Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6541
Revised: February 28, 2014
Accepted: March 12, 2014
Published online: June 7, 2014
Processing time: 238 Days and 3.8 Hours
AIM: To examine the expression of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) in rat esophageal lesions induced by reflux of duodenal contents.
METHODS: Thirty 8-week-old male Wistar rats were exposed to duodenal content esophageal reflux. All animals underwent an esophagoduodenal anastomosis (EDA) with total gastrectomy to elicit chronic esophagitis. In ten rats sham operations with only a midline laparotomy were performed (Control). The rats were sacrificed at the 40th week, their esophagi were taken for hematoxylin and eosin staining and for examination of expression of COX2, PGE2, and proliferating cell nuclear antigen (PCNA), and total bile acids in the esophageal lumen was measured.
RESULTS: After 40 wk of reflux, columnar dysplasia, squamous cell carcinoma and adenocarcinoma were observed. Total bile acids in the esophageal lumen were significantly increased in the EDA group compared with the sham operated rats. PCNA labelling index and esophageal tissue PGE2 levels were higher in dysplastic and cancer tissues than in control tissues. Overexpression of COX2 was observed in dysplastic and cancer tissues.
CONCLUSION: Reflux of duodenal contents induces COX2 expression and increases prostaglandin synthesis in dysplastic and cancer tissues. This result suggests a possible mechanism by which bile acids promote esophageal cancer.
Core tip: It is known that reflux of duodenal contents (bile acids) can induce mucosal injury, stimulate cell proliferation, and promote tumorigenesis. We examined the expression of cyclooxygenase-2 (COX-2) and prostaglandin E2 in rat esophageal lesions induced by reflux of duodenal contents. All animals underwent an esophagoduodenal anastomosis with total gastrectomy to elicit chronic esophagitis. We demonstrated that reflux of duodenal contents induces COX-2 and increases prostaglandin synthesis in dysplastic and cancer tissues. This result suggests a possible mechanism by which bile acids promote esophageal cancer.
- Citation: Hashimoto N. Effects of bile acids on cyclooxygenase-2 expression in a rat model of duodenoesophageal anastomosis. World J Gastroenterol 2014; 20(21): 6541-6546
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6541.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6541
Reflux of duodenal contents appears to contribute to the development of esophagitis and Barrett’s esophagus[1,2]. This idea is supported by several observations. In patients with gastroesophageal reflux disease, the concentration of bile acids in the esophageal refluxate correlates with the degree of esophageal mucosal injury[3]. In experimental animals, induction of a duodenoesophageal anastomosis led to esophagitis, Barrett’s esophagus and esophageal cancer[4]. The precise mechanism by which duodenal reflux causes esophageal injury and predisposes to neoplasia is uncertain. However, there is considerable evidence to suggest that bile acids contribute to this mechanism. Bile acids can induce mucosal injury[5], stimulate cell proliferation[6] and promote tumorigenesis[7].
Two isoforms of cyclooxygenase (COX), designated COX-1 and COX-2, catalyze the synthesis of prostaglandins (PGs) from arachidonic acid. COX-1 is a housekeeping gene that is expressed constitutively in most tissues. COX-2 is an immediate-early response gene that is induced by a variety of mitogenic and inflammatory stimuli[8]. Elevated levels of COX-2 have been detected in both inflammatory[9] and neoplastic conditions[10]. For example, COX-2 is up-regulated in peptic ulcer disease, Barrett’s esophagus and esophageal cancer. Taking this information together, it seems likely that COX-2 plays a role in the pathogenesis of duodenal reflux-related diseases of the esophagus.
In this study, we investigated the effects of bile acids and duodenal reflux on COX-2 expression in a rat model of duodenoesophageal anastomosis.
Eight-week-old male Wistar rats weighing approximately 300 g were used for the experiments. They were allowed to acclimatize for 2 wk prior to surgery. Solid food was withdrawn 1 d before and for 1 d after surgery. Esophagoduodenal anastomosis (EDA) was performed in 30 rats under general anesthesia (pentobarbital 50 mg/kg body weight, i.p.) through an upper midline incision. The gastroesophageal junction was ligated, and the distal esophagus was transected 2 mm above the ligature. Furthermore, the gastroduodenal junction was also ligated, and the proximal duodenum was transected 3 mm distal to the pylorus. A total gastrectomy was performed with the removal of the entire stomach, and end-to-end anastomosis of the esophagus and duodenum. The abdominal incision was closed in two layers (Figure 1). In 10 rats, sham operations with only a midline laparotomy (control group) were performed. Postoperatively, the rats were allowed to drink water after six hours and were fed the following day. This procedure was approved by the Animal Care and Facilities Committee, Kinki University.
All of the rats were killed as described previously[11]. Special care was taken to separate the esophagus from the duodenum based on the suture line. For the animals killed at the 40th week, all of the esophagi were cut longitudinally and fixed in 10% buffered formalin. The formalin-fixed esophagus was Swiss-rolled, processed and embedded in paraffin. Five-micron sections were mounted on glass slides and used for pathological and immunohistochemical analyses.
COX-2: Localization of COX-2 protein was determined by immunohistochemical staining with a specific antibody. The DAKO EnVision system (Dako Cytomation Japan Co. Ltd., Kyoto, Japan) was used with autoclave acceleration. After blocking endogenous peroxidase, deparaffinized sections were covered with a protein block and serum-free medium (Dako) and were incubated overnight at 4 °C with a primary anti-COX2 monoclonal antibody (1:50; BD Transduction Laboratories, San Jose, CA). Sections were treated with a secondary biotinylated antibody (Dako), 3,3’-diamonobenzidine tetrahydrochloride was used as the chromogen, and the sections were counterstained with hematoxylin.
Proliferating cell nuclear antigen: Immunohistochemical detection of proliferating cell nuclear antigen (PCNA) was performed by the avidin-biotin complex method using a mouse anti-human PCNA monoclonal antibody and the appropriate Histostain Gold AEC kit. The PCNA labeling index has been widely used to assess cell proliferation. In this study, the index was defined as the number of squamous epithelial cells with a PCNA-positive nucleus (or nuclei)/100 squamous epithelial cells (%).
Each tissue sample frozen at -80 °C was weighed and homogenized using a Polytron homogenizer PT-MR2100 in 0.5 mL of homogenization buffer (0.1 mol/L phosphate, pH 7.4, containing 1 mmol/L ethylenediamine tetraacetic acid and 10 μmol/L indomethacin) containing 100 mg tissue. Then, 2 volumes of acetone were added to the samples and vigorously spun. The samples were incubated at room temperature for 5 min, and centrifuged at 1500 ×g for 10 min to remove precipitated proteins. The supernatant was transferred into a clean tube, and dried to remove the acetone using a gentle stream of nitrogen. One mL of 1.0 mol/L acetate buffer (pH 7.4) was added to dissolve the samples, which were immediately affinity purified with an SPE cartridge (Cayman Chemical, Ann Arbor, MI). The samples were assayed using a PGE2 EIA kit (Cayman Chemical), according to the manufacturer’s instructions. PGE2 levels were shown as pg/mg of tissue.
After each rat was sacrificed, their esophagi were removed and lavaged with 0.5 mL of saline. The lavage was centrifuged at 1500 ×g at 4 °C for 5 min. The supernatant was frozen and stored. Total bile acid concentration was measured with an ENZa BILE kit (Daiichi Chemical, Tokyo).
Data are expressed as mean ± SD of each group. Student’s t test was used for statistical analysis. P < 0.05 was considered statistically significant.
A total of 37 of 40 (92.5%) rats completed the study. In the EDA group, 27 (90%) rats completed the study, and 3 rats died from complications, such as malnutrition and pneumonia. In the control group, 10 (100%) rats completed the study.
