Published online Jan 14, 2014. doi: 10.3748/wjg.v20.i2.539
Revised: September 29, 2013
Accepted: November 12, 2013
Published online: January 14, 2014
Processing time: 165 Days and 3.1 Hours
AIM: To investigate the correlations between serum amylase levels, intestinal permeability (IP), and pancreatic injury and to explore the mechanisms responsible for hyperamylasemia in double-balloon enteroscopy (DBE).
METHODS: A prospective study was conducted in 20 patients who underwent DBE from August 1, 2008 to February 28, 2009. Serum amylase was examined 0, 2, 6 and 24 h post-DBE, C-reactive protein and lipase were examined at 24 h, and urine lactulose, mannitol, and trypsinogen-II (TRY-II) levels were measured at 6 h. Lactulose/mannitol ratio indicated IP, and TRY-IIindicated pancreatic injuries. Procedure duration and enteroscope insertion length were recorded.
RESULTS: Twelve patients underwent oral DBE (M:F, 5:7; mean age 50.42 ± 11.11 years) and 8 underwent anal DBE (M:F, 5:3; mean age 44.75 ± 12.66 years). They all showed significantly increased post-DBE serum amylase. Amylase and lipase levels were higher in the oral DBE group (P < 0.05). Hyperamylasemia was diagnosed in 9 (75.0%) patients undergoing oral DBE. Only patients receiving oral DBE showed increased post-procedure IP, which correlated with increased serum amylase (r = 0.611, P = 0.035) and procedure duration (r = 0.668, P = 0.018). Adverse events included one oral case with pancreatic injury (elevated TRY-II) and two cases of abdominal discomfort in each group. Pancreatitis was not reported.
CONCLUSION: Hyperamylasemia correlates with increased IP and clinically undetectable pancreatic injuries. DBE could cause intestinal mucosa damage, which may result in IP elevation and increased amylase absorption, necessitating improvements and standardization of DBE methods.
Core tip: Hyperamylasemia, or increased serum amylase, and acute pancreatitis following double-balloon enteroscopy (DBE) have been reported in patients receiving diagnostic DBE, particularly oral DBE.
- Citation: Feng N, Dai J, Lu H, Li XB, Gao YJ, Ge ZZ. Hyperamylasemia is associated with increased intestinal permeability in patients undergoing diagnostic oral double-balloon enteroscopy. World J Gastroenterol 2014; 20(2): 539-545
- URL: https://www.wjgnet.com/1007-9327/full/v20/i2/539.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i2.539
Double-balloon enteroscopy (DBE) is a relatively safe digestive endoscopic technique for diagnosis and treatment of small bowel diseases. DBE can be performed either anally or orally and has been widely used in clinical practice for over 5 years[1]. DBE postoperative complication rate is about 4% in the Unites States[1] and has been reported to be as low as 1.4% worldwide[2]. Therapeutic endoscopy has been associated with severe complications (bleeding, perforation, and concomitant tissue necrosis), but these complications are rarely observed in diagnostic procedures[2,3]. However, hyperamylasemia and acute pancreatitis were reported in DBE patients undergoing diagnostic procedures, especially when performed orally[4-6]. Because both hyperamylasemia and acute pancreatitis are threatening conditions, a better understanding of these disorders is required to design effective preventative strategies.
DBE-induced acute pancreatitis was first reported by Groenen et al[4], and was later mainly associated with oral DBE[2,5,6]. Moreover, hyperamylasemia after oral DBE was also reported by Kopácová et al[7], who demonstrated that hyperamylasemia was much more frequent than acute pancreatitis. Damage to the intestine caused by local strain and friction in the small bowel during DBE may be central to the development of hyperamylasemia[8]. Thus, shorter BDE time, fewer passes, and cautious insertion may be useful in reducing small bowel injury[8]. On the other hand, some studies reported no association between small bowel damage and hyperamylasemia in DBE patients[9].
Damage to the bowel can be indicated by altered intestinal permeability (IP)[10]. Injuries to the intestinal mucosal barrier may change the inter-epithelial structure and increase permeability, increasing lactulose absorption, but without influence on mannitol absorption via the cell membrane. Therefore, the lactulose/mannitol (L/M) ratio can reflect the change in IP[10]. Intestinal barrier permeability defects caused by DBE may have other effects, such as altered biochemical parameters and increased bacterial translocation[11].
The present study was conducted to determine the relationships between serum amylase levels, IP (indicated by the L/M ratio), and pancreatic injury [indicated by urinary trypsinogen-II (TRY-II) levels] in patients undergoing oral or anal DBE. This study was designed to comprehensively investigate pancreatic injury and systemic abnormalities associated with DBE, and to clarify the mechanisms responsible for DBE-induced hyperamylasemia.
A prospective study was conducted in consecutive DBE patients treated at the Endoscopy Center of the Shanghai Jiaotong University School of Medicine from 1st August 2008 to 28th February 2009. The study protocol was approved by the Institutional Ethics Committee of the Shanghai Jiaotong University School of Medicine. All procedures were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Inclusion criteria were: (1) patients aged 17 to 70 years; and (2) patients who underwent diagnostic DBE within the study period. Exclusion criteria were: (1) patients who underwent any other recent endoscopic treatments for diagnostic or therapeutic purposes; (2) patients who were diagnosed with dysfunction of the liver, gallbladder, pancreas or kidneys; and (3) patients who exhibited current or previous enterostenosis, tumors, or inflammatory bowel disease. Based on these criteria, of 60 patients who were initially recruited and received the double-sugar solution, 40 were excluded after DBE.
Clinical data was recorded for each patient, including abdominal ultrasonographic findings and blood biochemical results. For each patient, levels of alanine transaminase, aspartate transaminase, total and direct bilirubin, γ-glutamyl transpeptidase, alkaline phosphatase, blood urea nitrogen and creatinine were recorded.
Hyperamylasemia was defined as increased serum amylase levels above the upper limit of normal (> 100 U/L) or 3 × higher than baseline (preoperative) levels[8]. Pancreatitis was defined in accordance with the consensus definitions for major complications of endoscopic retrograde cholangiopancreatography[12]. Briefly, acute pancreatitis was defined as occurrence of clinical signs that included typical epigastric pain with radiation to the back and/or nausea, and vomiting combined with amylase/lipase levels ≥ 3 × the upper limit of normal, requiring unplanned admission or prolongation of planned admission.
Indications for DBE were recorded. All patients were asked to drink 60 mL of double-sugar solution containing 10 g of mannitol and 5 g of lactulose after an overnight fast, anytime within the 3-d period prior to DBE, and pre-procedure urine samples were collected approximately 6 h after drinking, as previously described[10]. Right before the DBE procedure, baseline serum amylase was examined (which was marked as “0 h” below).
Oral and anal DBE procedures were performed by a single experienced endoscopist using an EN-450P5 DBE (Fujinon, Saitama, Japan). All patients received gas and intravenous anesthesia during the DBE procedures. For oral DBE, the endoscopist initiated inflation of the overtube balloon after the enteroscope had passed the ligament of Treitz, thus avoiding direct compression or injury to the duodenal papilla. For anal DBE, the enteroscope was inserted as far as possible beyond the ileocecal valve. After examinations were completed, patients in both groups were injected with 60 mL of double-sugar solution through the enteroscope into the duodenal bulb as the enteroscope was removed. We recorded procedure duration and the length of enteroscope insertion relative to the ligament of Treitz or ileocecal valve for both groups of patients. Therefore, when the enteroscope reached its deepest level, we pulled the endoscope, and the insertion length was calculated as: [reference point on the endoscope - (60 cm, oral or 65 cm, anal)] × 8.
After DBE, serum amylase levels were examined at 2, 6 and 24 h (normal range: 40-100 U/L). Serum C-reactive protein (CRP) (normal range: < 10.0 mg/L) and lipase (normal range: < 40 U/L) were examined 24 h after DBE. Post-procedure urine samples were collected 6 h after injection of double-sugar solution during DBE. Urine lactulose, mannitol, and TRY-II levels were measured. The L/M ratio was calculated to determine IP and TRY-II was used as an indicator of pancreatic injuries. After final samples were taken, asymptomatic patients were discharged, and patients with confirmed or suspected acute pancreatitis were admitted to the hospital.
After DBE, patients were assessed by routine clinical examinations at approximately 30 min intervals, and epigastric abdominal pain, distension, nausea, or vomiting was recorded. Adverse events (AEs) were reported, including abdominal signs and symptoms.
Continuous data are reported as mean ± SD and ranges. Statistical analysis was conducted using SAS 5.1 (SAS Institute, Cary, NY, United States). Continuous variables were compared using unpaired or paired t tests or, alternatively, using the Wilcoxon rank-sum test. Categorical variables were compared using the Chi-squared test. Correlation analysis was performed by linear regression test. A two-sided P value < 0.05 was considered statistically significant.
Of a total of 60 consecutive DBE patients, 40 (66.7%) were excluded due to liver, gallbladder, and/or pancreas diseases (n = 7); renal dysfunction (n = 6); enterostenosis (n = 6), tumors (n = 2); or inflammatory bowel disease (n = 19). The remaining 20 (33.3%) patients (M:F, 10:10; mean age 48.15 ± 11.77 years, ranging 26-70 years) were included in the study, including 12 undergoing the oral procedure (M:F, 5:7; mean age 50.42 ± 11.11 years, ranging 26-70 years) and 8 undergoing the anal procedure (M:F, 5:3; mean age 44.75 ± 12.66 years, ranging 26-60 years). No significant differences were observed in demographic characteristics between the two groups of patients. Indications for DBE included unexplained abdominal pain (n = 8), abdominal distension (n = 5), iron deficiency anemia (n = 4), and intermittent diarrhea with negative esophagogastroduodenoscopy and colonoscopy (n = 3) (Table 1).
| Oral DBE (n = 12) | Anal DBE (n = 8) | |
| Age (yr), mean ± SD | 50.42 ± 11.11 | 44.75 ± 12.66 |
| Gender | ||
| Male | 5 (41.7) | 5 (62.5) |
| Female | 7 (58.3) | 3 (37.5) |
| Indications | ||
| Abdominal pain | 5 (41.7) | 3 (37.5) |
| Abdominal distension | 4 (33.3) | 1 (12.5) |
| Anemia | 3 (25.0) | 1 (12.5) |
| Intermittent diarrhea | 0 (0.0) | 3 (37.5) |
| Procedure, mean ± SD | ||
| Duration (min) | 71.92 ± 20.19 | 65.63 ± 17.80 |
| Length of insertion (m) | 3.55 ± 1.42 | 2.8 ± 1.09 |
| Adverse events | ||
| Abdominal discomfort/distention | 2 (16.7) | 2 (25.0) |
| Pancreatic injury (elevated urinary trypsinogen-II) | 1 (8.3) | 0 |
Mean procedure duration was 71.92 ± 20.19 min for patients in the oral DBE group and 65.63 ± 17.80 min for those in the anal DBE group. Insertion length was 3.55 ± 1.42 m beyond the ligament of Treitz for patients in the oral DBE group, and 2.80 ± 1.09 m beyond the ileocecal valve for those in the anal DBE group (Table 1).
Nine (75.0%) patients undergoing oral DBE were diagnosed with hyperamylasemia, including 9 at 2 h, 7 at 6 h, and 6 at 24 h. By 6 h after DBE, 2 of these cases exhibited normalized amylase levels, and no new cases of hyperamylasemia were subsequently reported. Although post-procedure amylase levels were also significantly elevated in patients undergoing anal DBE at 2 h (P = 0.013) and 6 h (P < 0.001), no patients in the anal DBE group were diagnosed with hyperamylasemia.
In addition, compared with baseline values, serum amylase levels at 2 h after DBE were increased by 141.0% in the oral DBE group, but only by 19.0% in the anal DBE group. Patients in the oral DBE group exhibited amylase levels of 63.08 ± 25.13, 150.92 ± 87.98, 160.83 ± 99.61 and 90.75 ± 41.98 U/L at 0, 2, 6 and 24 h, respectively, after DBE, while patients in the oral DBE group exhibited amylase levels of 53.88 ± 17.35, 63.50 ± 19.82, 66.25 ± 20.89 and 57.38 ± 15.81 U/L at 0, 2, 6 and 24 h, respectively (Table 2). Notably, no correlations were observed between serum amylase levels and enteroscope insertion length.
| Oral DBE (n = 12) | Anal DBE (n = 8) | |
| Serum amylase (U/L) | ||
| 0 h | 63.08 ± 25.13 | 53.88 ± 17.35 |
| 2 h | 150.92 ± 87.98a | 63.50 ± 19.82a |
| 6 h | 160.83 ± 99.61a | 66.25 ± 20.89a |
| 24 h | 90.75 ± 41.98a | 57.38 ± 15.81 |
| Serum amylase ratio (vs at 0 h) | ||
| 2 h | 2.41 ± 1.08c | 1.19 ± 0.15 |
| 6 h | 2.54 ± 1.08c | 1.24 ± 0.11 |
| 24 h | 1.51 ± 0.53c | 1.10 ± 0.24 |
| Serum C-reaction protein (mg/L) at 24 h | 7.46 ± 6.501 | 6.91 ± 8.95 |
| Urine lactulose (mg/L) | ||
| 0 h | 0.571 ± 0.134 | 0.481 ± 0.096 |
| 6 h | 0.691 ± 0.139 | 0.294 ± 0.081 |
| Urine mannitol (mg/L) | ||
| 0 h | 7.881 ± 2.664 | 7.726 ± 2.247 |
| 6 h | 8.059 ± 2.131 | 4.308 ± 1.802 |
| Urine lactulose/mannitol ratio | ||
| 0 h | 0.083 ± 0.0482 | 0.067 ± 0.023 |
| 6 h | 0.095 ± 0.049a3 | 0.075 ± 0.028a |
Patients undergoing oral DBE and those undergoing anal DBE exhibited serum lipase levels of 37.92 ± 26.64 and 19.61 ± 8.96 U/L, respectively, 24 h after DBE. Serum lipase levels at 24 h after DBE were significantly increased in 5 (41.7%) patients receiving the oral DBE procedure, but no increased lipase levels were observed in the anal DBE group. Furthermore, serum lipase levels were significantly higher in the oral DBE group than in anal DBE group (P = 0.008). Furthermore, serum lipase at 24 h post-DBE appeared to be related to serum amylase levels at 2 h post-procedure, though this correlation was not statistically significant (r = 0.514, P = 0.08).
CRP was normal in 10 (83.3%) patients receiving oral DBE and 7 (87.5%) patients receiving anal DBE. Thus, only 2 (16.7%) patients receiving oral DBE and 1 (12.5%) patient receiving anal DBE had high CRP levels.
Elevated urine TRY-II was only observed in a 49-year-old male patient receiving oral DBE, who exhibited a TRY-II value of 30.59 μg/L. In this case, the duration of DBE procedure was 40 min and the insertion length was 2.8 m, with serum amylase peaking 2 h after the procedure at 312 U/L. Notably, no abdominal symptoms were observed in this patient, and serum lipase and IP were normal. Mean TRY-II level for other 19 patients was 2.6 ± 0.1 μg/L.
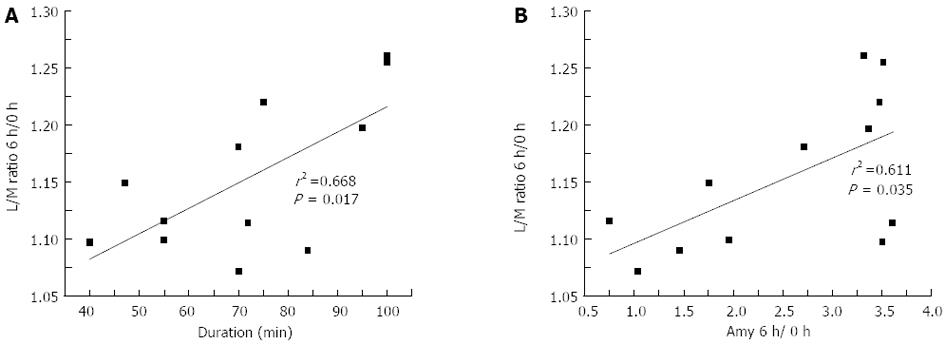
Post-DBE urine L/M ratios were elevated in both groups of patients, but the elevation was more significant in patients receiving oral DBE. In the oral group, urine mannitol levels were slightly increased, but lactulose levels were much more dramatically increased after the procedure. Although the absolute values of urine mannitol and lactulose concentrations in the anal group appeared to be decreased, their ratios increased compared with preoperative levels (P < 0.05) (Table 2). Increased IP indicated by urine L/M ratios in patients receiving oral DBE positively correlated with procedure duration (r = 0.668, P = 0.017) (Figure 1A). Moreover, L/M ratios and 6 h/0 h serum amylase ratios were significantly correlated in patients receiving oral DBE (r = 0.611, P = 0.035) (Figure 1B). Furthermore, in the oral group, IP was increased by a mean of 15%. In the anal group, although the absolute values of mannitol and lactulose levels after DBE decreased compared with the pre-DBE levels, the L/M ratio was increased by about 11% on average, compared with the preoperative value.
The procedures were successful in all patients. Four (20%) patients, including 2 (16.7%) in the oral DBE group and 2 (25.0%) in the anal DBE group, complained of abdominal discomfort. By 6 h after the procedure, abdominal distension disappeared in all affected patients and all of them were able to walk normally, leading to prompt discharge. Pancreatic injuries were observed only in 1 patient receiving oral DBE (elevated urinary TRY-II), and no pancreatitis cases were reported. No other acute abdominal symptoms, pancreatitis, or other serious AEs were reported.
In the present study, post-DBE serum amylase levels were increased in patients treated by diagnostic oral or anal DBE. This increase, along with the increase in lipase, was more significant in patients receiving oral DBE. Furthermore, a significant number of patients receiving oral DBE developed hyperamylasemia, and increased post-DBE IP correlated with increased serum amylase and procedure duration. Notably, no pancreatitis and only a single case of pancreatic injury were reported in the present study. These findings may have mechanistic implications for the development of DBE-induced hyperamylasemia in patients treated by oral DBE, suggesting that increased serum amylase and, hypothetically, increased amylase absorption due to DBE-induced IP elevation, may play a role in causing virtually undetectable pancreas injury leading to hyperamylasemia.
An international, multicenter retrospective study reported that the incidence of acute pancreatitis in 2362 patients who underwent DBE was 0.3% (n = 7)[2], demonstrating an extremely low rate of pancreatitis that may explain the lack of pancreatitis in the present study. Honda et al[13] reported that 46% of patients exhibited increased serum amylase 3 h after diagnostic oral DBE but, in the present study all patients had increased serum amylase by 6 h, which is likely due to the later sampling. Similarly, Kopácová et al[7] reported that post-DBE serum amylase levels were increased in 58% of patients, of whom 9 (25.7%) were diagnosed with hyperamylasemia, 1 (11%) with mild acute pancreatitis, and 5 (55.6%) with abdominal discomfort, which is very similar to results of the present study.
It has also been suggested that the development of hyperamylasemia and acute pancreatitis following DBE may be related, though associated acute pancreatitis generally appears mild and may represent only minor pathological changes in the pancreas[7]. Furthermore, hyperamylasemia tends to resolve shortly after DBE[7]. These findings indicate that measurement of serum amylase 2 h after DBE may be sufficient for clinical monitoring of abnormal serum amylase level and diagnosis of hyperamylasemia. Furthermore, Sugiyama et al[14] explored the risk factors associated with hyperamylasemia, and showed that the bile duct size, history of pancreatitis, and young age may play a role in the development of hyperamylasemia, though no relationship between hyperamylasemia and pancreatitis was determined. Some studies also demonstrated that the insertion technique can increase the risk of hyperamylasemia[8]. Indeed, the endoscopist may exert forces on the small intestine to correctly position the enteroscope, thus exerting pressure and friction on the intestinal wall that may mechanically and functionally damage intestinal mucosa and potentially raise the risk of infection and inflammation[15-18].
In the present study, the L/M ratios, indicating altered IP, were increased in both groups, though the extent was significantly greater in patients receiving oral DBE. These findings are consistent with those of previous studies, though the present study did not explore the clinical implications of these alterations, including increased bacterial proliferation and infection risk[10]. However, the present study demonstrated that IP was increased by about 15% and the L/M ratio by about 11%. Based on these findings, we postulate that damage to the small intestinal mucosa and intestinal mucosal barrier may lead to increased IP, though this hypothesis and its potential clinical implications requires further study. Thus, L/M ratio may be a clinically useful prognostic indicator in DBE patients.
Furthermore, the present findings demonstrated a correlation between IP and procedure duration in patients receiving oral DBE, which may indicate that prolonged procedures increase IP. As a result, high serum amylase levels may be attributable to increased intestinal absorption, but this hypothesis requires further verification. Notably, Aktas et al[8] and Pata et al[19] both reported that enteroscope length and procedure duration were correlated with serum amylase levels, and that balloon inflation time was an independent predictive factor of post-operative pancreatitis after oral DBE. Thus, it is reasonable to consider that longer and more extensive enteroscopy may be associated with greater damage to the intestinal mucosa, promoting changes in IP and the subsequent increase in serum amylase levels.
In the present study, some patients showed signs of pancreatic injury, indicated by elevated TRY-II levels, which is consistent with previous reports[20-23]. Notably, Kemppainen et al[24] detected TRY-II 6 h post-DBE and showed the high sensitivity and specificity (81% and 90%, respectively) of TRY-II-based diagnosis of pancreatic injury. Some pancreatic injuries may be too mild to be considered as acute pancreatitis, but elevated TRY-II may indicate that minor DBE-induced pancreatic injury may be a critical factor in the development of complications after DBE. Thus, more gentle insertion techniques, shortening of balloon time and reducing the number of passes were tried to decrease DBE complications[8,19,24].
Though our results are highly consistent with previous studies, it is important to note that the relatively small sample size and lack of long-term follow-up may limit the applicability of these findings. Longitudinal studies conducted in more diverse patient populations will be required to confirm these findings and to fully explore the mechanism and clinical implications associated with hyperamylasemia in patients undergoing diagnostic DBE.
In conclusion, increased serum amylase levels, or hyperamylasemia, were found to be significantly more common in patients undergoing oral DBE. In these patients, hyperamylasemia was correlated with increased IP, and increased serum amylase levels may be attributable to increased amylase absorption. Furthermore, TRY-II assessment demonstrated that, though mild in nature, pancreatic injuries that did not develop into full acute pancreatitis were sometimes detectable in DBE patients, particularly those treated by oral DBE. Based on these findings and contemporary literature, we suggest improvements (more gentle insertion techniques, shortening of balloon time and reducing the number of passes) and standardization of DBE to limit small intestine damage and subsequent increase in IP, thus reducing the occurrence of hyperamylasemia in patients undergoing diagnostic DBE.
Hyperamylasemia (increased serum amylase) and acute pancreatitis following double-balloon enteroscopy (DBE) have been reported in patients undergoing diagnostic DBE, particularly oral DBE.
Hyperamylasemia and acute pancreatitis were reported in DBE patients undergoing diagnostic procedures, especially when performed orally. Because both hyperamylasemia and acute pancreatitis are threatening conditions, a better understanding of these disorders is required to design effective preventative strategies.
Hyperamylasemia correlates with increased intestinal permeability (IP) and clinically undetectable pancreatic injuries. DBE could cause intestinal mucosa damage, which may result in IP elevation and increased amylase absorption, necessitating improvements and standardization of DBE methods.
This is a significant study which focused on the correlations between serum amylase level, IP and DBE. Though DBE-associated acute pancreatitis is rare, hyperamylasemia is common but its mechanism remains unknown. This is the first study to prove the hyperamylasemia was due to enhanced IP rather than the injury to pancreas.
P- Reviewers: Du YQ, Figueiredo P, Kita H S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Wu HL
| 1. | Mehdizadeh S, Ross A, Gerson L, Leighton J, Chen A, Schembre D, Chen G, Semrad C, Kamal A, Harrison EM. What is the learning curve associated with double-balloon enteroscopy? Technical details and early experience in 6 U.S. tertiary care centers. Gastrointest Endosc. 2006;64:740-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Mensink PB, Haringsma J, Kucharzik T, Cellier C, Pérez-Cuadrado E, Mönkemüller K, Gasbarrini A, Kaffes AJ, Nakamura K, Yen HH. Complications of double balloon enteroscopy: a multicenter survey. Endoscopy. 2007;39:613-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | May A, Nachbar L, Pohl J, Ell C. Endoscopic interventions in the small bowel using double balloon enteroscopy: feasibility and limitations. Am J Gastroenterol. 2007;102:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Groenen MJ, Moreels TG, Orlent H, Haringsma J, Kuipers EJ. Acute pancreatitis after double-balloon enteroscopy: an old pathogenetic theory revisited as a result of using a new endoscopic tool. Endoscopy. 2006;38:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Honda K, Mizutani T, Nakamura K, Higuchi N, Kanayama K, Sumida Y, Yoshinaga S, Itaba S, Akiho H, Kawabe K. Acute pancreatitis associated with peroral double-balloon enteroscopy: a case report. World J Gastroenterol. 2006;12:1802-1804. [PubMed] |
| 6. | Matsushita M, Shimatani M, Uchida K, Okazaki K. Mechanism of acute pancreatitis after peroral double-balloon enteroscopy. Endoscopy. 2007;39:480; author reply 481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Kopácová M, Rejchrt S, Tachecí I, Bures J. Hyperamylasemia of uncertain significance associated with oral double-balloon enteroscopy. Gastrointest Endosc. 2007;66:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Aktas H, Mensink PB, Haringsma J, Kuipers EJ. Low incidence of hyperamylasemia after proximal double-balloon enteroscopy: has the insertion technique improved? Endoscopy. 2009;41:670-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Teshima CW, Aktas H, Kuipers EJ, Mensink PB. Hyperamylasemia and pancreatitis following spiral enteroscopy. Can J Gastroenterol. 2012;26:603-606. [PubMed] |
| 10. | Marsilio R, D’Antiga L, Zancan L, Dussini N, Zacchello F. Simultaneous HPLC determination with light-scattering detection of lactulose and mannitol in studies of intestinal permeability in pediatrics. Clin Chem. 1998;44:1685-1691. [PubMed] |
| 11. | Hospital NTU. Balloon-assisted Enteroscopy and Bacteria. Available from: http//clinicaltrials.gov/ct2/show/NCT01065324. |
| 12. | Anderson MA, Fisher L, Jain R, Evans JA, Appalaneni V, Ben-Menachem T, Cash BD, Decker GA, Early DS, Fanelli RD. Complications of ERCP. Gastrointest Endosc. 2012;75:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 299] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 13. | Honda K, Itaba S, Mizutani T, Sumida Y, Kanayama K, Higuchi N, Yoshinaga S, Akiho H, Kawabe K, Arita Y. An increase in the serum amylase level in patients after peroral double-balloon enteroscopy: an association with the development of pancreatitis. Endoscopy. 2006;38:1040-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for acute pancreatitis and hyperamylasemia after endoscopic papillary balloon dilation. Gastrointest Endosc. 2003;57:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Sørensen SH, Proud FJ, Adam A, Rutgers HC, Batt RM. A novel HPLC method for the simultaneous quantification of monosaccharides and disaccharides used in tests of intestinal function and permeability. Clin Chim Acta. 1993;221:115-125. [PubMed] |
| 16. | van Elburg RM, Kokke FT, Uil JJ, Mulder CJ, de Monchy JG, Heymans HS. [Measurement of selective intestinal permeability using a new, simple sugar absorption test]. Ned Tijdschr Geneeskd. 1993;137:2091-2095. [PubMed] |
| 17. | Harris CE, Griffiths RD, Freestone N, Billington D, Atherton ST, Macmillan RR. Intestinal permeability in the critically ill. Intensive Care Med. 1992;18:38-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Trehan I, Shulman RJ, Ou CN, Maleta K, Manary MJ. A randomized, double-blind, placebo-controlled trial of rifaximin, a nonabsorbable antibiotic, in the treatment of tropical enteropathy. Am J Gastroenterol. 2009;104:2326-2333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Pata C, Akyüz U, Erzin Y, Mutlu N, Mercan A, Dirican A. Post-procedure elevated amylase and lipase levels after double-balloon enteroscopy: relations with the double-balloon technique. Dig Dis Sci. 2010;55:1982-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Kemppainen EA, Hedström JI, Puolakkainen PA, Sainio VS, Haapiainen RK, Perhoniemi V, Osman S, Kivilaakso EO, Stenman UH. Rapid measurement of urinary trypsinogen-2 as a screening test for acute pancreatitis. N Engl J Med. 1997;336:1788-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Hedström J, Korvuo A, Kenkimäki P, Tikanoja S, Haapiainen R, Kivilaakso E, Stenman UH. Urinary trypsinogen-2 test strip for acute pancreatitis. Lancet. 1996;347:729-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Kylänpää-Bäck M, Kemppainen E, Puolakkainen P, Hedström J, Haapiainen R, Perhoniemi V, Kivilaakso E, Korvuo A, Stenman U. Reliable screening for acute pancreatitis with rapid urine trypsinogen-2 test strip. Br J Surg. 2000;87:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Pezzilli R, Morselli-Labate AM, d’Alessandro A, Barakat B. Time-course and clinical value of the urine trypsinogen-2 dipstick test in acute pancreatitis. Eur J Gastroenterol Hepatol. 2001;13:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Kemppainen E, Hedström J, Puolakkainen P, Halttunen J, Sainio V, Haapiainen R, Stenman UH. Urinary trypsinogen-2 test strip in detecting ERCP-induced pancreatitis. Endoscopy. 1997;29:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |