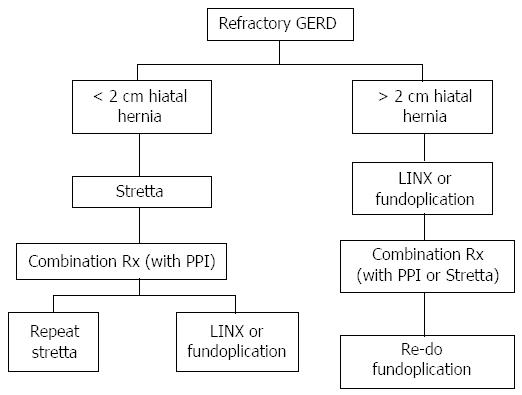
Nevertheless, as the precise physiologic dysfunction exhibited by GERD sufferers is not completely understood, it stands to reason that multiple therapeutic modalities - used alone or even in combination - may contribute to symptom control. The abundance of clinical data on Stretta confirms the following: (1) tissue destruction and creation of fibrosis does not occur; (2) symptom control in PPI dependent patients occurs consistently; (3) a variety of functional improvements occur in the distal esophagus including improved acid sensitivity and tissue compliance; and (4) the procedure is exceedingly safe, durable and reproducible. As such, Stretta provides an invaluable adjunct in the treatment of GERD that spans the “gap” between PPI responders and surgical candidates (Figure 3). There is unquestionably an unmet need for the many sufferers of GERD, particularly the refractory ones, where Stretta has been shown to offer significant improvements by both objective and subjective criteria. Stretta is safe, effective, durable, and repeatable if necessary. Further, it does not preclude any other alternative (repeat Stretta, PPI addition, LINX or fundoplication) and is the least expensive alternative to medical therapy (Figure 4). Today more than ever, clinicians will benefit from the addition of Stretta to the treatment armamentarium for their GERD patients.
P- Reviewer: Jonaitis L S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179-1187.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1467] [Article Influence: 112.8] [Reference Citation Analysis (1)] |
| 2. | Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700-1707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Hershcovici T, Fass R. An algorithm for diagnosis and treatment of refractory GERD. Best Pract Res Clin Gastroenterol. 2010;24:923-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656-664. [PubMed] |
| 5. | Kahrilas PJ, Boeckxstaens G. Failure of reflux inhibitors in clinical trials: bad drugs or wrong patients? Gut. 2012;61:1501-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Triadafilopoulos G. Stretta: an effective, minimally invasive treatment for gastroesophageal reflux disease. Am J Med. 2003;115 Suppl 3A:192S-200S. [PubMed] |
| 7. | Auyang ED, Carter P, Rauth T, Fanelli RD. SAGES clinical spotlight review: endoluminal treatments for gastroesophageal reflux disease (GERD). Surg Endosc. 2013;27:2658-2672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Leeds S, Reavis K. Endolumenal therapies for gastroesophageal reflux disease. Gastrointest Endosc Clin N Am. 2013;23:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Kaplan-Machlis B, Spiegler GE, Revicki DA. Health-related quality of life in primary care patients with gastroesophageal reflux disease. Ann Pharmacother. 1999;33:1032-1036. [PubMed] |
| 10. | Scholten T. Long-term management of gastroesophageal reflux disease with pantoprazole. Ther Clin Risk Manag. 2007;3:231-243. [PubMed] |
| 11. | Corley DA, Katz P, Wo JM, Stefan A, Patti M, Rothstein R, Edmundowicz S, Kline M, Mason R, Wolfe MM. Improvement of gastroesophageal reflux symptoms after radiofrequency energy: a randomized, sham-controlled trial. Gastroenterology. 2003;125:668-676. [PubMed] |
| 12. | Aziz AM, El-Khayat HR, Sadek A, Mattar SG, McNulty G, Kongkam P, Guda MF, Lehman GA. A prospective randomized trial of sham, single-dose Stretta, and double-dose Stretta for the treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Triadafilopoulos G, DiBaise JK, Nostrant TT, Stollman NH, Anderson PK, Wolfe MM, Rothstein RI, Wo JM, Corley DA, Patti MG. The Stretta procedure for the treatment of GERD: 6 and 12 month follow-up of the U.S. open label trial. Gastrointest Endosc. 2002;55:149-156. [PubMed] |
| 14. | Liu HF, Zhang JG, Li J, Chen XG, Wang WA. Improvement of clinical parameters in patients with gastroesophageal reflux disease after radiofrequency energy delivery. World J Gastroenterol. 2011;17:4429-4433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Perry KA, Banerjee A, Melvin WS. Radiofrequency energy delivery to the lower esophageal sphincter reduces esophageal acid exposure and improves GERD symptoms: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 2012;22:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Milkes D, Gerson LB, Triadafilopoulos G. Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal and intragastric pH in patients with gastroesophageal reflux disease (GERD). Am J Gastroenterol. 2004;99:991-996. [PubMed] |
| 17. | Gersin K, Fanelli R. The Stretta procedure: Review of catheter and technique evolution, efficacy and complications 2 years after introduction. Surg Endosc. 2002;16:PF199 (abstract). |
| 18. | Utley DS, Kim M, Vierra MA, Triadafilopoulos G. Augmentation of lower esophageal sphincter pressure and gastric yield pressure after radiofrequency energy delivery to the gastroesophageal junction: a porcine model. Gastrointest Endosc. 2000;52:81-86. [PubMed] |
| 19. | Kim MS, Holloway RH, Dent J, Utley DS. Radiofrequency energy delivery to the gastric cardia inhibits triggering of transient lower esophageal sphincter relaxation and gastroesophageal reflux in dogs. Gastrointest Endosc. 2003;57:17-22. [PubMed] |
| 20. | Arts J, Bisschops R, Blondeau K, Farré R, Vos R, Holvoet L, Caenepeel P, Lerut A, Tack J. A double-blind sham-controlled study of the effect of radiofrequency energy on symptoms and distensibility of the gastro-esophageal junction in GERD. Am J Gastroenterol. 2012;107:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Tam WC, Schoeman MN, Zhang Q, Dent J, Rigda R, Utley D, Holloway RH. Delivery of radiofrequency energy to the lower oesophageal sphincter and gastric cardia inhibits transient lower oesophageal sphincter relaxations and gastro-oesophageal reflux in patients with reflux disease. Gut. 2003;52:479-485. [PubMed] |
| 22. | Orlando RC. Current understanding of the mechanisms of gastro-oesophageal reflux disease. Drugs. 2006;66 Suppl 1:1-5; discussion 29-33. [PubMed] |
| 23. | Arts J, Sifrim D, Rutgeerts P, Lerut A, Janssens J, Tack J. Influence of radiofrequency energy delivery at the gastroesophageal junction (the Stretta procedure) on symptoms, acid exposure, and esophageal sensitivity to acid perfusion in gastroesophagal reflux disease. Dig Dis Sci. 2007;52:2170-2177. [PubMed] |
| 24. | Triadafilopoulos G. Changes in GERD symptom scores correlate with improvement in esophageal acid exposure after the Stretta procedure. Surg Endosc. 2004;18:1038-1044. [PubMed] |
| 25. | Richards WO, Houston HL, Torquati A, Khaitan L, Holzman MD, Sharp KW. Paradigm shift in the management of gastroesophageal reflux disease. Ann Surg. 2003;237:638-47; discussion 648-9. [PubMed] |
| 26. | DiBaise JK, Brand RE, Quigley EM. Endoluminal delivery of radiofrequency energy to the gastroesophageal junction in uncomplicated GERD: efficacy and potential mechanism of action. Am J Gastroenterol. 2002;97:833-842. [PubMed] |
| 27. | Reymunde A, Santiago N. Long-term results of radiofrequency energy delivery for the treatment of GERD: sustained improvements in symptoms, quality of life, and drug use at 4-year follow-up. Gastrointest Endosc. 2007;65:361-366. [PubMed] |
| 28. | Noar MD, Lotfi-Emran S. Sustained improvement in symptoms of GERD and antisecretory drug use: 4-year follow-up of the Stretta procedure. Gastrointest Endosc. 2007;65:367-372. [PubMed] |
| 29. | Dughera L, Navino M, Cassolino P, De Cento M, Cacciotella L, Cisarò F, Chiaverina M. Long-Term Results of Radiofrequency Energy Delivery for the Treatment of GERD: Results of a Prospective 48-Month Study. Diagn Ther Endosc. 2011;2011:507157. [PubMed] |
| 30. | Noar MD, Squires P, Noar E. Sustained Improvement in GERD-HRQL, patient satisfaction, and anti-secretory drug use 10 years after Stretta for medically refractory GERD. Gastroenterology. 2013;144:S-1077 (abstract). |