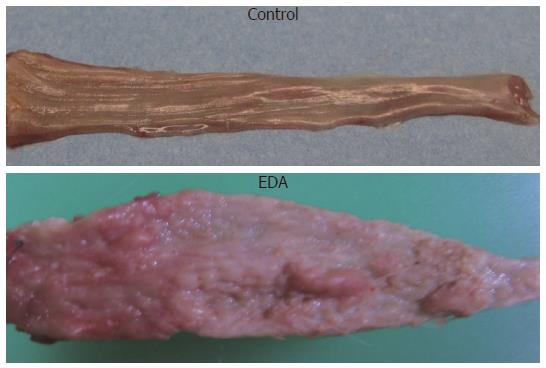
The middle and lower esophagus of animals in the EDA group was wide and thickened. There was gross evidence of severe esophageal mucosal injury in the EDA group, which included epithelial thickening and extensive hyperplasia of the lower two thirds of the esophagus. Ulceration was frequently present in the area above the anastomosis (Figure 2).
There was a small polyploid tumor in the lower esophagus in the EDA group. The tumor was squamous cell carcinoma and adenocarcinoma. Most of the nodular lesions were also associated with carcinomas, and the others with esophagitis. In addition, there were grossly normal tissues in the control group.
The esophagi of the control rats did not reveal any pathological findings, but various squamous cell lesions were observed in the middle and lower esophagus in the EDA group (Figure 3).
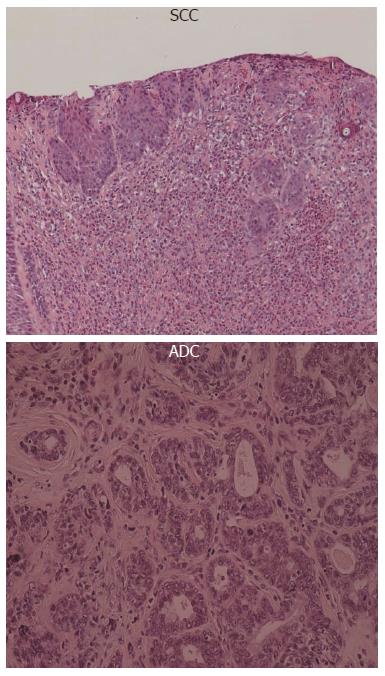
All animals from the EDA group showed histological features of esophagitis, including marked hyperplastic changes with increased thickness of the squamous epithelium, hyperkeratosis and regenerative changes with papillomatosis, and basal cell hyperplasia. These features were not found in the control group. Columnar lined epithelium (CLE) and epithelial ulceration were frequently present adjacent to the anastomosis. CLE was observed in 40% of rats at the 40th week. Sever dysplasia in the lower esophagus occurred in 100%, squamous cell carcinoma (SCC) was observed in 40% and adenocarcinoma (ADC) was observed in 30% at the 40th week.
To assess the biological behavior of various squamous lesions, we performed immunohistochemical staining for PCNA because the proliferative index is often increased in dysplastic and cancerous tissues. The PCNA labeling index of dysplasia and cancer (75% ± 5%) was higher than that of control (30% ± 5%) (P < 0.05) (Figure 4).
Total bile acids in the esophageal lumen were significantly increased in the EDA group (180 ± 50) compared with the sham operated rats (35 ± 5) (P < 0.05).
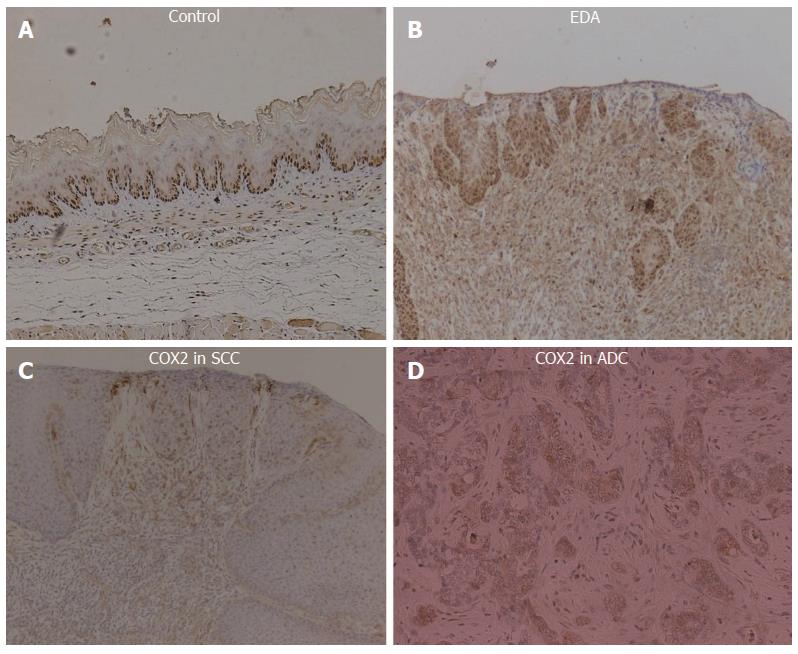
Every animal that suffered from reflux demonstrated COX-2 protein expression in the lower esophagus. COX-2 was abundantly expressed in both inflammatory and proliferative esophageal mucosa of rats exposed to chronic EDA. Some SCC and ADC epithelial cells strongly expressed the COX-2 protein (Figure 4).
PGE2 synthetic activity was significantly increased in the EDA group (260 ± 50) compared with the sham operated group (25 ± 5) (P < 0.01).
Overexpression of COX-2 has been linked to a variety of inflammatory and neoplastic conditions. Hence, it is logical to postulate that endogenous inducers of COX-2 could predispose to inflammation and malignancy. Previously, Song et al[12] reported that unconjugated bile acids induced COX-2 expression.
Recent evidence suggests that bile acids, major constituents of the duodenogastroesophageal refluxate, can also promote the development of Barrett’s esophagus and esophageal cancer. Bile reflux is particularly common in individuals with gastroesophageal reflux disease who subsequently develop Barrett’s esophagus[13,14]. Barrett’s esophagus also develops in patients who have undergone total gastrectomy, a situation in which bile reflux is common. Development of Barrett’s esophagus and subsequently esophageal cancer occurs in a rat model of esophagoduodenostomy. The present study demonstrates that it is duodenal contents, not gastric contents, that induce esophageal carcinogenesis through reflux. Because this carcinogenesis required no administration of carcinogens, and because spontaneous esophageal carcinoma is rare in animals, duodenal contents are most likely carcinogenic in the development of esophageal carcinoma.
The histological pattern of esophageal carcinoma induced in the present study was classified into 2 types; ADC and SCC.
ADC always occurred near the esophagoduodenostomy and always in the columnar lined epithelium. Human esophageal ADC mostly arises in the lower third of the esophagus, and when it does occur, it is usually associated with Barrett’s esophagus. The majority of Barrett’s esophagus cases result from chronic gastro-esophageal reflux. SCC was observed distant from the site of the anastomosis, and was surrounded by chronic squamous esophagitis with features of basal-cell hyperplasia and regenerative thickening.
It is widely accepted in humans that regurgitation of duodenal contents is closely linked to Barrett’s esophagus and to the development of esophageal ADC; however, esophageal SCC is not reported to be related to reflux[15], but is strongly associated with tobacco smoking and alcohol consumption. Gastroesophageal reflux does not appear to be an independent risk factor for esophageal SCC, but it may enhance the acknowledged risk factors such as tobacco smoking and alcohol consumption. In contrast, results from several studies using rat duodenal content reflux models have shown the development of esophageal carcinomas including SCC[16]. In this study, the incidence of pure ADC is lower than that of SCC. It is unclear what factors lead to the formation of carcinomas of specified histology.
The precise mechanisms by which duodenal reflux causes esophageal injury and predisposes to esophageal cancer are uncertain. There is considerable evidence, however, that bile acids contribute to this process. Total bile acids in the esophageal lumen were significantly increased in the EDA group compared with the control group. Bile acids induce AP-1-mediated gene transcription[17-19] and enhance the activity of protein kinase C[20]. Recent evidence has linked bile acid induced tumorigenesis to increased activity of COX-2. It is also unclear which bile acids in the refluxate contribute to COX-2 induction.
As discussed above, bile acids represent one of the important constituents of duodenal fluid that has been implicated in esophageal mucosal injury[21]. Bile acids strongly induce COX-2 by either transcriptional or post-transcriptional mechanisms in multiple gastrointestinal tract cancers, including cancer of the colon, pancreas, stomach, liver, esophagus and bile duct[22].
An animal model was used to determine whether duodenoesophageal reflux caused induction of COX-2. We observed markedly enhanced expression of COX-2 in dysplastic and cancerous mucosa obtained from rats in which an esophagoduodenal anastomosis had been created. In contrast, COX-2 was undetectable in esophageal and duodenal mucosa from the control rats. Esophageal tissue PGE2 levels were significantly increased in rats that developed dysplasia and cancer. This result suggests a possible mechanism by which bile acids promote esophageal cancer.
Bile acids increase cellular proliferation and the number of mitotic events in colonic mucosa[23]. Enhanced DNA synthesis has been demonstrated in the epithelium of the large intestine of rats treated with bile acids[24]. Reduced susceptibility to apoptosis occurs in animal and human models of colon cancer following bile acid treatment[25]. Taken together, the data suggest that bile acids are important mediators of carcinogenesis.
In conclusion, our findings suggest that reflux of bile acids induced the development of esophageal cancers. COX-2 induced by bile acids might be responsible for tumor angiogenesis, an important process in the development of esophageal cancers.
It is known that reflux of duodenal contents (bile acids) can induce mucosal injury, stimulate cell proliferation, and promote tumorigenesis.
In this study, bile reflux of duodenal contents induces cyclooxygenase-2 (COX-2) expression and increases prostaglandin synthesis in dysplastic and cancer tissues. This result suggests a possible mechanism by which bile acids promote esophageal cancer.
Hematoxylin eosin, COX-2, PGE2, and proliferating cell nuclear antigen.
This is an excellent experimental study which probably adds to the existing literature.
P- Reviewers: Cai SY, Shimi SM, Tovar JA S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Ma S
| 1. | Kauer WK, Peters JH, DeMeester TR, Ireland AP, Bremner CG, Hagen JA. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized. Ann Surg. 1995;222:525-531; discussion 531-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 261] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Gillen P, Keeling P, Byrne PJ, Healy M, O’Moore RR, Hennessy TP. Implication of duodenogastric reflux in the pathogenesis of Barrett’s oesophagus. Br J Surg. 1988;75:540-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut. 1999;44:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 299] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Goldstein SR, Yang GY, Curtis SK, Reuhl KR, Liu BC, Mirvish SS, Newmark HL, Yang CS. Development of esophageal metaplasia and adenocarcinoma in a rat surgical model without the use of a carcinogen. Carcinogenesis. 1997;18:2265-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Kivilaakso E, Fromm D, Silen W. Effect of bile salts and related compounds on isolated esophageal mucosa. Surgery. 1980;87:280-285. [PubMed] |
| 6. | Craven PA, Pfanstiel J, DeRubertis FR. Role of activation of protein kinase C in the stimulation of colonic epithelial proliferation and reactive oxygen formation by bile acids. J Clin Invest. 1987;79:532-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 144] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Narisawa T, Magadia NE, Weisburger JH, Wynder EL. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N’-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974;53:1093-1097. [PubMed] |
| 8. | Subbaramaiah K, Telang N, Ramonetti JT, Araki R, DeVito B, Weksler BB, Dannenberg AJ. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 1996;56:4424-4429. [PubMed] |
| 9. | Crofford LJ, Wilder RL, Ristimäki AP, Sano H, Remmers EF, Epps HR, Hla T. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. J Clin Invest. 1994;93:1095-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 498] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 10. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [PubMed] |
| 11. | Fein M, Peters JH, Chandrasoma P, Ireland AP, Oberg S, Ritter MP, Bremner CG, Hagen JA, DeMeester TR. Duodenoesophageal reflux induces esophageal adenocarcinoma without exogenous carcinogen. J Gastrointest Surg. 1998;2:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Song S, Guha S, Liu K, Buttar NS, Bresalier RS. COX-2 induction by unconjugated bile acids involves reactive oxygen species-mediated signalling pathways in Barrett’s oesophagus and oesophageal adenocarcinoma. Gut. 2007;56:1512-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Stein HJ, Kauer WK, Feussner H, Siewert JR. Bile reflux in benign and malignant Barrett’s esophagus: effect of medical acid suppression and nissen fundoplication. J Gastrointest Surg. 1998;2:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 133] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | DeMeester TR. Antireflux surgery in the management of Barrett’s esophagus. J Gastrointest Surg. 2000;4:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2028] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 16. | Kumagai H, Mukaisho K, Sugihara H, Miwa K, Yamamoto G, Hattori T. Thioproline inhibits development of esophageal adenocarcinoma induced by gastroduodenal reflux in rats. Carcinogenesis. 2004;25:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Jaiswal K, Lopez-Guzman C, Souza RF, Spechler SJ, Sarosi GA. Bile salt exposure increases proliferation through p38 and ERK MAPK pathways in a non-neoplastic Barrett’s cell line. Am J Physiol Gastrointest Liver Physiol. 2006;290:G335-G342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Qiao D, Chen W, Stratagoules ED, Martinez JD. Bile acid-induced activation of activator protein-1 requires both extracellular signal-regulated kinase and protein kinase C signaling. J Biol Chem. 2000;275:15090-15098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Hirano F, Tanada H, Makino Y, Okamoto K, Hiramoto M, Handa H, Makino I. Induction of the transcription factor AP-1 in cultured human colon adenocarcinoma cells following exposure to bile acids. Carcinogenesis. 1996;17:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Pongracz J, Clark P, Neoptolemos JP, Lord JM. Expression of protein kinase C isoenzymes in colorectal cancer tissue and their differential activation by different bile acids. Int J Cancer. 1995;61:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Palanca-Wessels MC, Barrett MT, Galipeau PC, Rohrer KL, Reid BJ, Rabinovitch PS. Genetic analysis of long-term Barrett’s esophagus epithelial cultures exhibiting cytogenetic and ploidy abnormalities. Gastroenterology. 1998;114:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Tucker ON, Dannenberg AJ, Yang EK, Fahey TJ. Bile acids induce cyclooxygenase-2 expression in human pancreatic cancer cell lines. Carcinogenesis. 2004;25:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | DeRubertis FR, Craven PA. Relationship of bile salt stimulation of colonic epithelial phospholipid turnover and proliferative activity: role of activation of protein kinase C1. Prev Med. 1987;16:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Takano S, Akagi M, Bryan GT. Stimulation of ornithine decarboxylase activity and DNA synthesis by phorbol esters or bile acids in rat colon. Gann. 1984;75:29-35. [PubMed] |
| 25. | Magnuson BA, Shirtliff N, Bird RP. Resistance of aberrant crypt foci to apoptosis induced by azoxymethane in rats chronically fed cholic acid. Carcinogenesis. 1994;15:1459-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |