INTRODUCTION
More than 2 million human tissue transplants (bone, tendon, cartilage, skin, cornea, amniotic membrane, stem cells, heart valve, blood vessel, etc.), are performed worldwide every year. Cells and tissues are shared between countries with different regulations and laboratory equipment, and represent a risk for hepatitis B virus (HBV) transmission that has become a global safety concern. While the risk of transfusion-transmitted HBV infection per blood donations has been estimated[1], the rate of HBV transmission from donors to recipients of allografts is unknown and varies among different tissues. Infectious disease transmission occurs in less than 1% of solid organ recipients and is believed to be at a lower rate for tissue and cell recipients[2,3]. In fact, the level of safety in tissue transplantation has been significantly increased and disease transmission can be considered a rare event when comparing reports of infection and the number of allografts transplanted per year. This outcome has been achieved as a result of the experience gained over the last 50 years in this field.
The presence of hepatitis B surface antigen (HBsAg) and HBV DNA has been described in corneas from HBsAg-positive donors. Nevertheless, controversial results have been reported on their potential for disease transmission[4-7]. Morris et al[8] showed in 1990 their experience using aortic valve allografts from HBsAg-positive donors. The reason for accepting these donors was the scarcity of heart valve donors and the relatively high prevalence of HBV infection in their own country. In the case of HBsAg-positive and anti-HBe-negative donors, they used prophylactic administration of hepatitis B immunoglobulin and/or hepatitis B vaccine. In their series, only 1 of 9 recipients seroconverted to HBV (positive for anti-HBc, anti-HBs and anti-HBe, but negative for HBsAg).
In a recent paper, Hinsekamp et al[9] have reviewed the adverse reactions and events related to musculoskeletal allografts which is the most demanded tissue. They analyzed medical literature, reports from professional organizations and tissue banks. Wang et al[10] used FDA’s MedWatch reporting system to review reports on adverse events attributed to allografts of several kinds of tissue, during 2001-2004. No cases of hepatitis B transmission were reported. The review of the literature by Pruss et al[11] considered this risk as very rare. Nevertheless, Mallick et al[12], reported 9 cases of hepatitis B transmission from tissue allografts. This last article showed the importance of the implementation of Current Good Tissue Practice rules in the task of reporting infections.
In the last 20 years, stocks of human tissues in tissue banks have been significantly increased and refined strategies for the assessment of HBV transmission risk have been developed. A balance between safety and availability must be achieved but tissue availability must never jeopardizes biosafety. The adoption of algorithms for decision making must be based on medical evidence, avoiding the unjustified loss of products in tissue banks[13,14].
CRITICAL POINTS TO REDUCE HBV TRANSMISSION RISK BY CELL AND TISSUE TRANSPLANTATION
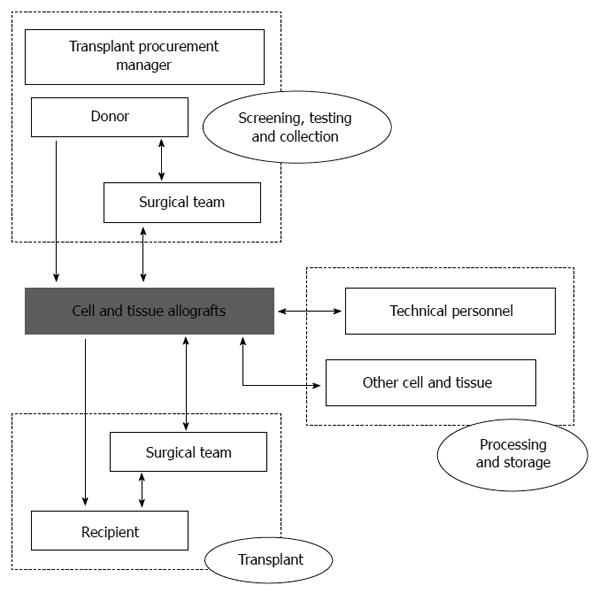
What are the most important areas in which particular attention has been given in order to reduce transmission risk? Figure 1 shows several potential sources of transmission: donor, tissue allograft, surgical team (during collection or transplant), processing team and other tissues (during processing or storage). However, donor screening and donor testing remain as the main issues for preventing HBV transmission.
Figure 1 Activities and professionals to take into account when assessing risk factors for disease transmission associated to tissue transplantation.
Arrow heads show the interactions among elements.
Donor screening
This is the first factor for preventing hepatitis B transmission. It includes different sources of information to assess donor suitability: medical history, physical examination, social behaviour, and other available medical records considered as relevant risk factors[15]. HBV infectious risk is inferred from the donor’s medical and social history and based on guidelines for preventing HIV transmission through tissue and organ transplantation[16].
Generally, for living donors when the femoral head (as a source of cancellous bone for use in impaction grafting procedures) is obtained from elderly patients undergoing hip surgery, the donor selection is assumed by health personnel in the orthopedic surgery department (nurses or surgeons who obtain data directly from the donor themselves), who are sometimes untrained in this task or with limited time available.
For deceased donors, this function is performed by the transplant procurement manager, who is a professional trained in the field of donor selection and responsible for the coordination of donation-transplantation activities, which implies health care professionals and services from a variety of specialties. Collecting data on medical and behavioural history is an essential step in analyzing risk factors, but, sometimes, in asymptomatic patients, hepatitis is difficult to detect.
Donor testing: General considerations
HBV transmission is due to the collection of cell and tissues from window period donors. However, in recent years, the HBV transmission risk has been significantly reduced with a greater accuracy in the performance of serological assays for infectious disease testing and the inclusion of DNA-based techniques. Human error has, therefore, become a major cause in cases of disease transmission and efforts addressed to avoid it must be increased.
The final objective of donor testing is the detection of potentially transmissible disease. This task will become ineffective if other relatively simple issues are not considered. Some additional important considerations related to the characteristics of the donor’s blood sample include: (1) Quantity: insufficient sample volume to complete testing; and (2) Quality: hemolysed or samples that are too diluted, which may influence the reliability of the results, especially in phases when the viral load is low (e.g., due to the infusion of blood, colloid and crystalloid solutions in patients with massive blood loss who finally become cadaveric tissue donors) and before the antibodies are detectable (window period)[11,14,17,18]. In the case of deceased donors, if the pre-mortem specimens are not available and the only alternative is to use post-mortem blood, an increase in false serological results can be expected (especially for false positive cases)[19]. Not all available systems for serological testing are validated for use with samples from post-mortem blood samples. Baleriola et al[20] compared the efficacy of viral marker detection in paired pre- and post-mortem samples and did not find a reduction of sensitivity between them. Traceability: unlabelled or not clearly identified samples cannot be associated with a donation.
In cases of deceased donors, an important number of tissues may be obtained from each one. The implementation of strategies to minimize the incidence of unsuitable samples will then improve the activity of the tissue bank. Kitchen et al[14] have proposed the use of a sequential serology screening algorithm in order to reduce the number of tissues which are rejected due to non-specific screen reactivity. Using this strategy, they have improved the specificity of serology screening, significantly decreasing screening losses. For these authors, when comparing blood donors with deceased tissue donors, in terms of infection risk, the latter should be considered as first time blood donors. Zou et al[21] reported a higher estimated probability of undetected viremia among tissue donors than among first-time blood donors but lower than those attributed to the general population. They point out that the donor selection procedure for tissue donors (medical history, physical examination, and interviews with the next of kin) is not as effective as the face-to-face interview that is carried out with blood donors.
Donor testing: Specific HBV tests
HBV detection tests are included in the routine screening tests for cell and tissue donors. The standard test for preventing transplant-transmitted hepatitis B is hepatitis B surface antigen (HBsAg)[11]. Since this immunoassay test was introduced in 1971 its analytical sensitivity has now improved dramatically, and the current tests can sense HBsAg at concentrations lower than 0.1 ng/mL and 0.62 ng/mL[22].
The probability of finding a tissue donor with undetectable viremia by immunoassay testing has been calculated from human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV)[23] using the incidence-window period model[24]. The risk of detecting a negative serology test in an infected tissue donor has been shown to be higher for these two viruses with lower incidence rates and longer window periods (HBV and HCV), than for the HIV virus (higher incidence rate and shorter window period (HIV)[21].
The implementation of methods involving nucleic acid amplification (NAT) and the new generation of reactives to detect viral antibodies or antigens with immunoassay, has increased the sensitivity and the specificity of the screening tests. The window period length for HBsAg test has fallen from 59 d[23] using enzymoimmunoassay (EIA) to 36-38 d[24] using the new EIAs and chemolumynoimmunoassay (ChLIA) tests. Anti-hepatitis B core antigen (anti-HBc) arises between one and two weeks later that HBsAg[25] while HBV DNA, measured by nucleic acid testing in individual donation (ID-NAT), arises around 21 d after the donor becomes infectious and 15 d before the serologic testing detects the HBV infection[26].
Despite the continuous progress in screening tests, three causes of transfusion and transplant-transmitted hepatitis B remain: window period donation, donor carrying an occult hepatitis B infection (OBI) and virus with mutations that are undetectable by the available screening tests[27]. NAT, especially ID-NAT, provides the shorter window period and defines the OBI[28] (HBV DNA presence, without HBsAg and with or without anti-HBc and/or anti-hepatitis B surface antigen, anti-HBs). There are two types of window period (early infection and what occurs after acute infection, with undetectable HBsAg), three OBI types (only with DNA, with DNA and anti-HBc, with DNA and anti-HBs) and two chronic infection types (NAT+ and NAT-). Every variety of HBV related conditions, except chronic infection NAT-, is detectable by NAT, while the HBsAg test does not detect five of the conditions, while the anti-HBc test does not detect three of them[27].
Once the advisability of HBV NAT testing for screening of the cell and tissue donations is well established, it is mandatory to decide whether it should be in ID-NAT or in minipool (MP-NAT). Since HBV NAT is available, we are aware that the repeat testing results of a sample with low DNA concentration are often discordant, even in the same watch[29]. Recently, a donation with west nile virus (WNV) and low concentration RNA included in a minipool with a positive result, was negative in individual retesting, and its transplantation caused WNV transmission with a fatal outcome[30]. It is well known during the last few years that the length of the window period with ID-NAT is shorter than MP-NAT[31]. Since the prudent attitude seems to be to wast every donations included in a MP-NAT that is initially reactive, NAT screening as ID-NAT must be performed.
Once established that HBV ID-NAT is mandatory, the need to maintain serologic tests is an interesting question. In the case of an affirmative answer, which serologic tests should be performed? In order to resolve this question, it has to be realized that the analytical sensitivity of VHB ID-NAT is 10 IU/mL (50 copies/mL)[32] and a concentration as low as 1 copy/20 mL can transmit HBV infection[26].
HBsAg becomes positive about two weeks later than ID-NAT tests and, by definition, is negative in OBI. So, one could envisage the scandalous possibility of removing HBsAg as a mandatory test in HBV screening if HBV ID-NAT is performed[33]. Both anti-HBc and ID-NAT tests cover serologic window periods and the vast majority of NAT negative VHB chronic infections. In countries with a prevalent high HBV infection it is feasible to minimize the tissue loss by adding the anti-HBs determination, so that donations ID-NAT negative, anti-HBc positive with anti-HBs > 10 IU/L can be delivered[34]. If ID-NAT is not performed, the anti-HBs concentration to allow tissue delivery must be > 100 IU/L[35].
In summary, the optimum screening HBV tests should be adapted to current knowledge. Until the HBV NAT with an analytical sensitivity around 1 copy/20 mL becomes available, anti-HBc must be performed, allowing for the delivery of NAT negative, anti-HBc negative and the NAT negative, and anti-HBc positive with anti-HBs > 10 IU/L donations. On the contrary, NAT positive and NAT negative with anti-HBc positive and anti-HBs < 10 IU/L donations must be discarded. The HBsAg determination is not useful if ID-NAT is routinely performed. In fact, several studies have concluded that NAT should be a routine test for donations and confirmed the value of maintaining anti-HBc for the detection of low-level HBV DNA-positive donors and observed that HBsAg screening showed no blood safety value[11,21,36,37].
In the case of living donors (i.e., femoral head), if NAT testing is not used, tissue can be quarantined for at least 180 d[38], until the donor is retested for serological markers. Sometimes this retesting is not possible (it entails additional discomfort for elderly patients) and the tissue must be rejected. Westby et al[39] calculated the cost comparison between 180 d retesting and NAT testing, concluding that NAT implementation was much more cost effective.
Surgical teams
Tissue collection and tissue transplant are highly dependent on human intervention. The professionals participating in these activities can be the source for hepatitis B transmission. Some cases of hepatitis B transmission from a surgeon have been reported[40,41].
Processing
One of the most frequently reported acquired infections by laboratory staff is the HBV infection as it is several times greater in laboratory staff than the general population and is one of the most frequently reported laboratory acquired infections[42].
In the mid 50’s, when the first tissue banks begun operating, the effect of freezing on viruses was not clear. It was suggested that after long-term storage (> 5 years) viruses could become inactive[43]. This last reference is considered as the first report of the transmission of hepatitis by frozen bone. Since then, it has been really difficult to find more cases of this disease due to tissue transplants. As an alternative to freezing, some tissues (such as cancellous bone) can be dehydrated, using freeze-drying or chemical agents. These dehydrated tissues have the advantage of being stored at room temperature. The dehydration process and the importance of water in the maintaining of a viral envelope could explain the reduction of viral infectivity observed in some cases, hindering the fusion with the cell membrane[44]. Several authors have reported cases of disease transmission with organ and fresh-frozen tissue transplantation but not with processed tissues from the same donor[45]. In addition, dehydrated/lyophillized tissues undergo secondary sterilization (e.g., gamma irradiation). However, for some tissues, the level of radiation must be limited to avoid undesiderable effects on their biological and/or biomechanical properties[46-48].
Nevertheless, the low temperature and the addition of cryoprotective agents which maintain the stability of lipidic membranes (e.g., albumin and sucrose), can enlarge the infective period (even for lyophillized tissues)[49,50]. DNA is more stable than RNA and, hence, more resistant to the effects of environmental conditions. Baleriola et al[51] have observed a significant loss of HBV load in samples stored at -70 °C for 9 years. However, the viral titter after storage was adequate for the detection of HBV nucleic acid with NAT. Bond et al[52] have determined that HBV can survive and remain infectious on environmental surfaces for up to 7 d.
Tissues are processed in tissue banks inside flow cabinets placed in clean rooms, with stringent environmental monitoring. During this phase, measures to avoid cross-contamination among tissues from different donations must be implemented.
Storage
Nitrogen is commonly used in cell and tissue banks in order to achieve ultralow temperatures to ensure long term storage, avoiding significant changes in the stored products. However, the liquid phase of nitrogen (-196 °C) has been reported as a vehicle for hepatitis B transmission thus the vapour phase has been recommended to prevent it[53-55]. In addition, the use of double-bagging protects stored material against cross-contamination risk in case of leakage.
Information and quality system
Sometimes, the lack of a system to share data among professionals implied within the different activities performed from donation to transplant, is the cause of delivery of non-conforming tissues. The importance of rapid communication was showed in the case described by Tugwell et al[56] in which tissues from a hepatitis C positive donor were transplanted in spite of some tissues from this donor, who a year before had been shown as the origin of disease transmission (but these infections were not reported).
Considering the significant improvement achieved in the detection of viral markers, the incidence of human errors has emerged as the main risk for hepatitis B transmission. Taking advantage, therefore, of the broad experience accumulated in the field of allograft transplantation, the implementation of a quality system especially in tissue banks and surgical units, as well as a risk management system based on preventive action (proactive criteria), have contributed to minimizing this risk. Failure mode and effect analysis (FMEA, FMECA) are two widely used approaches to identify and eliminate events which can lead to adverse events, before they occur. Both strategies are based on the quantification of the ability to note the cause of the failure (detectability) and the probability of taking place (occurrence). The main difference between them is that FMECA incorporates a classification for the severity of the consequences. This system yields a hierarchization which enables decision making regarding the priorities to adopt. The EUSTITE (European Union Standards and Training for the Inspection of Tissue Establishments) Project has provided useful grading for the parameters to be considered in risk assessment.
FOLLOW-UP OF CELL AND TISSUE RECIPIENTS
In general, tissue banks have shown an inability to track the outcomes of the patients receiving their tissues, which is an important issue in order to detect adverse events from their use. Sometimes, the reason is the difficulty in confirming the association between an infectious disease with the transplanted tissue. The development of measures to solve the problem of this lack of communication requires efficiency on the part of the various participants: organ and tissue procurement organizations, tissue banks, transplant centers, professional organizations, public health agencies. Examples of this collaboration are the Transplantation Sentinel Network (TSN) in United States, and in Europe, the SOHOVS (Vigilance and Surveillance of Substances of Human Origin) Project[9,12,56-59].
As previously mentioned, the knowledge of adverse reactions and events associated with tissue allografts has led to risk management analysis and the implementation of corrective actions, which have significantly improved the safety of tissue transplantation. The systems for surveillance and the creation of communication networks for reporting incidences related to disease transmission have been the best source for acquiring the experience necessary in risk management. In 2010, as a consequence of the Sixty-third World Health Assembly, the World Health Organization launched Project Notify. The aim of this project was to provide a global interface for the vigilance and surveillance of substances of human origin[59].
Dhakal et al[60] have proposed an interest in using Medicare data to facilitate the access to information related to tissue allograft outcomes.
In this scenario, tissue banks appear as the link for providing the information generated from donation to transplantation, ensuring traceability in both trace-back and trace-forward analysis. Indeed, tissue processors are the main source of reports.
Authorization/inspection/audit
Public health care authorities have the competence to develop regulations regarding activities performed from donation to transplantation, with special attention given to cell and tissue banking. In addition, control measures must be implemented in order to ensure compliance with these regulations.
This task should be supported with the incorporation of ethical and technical standards developed from scientific associations involved in the different activities (donor selection, donor testing, tissue procurement, tissue processing, tissue storage, tissue transplant, quality control, data management, communication system, risk management).
As an example, the EQSTB (European Quality System for Tissue Banking) project has been developed with the aim of analysing the factors that may influence the quality and safety of cells and tissues for transplantation[61]. A useful guide for auditing tissue establishments was developed as a result of this project. In the United States, the FDA has edited guides for good tissue practices with recommendations.