Published online May 14, 2014. doi: 10.3748/wjg.v20.i18.5461
Revised: December 12, 2013
Accepted: March 7, 2014
Published online: May 14, 2014
Processing time: 193 Days and 6.5 Hours
Helicobacter pylori (H. pylori) infection is well known to be associated with the development of precancerous lesions such as chronic atrophic gastritis (AG), or gastric intestinal metaplasia (GIM), and cancer. Various molecular alterations are identified not only in gastric cancer (GC) but also in precancerous lesions. H. pylori treatment seems to improve AG and GIM, but still remains controversial. In contrast, many studies, including meta-analysis, show that H. pylori eradication reduces GC. Molecular markers detected by genetic and epigenetic alterations related to carcinogenesis reverse following H. pylori eradication. This indicates that these changes may be an important factor in the identification of high risk patients for cancer development. Patients who underwent endoscopic treatment of GC are at high risk for development of metachronous GC. A randomized controlled trial from Japan concluded that prophylactic eradication of H. pylori after endoscopic resection should be used to prevent the development of metachronous GC, but recent retrospective studies did not show the tendency. Patients with precancerous lesions (molecular alterations) that do not reverse after H. pylori treatment, represent the “point of no return” and may be at high risk for the development of GC. Therefore, earlier H. pylori eradication should be considered for preventing GC development prior to the appearance of precancerous lesions.
Core tip: This review provides a current understanding on Helicobacter pylori, pathogenesis of chronic gastritis, gastric intestinal metaplasia, gastric carcinoma, and prevention strategy.
-
Citation: Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, Miwa H, Lim KJ, Das KM.
Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol 2014; 20(18): 5461-5473 - URL: https://www.wjgnet.com/1007-9327/full/v20/i18/5461.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i18.5461
Helicobacter pylori (H. pylori) is the most common chronic infection worldwide, with a prevalence of approximately 50%; however, the majority of the infected individuals are asymptomatic[1]. The country-to-country variance in prevalence ranges from as low as 30% in the United States to as high as 90% in developing countries such as Iran[2,3]. This large reservoir of asymptomatic carriers renders H. pylori a difficult infection to eradicate. Many factors determine a country’s infection rate, with socioeconomic status and living conditions in early childhood as the principle determinants. Another important factor is mode of transmission, with horizontal transmission amongst the general population as the predominant mode in developing countries verses transmission via family members in industrialized countries[4,5]. The person-to-person transmission of this infection is thought to occur via multiple routes: fecal-oral[6], oral-oral[7] as well as environmental transmission through a contaminated water supply[8]. There is evidence of earlier infection in developing nations compared to industrialized nations[9]. There are also reports of a higher prevalence of H. pylori in industrialized Asian countries compared to their western counterparts. For example the prevalence of H. pylori was noted to be 39% in Japan and 60% in South Korea[2], which are both industrialized, affluent countries with safe water supplies. One hypothesis is the potential increase in oral-oral transmission[7] of this infection due to the “family-style” sharing of meals and plates etc. typical in Asian countries.
Here we review H. pylori infection, diagnosis, and clinical syndromes with a primary focus on its effect on precancerous lesions, i.e., chronic atrophic gastritis (AG) and gastric intestinal metaplasia (GIM), development of gastric cancer (GC) and discuss early diagnosis, efficient preventive strategies for GC based on the clinical literature, through original contributions, systematic reviews, and meta-analyses.
H. pylori is a Gram-negative microaerophilic spiral-shaped bacterium with a flagella that enables it to colonize the human gastrointestinal tract. It has evolved various mechanisms to promote its survival in the stomach’s acidic environment and increase its ability to cause infection. One such adaptation is urease, an enzyme that hydrolyzes urea and releases ammonia, which in turn neutralizes gastric acid, allowing H. pylori to survive and colonize the gastric mucosa[10]. The other main feature of H. pylori is its ability to adhere to the gastric epithelium, which is achieved through receptor-mediated adhesion[11] via an array of outer membrane proteins. These proteins include adherence lipoprotein A and B (AlpA/B), blood group antigen binding adhesion (BabA), outer inflammatory protein A (OipA) and sialic acid binding adhesion (SabA). Although many other proteins in this class may play a role in cell adhesion and infection, the aforementioned are the major players. Additionally, cellular damage is achieved predominantly through the effects of two genes: vacuolating cytotoxin A (VacA) and cytotoxin associated gene A (CagA) which mechanism of action and interaction will be discussed in depth later in this article. Even though H. pylori is considered a non-invasive bacterium, there is some data supports its ability for intracellular invasion through mechanisms not yet fully understood[12]. H. pylori is a truly heterogeneous bacterium, expressing a wide array of multiple clinically important virulence factors that also seem to have a geographic influence.
The American College of Gastroenterology guideline in 2007 recommended testing for H. pylori only with the intention to treat a positive test result[13]. Absolute indications for testing and treatment include patients with active peptic ulcer disease, confirmed history of peptic ulcer but not previously treated, gastric mucosa-associated lymphoid tissue (MALT), and early GC. Relative indications include testing in patients with unexplained dyspepsia without alarming features. In the United States, a majority of these patients may have symptoms of gastroesophageal reflux disease (GERD) rather than H. pylori infection. Also testing for H. pylori prior to initiating non-steroidal anti-inflammatory drug treatment is usually not recommended in areas with low prevalence of H. pylori. Rarely unexplained iron deficiency anemia[14] or immune thrombocytopenic purpura can be reversed with H. pylori treatment, again the incidence of these finding would be low in the United States[15,16]. For asymptomatic patients, if they have a first degree relative with GC or are of high risk populations (Asians, Eastern Europeans, or Mesoamerican descent), are considered as higher risk populations for the development of GC[17].
Diagnostic testing for H. pylori can be divided into endoscopic and nonendoscopic techniques. The techniques could be direct (culture, histology, or detection of bacterial antigen in the biopsy tissue or stool) or indirect (using urease breath test, or an antibody response as a marker of disease). Serologic test for antibody indicates exposure to bacteria but does not help to assess active infection. The choice of a suitable test depends upon a variety of issues such as cost, accessibility, clinical situation, any family history of GC, and pretest probability of infection which is affected by population prevalence of infection. Also factors include the use of proton pump inhibitors, antibiotics or bismuth-containing compounds that may influence the accuracy of some test results.
Although the majority of H. pylori infections are thought to be asymptomatic, their clinical manifestations can be confounded by other disease processes such as functional dyspepsia and GERD. However a certain population of individuals infected with H. pylori can develop pathological findings of non-malignant and malignant diseases.
The relationship between H. pylori and a host of other gastrointestinal diseases is currently under investigation. Functional dyspepsia is a common ailment which occurs in 10%-30% of the population each year with no clear understanding of the pathophysiology of this disorder[18]. The underlying cause of functional dyspepsia is likely multi-factorial and the role of H. pylori is unclear. However, a large meta-analysis which examined 14 randomized controlled trials demonstrated that treating H. pylori improved dyspeptic symptoms up to 1 year later with an OR of 1.38; 95%CI: 1.18-1.62[19]. Due to these studies that evidenced improvement in dyspeptic symptoms with H. pylori eradication, many guidelines now recommend treating patients who test positive for the bacterium and suffer from functional dyspepsia[20].
GERD is an extremely common disease and its relationship with H. pylori is somewhat controversial. Several studies demonstrated an increase in GERD symptoms and esophagitis even after successful eradication of H. pylori[21]. However, upon further review, this relationship did not hold true in a meta-analysis comparing 10 trials and showed that reflux symptoms were similar in the H. pylori-treated groups and the controls. Although, improvement of reflux symptoms post treatment is also reported[22]. It is evident that H. pylori may be involved in more disease processes than previously thought, however before any definitive relationship can be established, more research is needed for clarification.
Gastritis is defined by inflammation of the stomach lining associated with mucosal injury. The duration of mucosal inflammation can be used to separate this condition from acute gastritis and chronic active gastritis. H. pylori is the most common infectious etiology associated with gastritis.
The majority of patients infected with H. pylori develop acute gastritis which may spontaneously resolve. The ability of H. pylori to cause acute gastritis is best demonstrated from studies where healthy volunteers have been intentionally infected with the organism. This acute infection is associated with the development of hypochlorhydria and neutrophilic infiltration on gastric biopsy[23-25].
After an acute H. pylori infection, the majority of acute gastritis evolves into chronic active gastritis that is histologically characterized by mononuclear cells, predominantly lymphocytes, plasma cells and macrophages. Lymphoid follicles with germinal centers are frequently seen and are characteristic of an H. pylori infection[26-28]. Three types of chronic gastritis are recognized: pangastritis, antrum predominant, and corpus predominant. Diffuse antral gastritis with normal or increased acid secretion. This is associated with little or no gastric atrophy and duodenal ulcers (DUs). Persistent inflammation results in the development of gastric atrophy with hypochlorhydria, or achlorhydria[29,30]. These changes facilitate the proximal migration of the bacteria, leading to corpus or multifocal gastritis, which tends to progress through intestinal metaplasia, then to intestinal type GC.
There is a clear association between H. pylori infection and the development of peptic ulcer disease. The prevalence rate of peptic ulcer disease caused by H. pylori remains high in Asia at about 93%[31]. In the United States and in Western Europe the prevalence rate of peptic ulcer disease have been lowered in the range of 40%-75% due to a declining occurrence of H. pylori infection[32-34].
Certain H. pylori genes and virulent factors have been suggested for the development of peptic ulcer disease. Virulent factor such as VacA m1 is possibly associated with an increased risk of peptic ulcer disease[35]. In China, the prevalence of dupA gene was highest in DU and inversely related to gastric ulcer and GC[36,37].
H. pylori eradication has been shown to be a cost effective approach to reduce peptic ulcer disease recurrence and increase DU healing rate[38-41]. According to a recent prospective, long-term study that 1000 patients were followed up for at least 12 mo to assess ulcer rebleeding rate after H. pylori eradication, the cumulative incidence of rebleeding was 0.5% (95%CI: 0.16%-1.16%), and the incidence rate of rebleeding was 0.15% (95%CI: 0.05%-0.36%) per patient-year of follow up. The authors concluded that peptic ulcer rebleeding virtually does not occur in patients with complicated ulcers after H. pylori eradication. Maintenance antisecretory therapy is not necessary if eradication is achieved[42]. Similarly, a recent systematic review and meta-analysis of five randomized controlled trials with 401 patients were performed to evaluate the effects of H. pylori eradication on prevention of ulcer recurrence after simple closure of perforated peptic ulcers. A high prevalence of H. pylori infection was found in patients with perforated peptic ulcers. Eradication of H. pylori has significantly reduced the incidence of ulcer recurrence at 8 wk (RR = 2.97; 95%CI: 1.06-8.29) and 1 year (RR = 1.49; 95%CI: 1.10-2.03) post-operation[43].
It has been postulated that H. pylori infection causes chronic gastritis, AG, usually with GIM and dysplasia, and GC, especially intestinal-type[44-46]. The stepwise course of this infection, which usually continues over decades, has been defined as a sequence of histological events that confer an increasing risk of malignant transformation, as described in Correa’s hypothesis[44]. A meta-analysis showed that H. pylori eradication seems to reduce GC[47]. However, a recent study from Japan showed that there is a risk of developing GC of both the intestinal (0.17% per year) and diffuse (0.13% per year) types even after the cure of H. pylori infection and extinction of gastric inflammation[48]. It has been also reported from Japan that H. pylori treatment reduces the risk of developing new GC in patients who have a history of GC and are thus at high risk for this development[49]. However, recent retrospective studies show that H. pylori eradication does not reduce the incidence of metachronous GC[50,51]. These results indicate that the establishment of predictable markers for the development of GC from patients cured of H. pylori infection is needed.
Up to now, a number of genetic and epigenetic alterations of tumor suppressor and tumor related genes involve the development or progression of precancerous lesions as well as GC have been reported.
GC is the second-leading cause of cancer death both worldwide and in Japan. GC is histologically divided into two types, intestinal and diffuse types[52]. One of the main risk factors for the development of both type of GC is considered to be H. pylori infection[53-57]. Therefore, in uninfected persons GC does not develop[58] besides cardia/junctional GC[59].
Observations of geographical differences in the prevalence of H. pylori and its related diseases, especially in Asia, have been intriguing. Although there is a strong link between H. pylori infection and GC in East Asia including Japan, the prevalence of H. pylori infection is high in some countries such as India and Bangladesh with low GC rates. There are several possible factors that affect this enigma[60-62]. First, the presence of virulent factors in the infecting H. pylori strain is a known determinant factor of the outcome of the infection. The CagA and VacA of H. pylori, have been associated to phenotypic characteristics of virulence. It has long been noted that patients infected with strains with an intact cag pathogenicity island have a more intense inflammatory response and this was also associated with an increased chance of developing GC or peptic ulcer disease[63]. Infection with Cag-positive VacA s1/m1 strains is associated with precancerous lesions and the development of GC, while persistent non-atrophic gastritis associated to Cag negative VacA s2/m2 does not increase the risk of cancer[64]. Most H. pylori clinical isolates in Japan and South Korea have been reported to possess both CagA and VacA genes with VacA genotype s1/m1, and this genotype is associated with progression of gastric preneoplastic lesions and cancer risk[65-68]. In contrast, VacA genotype in India and Taiwan, where the prevalence of H. pylori is high and that of GC is low, is different[69,70]. Taken together, previous reports show a potential role of H. pylori strain genotype diversity in various presentation of gastric disease in different regions and populations.
Besides genetic diversity of the infecting H. pylori strains, other factors that may influence the etiology of GC include differences in the host genetic background in various ethnic groups, i.e., gastric acid secretion and genetic polymorphisms in pro-inflammatory cytokines. These factors, in addition to environmental factors, such as personal hygiene and dietary habits, reflect the multifactorial etiology of GC[61].
AG is characterized by chronic inflammatory processes of gastric mucosa that leads to the loss of appropriate glands[71] and a reduction of gastric secretory function. The extensive spread AG, which is associated with the state of achlorhydria or hypochlorhydria, is known to be a significant risk of GC[72,73]. The relation between H. pylori and GC depends on the factors that determine the severity and rate of progression of AG. Several studies show, furthermore, that precancerous conditions including AG and GIM are indicators of an increased risk for GC as compared with chronic gastritis in the absence of these lesions[73-75]. A prospective study by Uemura et al[58] also has showed that RR for GC was 1.7 (95%CI: 0.8-3.7) in moderate AG and 4.9 (95%CI: 2.8-19.2) in severe AG compared to none or mild AG (control).
It is generally considered that AG has a relatively high prevalence rate in countries with a higher prevalence of H. pylori infection and GC[74]. However, despite a high rate of H. pylori infection, there are some regions with a low prevalence of precancerous lesions and GC. This phenomenon is reported as the geographical enigmas (African, Asian, Indian and Costa Rican enigmas)[60-62]. Moreover, GC and DU occupy opposite ends of the spectrum of H. pylori-related disease. Most DU are categorized as antral-predominant gastritis[58] or nonatrophic gastritis[76], which have a low risk for GC and different from gastric and gastro-duodenal ulcers in pathophysiology[58,77].
Up to now, Miki et al[78] have reported that progression of AG correlates strongly with stepwise reductions in serum pepsinogen (PG) I levels and the PG I/II ratio. In other words, measuring serum PG I and the PG I/II ratio offers the opportunity to evaluate the progression of AG, a precursor of GC[79]. As criteria for the serum PG test used for GC screening, the combination of PG I ≤ 70 ng/mL and PG I/II ≤ 3.0 is widely accepted as a reference value[79,80]. According to a recent meta-analysis of PG test, pooled sensitivity and specificity for GC detection were 77.3% (95%CI: 69.8-83.8) and 73.2% (95%CI: 72.8-73.6), respectively[81]. Thereafter, a combination of the serum PG test and H. pylori infection diagnosis was conducted to overcome the low sensitivity for GC detection[82,83]. The stage of H. pylori-related chronic gastritis was classified into 4 stages based on the combination of both test results: Group A [H. pylori (-), PG (-)]; Group B [H. pylori (+), PG (-)]; Group C [H. pylori (+), PG (+)]; and Group D [H. pylori (-), PG (+)]. As a result, annual incidences of GC development were: Group A, 0%; Group B, 0.11%; Group C, 0.24%; and Group D, 1.31%. Thus, with H. pylori infection and AG progression, the rate increased in a stepwise and significant manner.
In histological diagnosis of AG, the Sydney System and its Houston updated version provide a uniform nomenclature for gastritis, as well as visual analogue scales[71] The system, however, lacks the element of prognosis and the same pathologists who use it in their research activities found it too cumbersome for routine diagnostic activities[84]. In 2005, Operative Link for Gastritis Assessment (OLGA) staging system was proposed by an international group of gastroenterologists and pathologists (Table 1)[84,85]. As the risk of GC directly relates to the extent of AG, an atrophy based staging system will provide implications for the prognosis and, possibly, the management of patients. This OLGA staging system may offer clinicians an immediate overall perception of the extent of gastric disease and also provides information regarding cancer risk, especially intestinal-type. In this system, stages III and IV are significantly associated with GC development[86-88], thus being consistent with the biological assumption that the extent and location of atrophy correlate with the risk of cancer[58,89,90]. It has been reported, furthermore, that a significant association emerged between mean PG I/II values and OLGA stage (the lower the ratio, the higher the stage; by ordinal logistic regression: OR = 0.82; 95%CI: 0.72-0.94; P < 0.006), and the mean PG I/II ratio declined significantly as the OLGA stage increased (test for trend; P < 0.001)[88].
| Atrophy score | Corpus | ||||
| No atrophy | Mild atrophy | Moderate atrophy | Severe atrophy | ||
| (score 0) | (score 1) | (score 2) | (score 3) | ||
| Antrum | No atrophy (score 0) (including incisura angularis) | Stage 0 | Stage I | Stage II | Stage II |
| Mild atrophy (score 1) (including incisura angularis) | Stage I | Stage I | Stage II | Stage III | |
| Moderate atrophy (score 2) (including incisura angularis) | Stage II | Stage II | Stage III | Stage IV | |
| Severe atrophy (score 3) (including incisura angularis) | Stage III | Stage III | Stage IV | Stage IV | |
GIM is defined as the replacement of the gastric epithelium by two types of intestinal-type epithelium, which can be seen by Haematoxylin-eosin staining: (1) absorptive enterocytes with brush border along with goblet cells; and (2) columnar cells with foamy cytoplasm but lacking brush border[91]. Furthermore, it is divided into three phenotypes by alcian blue and high-iron diamine (AB/HID) staining as described by Filipe and Jass[92], namely type I (complete or small intestinal type) and types II and III (incomplete or colonic type). When more than one type of GIM coexisted in a given sample, the case was classified according to the dominant type present in the section[92]. GIM is generally considered to be a condition that predisposes to malignancy, and also the presence of incomplete-type GIM (type III) and a higher proportion of this type indicate a higher cancer risk, especially intestinal-type GC[91-94]. Shiotani et al[95] reported that incomplete GIM in the corpus lesser curve (OR = 6.4; 95%CI: 2.0-21, P = 0.002) is associated with an increased risk factor for GC. In contrast, there are opposite studies that detection of incomplete-type GIM (type III) as detected by AB/HID staining is of limited value as an indicator of risk of GC, and AB/HID subtyping does not provide useful information to the clinician[96-98]. Therefore, the pattern, extent, and severity of atrophy with/without GIM may be the most important predictor of increased cancer risk than GIM subtype.
Some investigators consider incomplete-type GIM to be a mild form of dysplasia[99]. However, there are some other reports which intestinal-type GC does not necessarily arise from GIM, thus it remains controversial whether GIM is actually a precancerous lesion or not[100-102]. Indeed, previous studies have showed that GIM was not always noted in the surrounding mucosa of minute intestinal-type GC[102], indicating that GIM may be a paracancerous lesion, but not a precancerous lesion.
GC develops through the accumulation of genetic and epigenetic alterations, but the mechanisms of induction have remained unknown. Similarly, molecular alterations are identified even in precancerous lesions including AG and GIM[103]. Preferential expression of COX-2 in colonic-phenotype (type III) intestinal metaplasia associated with H. pylori and GC has been reported by Sun et al[104]. This has been further confirmed by using a colon epithelial specific antibody Das-1 as described below[105].
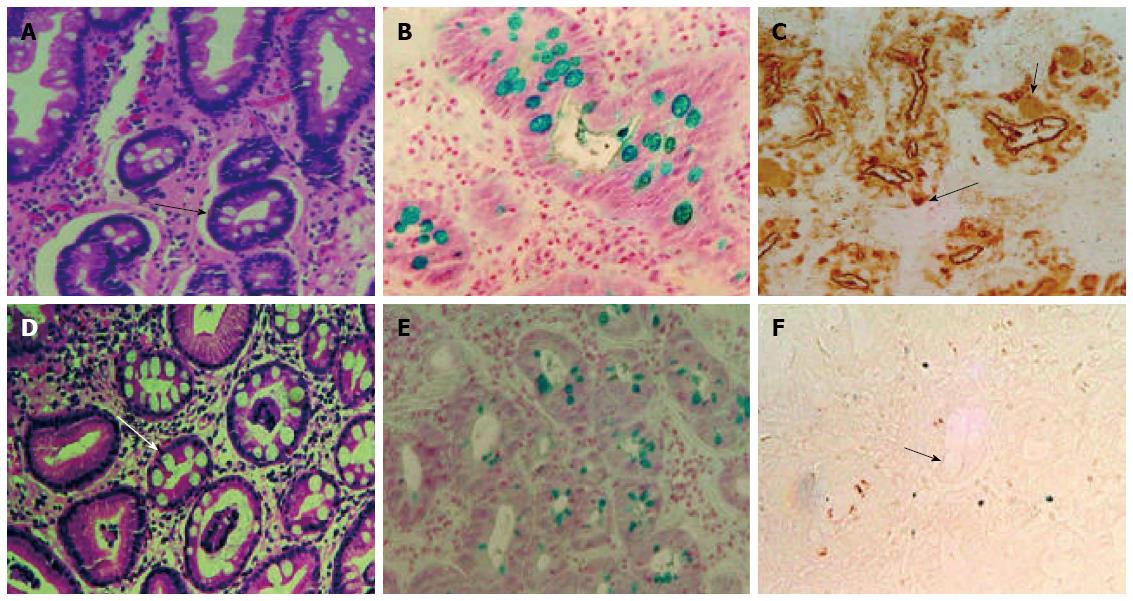
We have developed a novel monoclonal antibody (mAb), Das-1 (formerly known as 7E12H12, IgM isotype), which specifically reacts with the colonic epithelium[105], and have reported that GIM of a colonic phenotype, detected by mAb Das-1, is strongly associated with GC (Figure 1)[106,107]. Non-cancerous samples from 93% of patients having GIM as well as GC were found to react with mAb Das-1, whereas GIM samples from patients without GC reacted less frequently (35%) with the mAb (P < 0.0001)[106]. Subsequently, in a prospective study using a large cohort of patients infected with H. pylori and with and without chronic gastritis who were followed up to 4 years showed a change in the phenotype of metaplasia which may be an important factor in the induction of GC. These results suggested that mAb Das-1 positivity in GIM could be a risk marker related to gastric carcinogenesis[106,107]. It has been reported that microsatellite instability (MSI) are frequently detected in GIM[108,109] and chronic gastritis mucosa[110], but there is little evidence of mismatch repair defects in these tissues[111]. We have also found that genomic instability, including MSI and loss of heterozygosity (LOH) in GIM may be associated with gastric carcinogenesis[57,58,112], and MSI or mAb Das-1 reactivity in GIM. This strongly predicts that the development of metachronous GC after endoscopic treatment to early stage GC is irrespective of the eradication of H. pylori[113].
Intestine-specific homeobox genes, caudal-type homeobox (Cdx1) and Cdx2, are transcription factors that regulate both proliferation and differentiation in intestinal epithelial cells[114,115]. CDX2 expression in the gastric mucosa is found in patients with chronic gastritis and is closely associated with GIM[116]. As to the important role of Cdx2 in transdifferentiation of the gastric epithelial cells into GIM, Mutoh et al[117] reported that Cdx2-expressing transgenic mice induced GIM with an increase of epithelial cell proliferation. Also, they showed that invasive GC developed from GIM in Cdx2-transgenic mice[118], thus suggesting GIM itself may play a significant role in the genesis and progression of GC.
There have been only a few reports of this oncogene in H. pylori-associated chronic gastritis and GIM either with or without GC[119,120]. There is an interesting report that individuals with baseline K-ras mutations were more likely to progress from either atrophy to metaplasia or from complete-type GIM (type I) to incomplete-type GIM (type III); and those individuals with G→A transitions (Gly→Ser) were more likely to progress from atrophy to GIM than those individuals who lacked this mutation[119]. Similarly, mutations with AGT (Ser) were considered more likely to be advantageous in K-ras gene alterations and are important in gastric tumorigenesis in our study[121].
Epigenetic abnormalities are also important as cancer gene abnormalities in addition to gene structural abnormalities such as mutations and chromosomal deletions. In GC, inactivation of various genes because of methylation is more frequently observed compared to inactivation due to mutations or chromosomal deletions[122]. Even in non-cancerous mucosa, aberrant methylation can be present. These findings suggest that aberrant methylation is deeply involved in gastric carcinogenesis, and aberrant methylation seems to be useful as a new target for diagnostics and prevention of GC[122]. Epigenetic methylation-associated inactivation of the hMLH1 mismatch repair gene is a potent trigger of MSI, especially high-frequency MSI (MSI-H)[123]. DNA methylation of hMLH1 promoter region CpG island is tightly associated with the loss of hMLH1 expression in GC exhibiting MSI[124]. In contrast, there are a few reported that mean methylation levels for the tumor suppressor genes CDKN2A (p16) and hMLH1 were very low, thus evaluating the correlation with GC risk was difficult[125,126]. Genes methylated by H. pylori infection show specificity. With H. pylori infection, resistant genes show no methylation at all while susceptible genes display a high frequency of methylation[127]. The hypermethylation of E-cadherin gene is accelerated by H. pylori infection[128].
Some studies reported that the precancerous lesions including AG and GIM had improved after eradication, but other studies did not find any change[107]. Therefore, little consensus has been obtained as to the improvement of AG or GIM after eradication. Some of the reasons for these discrepancies may be ethnic variations, completeness of eradication, stage of the disease when treatment was initiated, and the short follow-up period (the follow-up period did not exceed 1 year). When evaluating the studies followed-up more than 5 years following H. pylori eradication, AG and GIM tended to improve histologically especially in the corpus (Table 2)[129-134].
| Ref. | Authors | Year | Country | Number of patients | Study design | Observation period (yr) | Gastric atrophy | Intestinal metaplasia | ||
| Antrum | Corpus | Antrum | Corpus | |||||||
| 129 | Forbes | 1996 | Australia | 54 | Prospective | 7.1 | No | No | ||
| 130 | Ito | 2002 | Japan | 22 | Prospective | 5.0 | Yes | Yes | Yes | Yes |
| 131 | Zhou | 2003 | China | 552 | RCT | 5.0 | No | No | Yes | No |
| 132 | Leung | 2003 | Hong Kong | 435 | RCT | 5.0 | ND | Yes | ||
| 133 | Vannella | 2010 | Italy | 300 | Observational | 5.2 | No | Yes | No | Yes |
| 134 | Kodama | 2012 | Japan | 323 | Prospective | 10.0 | Yes | Yes | No | Yes |
Up to now, there are two meta-analyses regarding the long-term effects of H. pylori eradication on gastric histology[135,136]. According to a meta-analysis by Rokkas et al[135], for histological changes of AG, the pooled OR with 95%CI was 0.554 (0.372-0.825) (P = 0.004) in antrum and corpus 0.209 (0.081-0.538, P < 0.001) respectively. However, no histological improvement of GIM was seen; the pooled OR = 0.795, 95%CI: 0.587-1.078 (P = 0.14) in the antrum and the pooled OR = 0.891, 95%CI: 0.663-1.253 (P = 0.506) in the corpus. Their results showed significant improvement of AG, whereas improvement was not shown for GIM. In contrast, another meta-analysis by Wang et al[136] showed that comparing the histological alterations before and after H. pylori eradication, the pooled weighted mean difference (WMD) with 95%CI was 0.12 (0.00-0.23) (P = 0.06) for antral AG and 0.32 (0.09-0.54) (P = 0.006) for corpus AG; whereas the pooled WMD was 0.02 (-0.12-0.16) (P = 0.76) for antral GIM and -0.02 (-0.05-0.02) (P = 0.42) for corpus GIM, respectively. Their result has revealed that H. pylori eradication significantly improved AG in the corpus but not in the antrum; it also did not improve GIM[136]. As to difference of the results between these 2 meta-analysis, Wang et al[136] discussed that the study by Rokkas et al[135] used different selection criteria, extracted different data from each article, and did not include a recent trial with negative results.
We previously reported that although H. pylori eradication does not reduce the histologic GIM score, but it changes the cellular phenotype of GIM, as identified by mAb Das-1 and TC22-4 which related to carcinogenesis of colon epithelium→colon cancer[137]; therefore this change of phenotype may be an important factor in the reduction of cancer incidence after eradication of H. pylori[107]. It has bee also reported that H. pylori eradication reduced MSI in GIM[57,113].
Chan et al[138] and Leung et al[139] showed that H. pylori eradication therapy could reverse methylation of E-cadherin gene in patients with chronic gastritis. In addition, decreased methylation levels of other genes after H. pylori eradication have been confirmed in specific genes[140]. Once methylation has occurred in a cell, it is difficult to conceive that demethylation would again occur in the same region. The decrease in methylation levels observed after H. pylori eradication is thus probably due to cell turnover (temporary methylation). Residual aberrant methylation even after eradication is thought to reflect methylation in gastric gland stem cells (permanent methylation)[141]. Therefore, individuals with residual methylation after H. pylori eradication have a risk of GC.
According to a systematic review in total of 15 papers by Ito et al[142], the H. pylori eradication statistically reduces the prevalence of clinical GC by approximately one-third. Interestingly, the studies from Japan support this conclusion; however, studies from other countries have reported conflicting results. GC that developed after eradication revealed a mainly intestinal-type histology and depressed-type appearance. They mentioned, furthermore, that the following are possible reasons for reduction of GC: (1) eradication therapy inhibits the new occurrence of GC; (2) eradication regresses or inhibits the growth of GC; and (3) eradication interferes with the discovery of GC[142]. A recent meta-analysis of randomized, controlled trials (n = 7), mostly in Asia (n = 6), also show that H. pylori treatment seems to reduce GC risk (RR = 0.65, 95%CI: 0.43-0.98)[47]. Wong et al[143] found that although no overall reduction was observed in participants who received H. pylori treatment compared with those who did not, in the subgroup of H. pylori carriers without precancerous lesions, i.e., AG or GIM, eradication of H. pylori significantly decreased the development of GC. Thus, they emphasized the concept of “point of no return”[144], in which the benefit of H. pylori eradication diminish after GIM stage was reached (in which many molecular changes had been detected).
In 1997, Uemura et al[145] reported that eradication of H. pylori after endoscopic resection of early GC reduced the development of metachronous GC by a non-randomised study. Thereafter, Japan Gast Study Group concluded by a multi-center, open-label, randomized controlled trial that prophylactic eradication of H. pylori after endoscopic resection of early GC should be used to prevent the development of metachronous GC[49]. However, two retrospective studies from Japan showed that H. pylori eradication does not reduce the incidence of metachronous GC[50,51]. As mentioned by these studies, it should be noted that follow-up time longer than 5 years might be determined to be one of the independent risk factors for metachronous GC.
Recent study by de Vries et al[146] showed the incidence of pre-malignant gastric lesions such as AG and GIM is declining and a further decrease of at least 24% in the incidence of GC in the coming decade may be anticipated in Western countries without specific intervention. The precancerous lesions with molecular alterations may represent the “point of no return” when the development of GC can no longer be prevented by H. pylori eradication. Thus, earlier eradication of H. pylori is considered to be more effective in preventing GC by inhibiting the progression of AG or GIM[147]. As current surveillance of patients with precancerous lesions is inconsistent with their cancer risk, development of guidelines may be indicated[148]. A recent systematic review also indicates that H. pylori serology or endoscopic population screening is cost-effective, while endoscopic surveillance of precancerous lesions presents conflicting results, therefore better implementation of published guidelines with the addition of molecular markers may provide more efficient and cost effective outcomes[149].
P- Reviewers: Kanda T, Ricci V, Smith SM S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Cave DR. Transmission and epidemiology of Helicobacter pylori. Am J Med. 1996;100:12S-17S; discussion 17S-18S. [PubMed] |
| 2. | Siao D, Somsouk M. Helicobacter pylori: evidence-based review with a focus on immigrant populations. J Gen Intern Med. 2014;29:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Hosseini E, Poursina F, de Wiele TV, Safaei HG, Adibi P. Helicobacter pylori in Iran: A systematic review on the association of genotypes and gastroduodenal diseases. J Res Med Sci. 2012;17:280-292. [PubMed] |
| 4. | Schwarz S, Morelli G, Kusecek B, Manica A, Balloux F, Owen RJ, Graham DY, van der Merwe S, Achtman M, Suerbaum S. Horizontal versus familial transmission of Helicobacter pylori. PLoS Pathog. 2008;4:e1000180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Magalhães Queiroz DM, Luzza F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2006;11 Suppl 1:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Thomas JE, Gibson GR, Darboe MK, Dale A, Weaver LT. Isolation of Helicobacter pylori from human faeces. Lancet. 1992;340:1194-1195. [PubMed] |
| 7. | Axon AT. Review article: is Helicobacter pylori transmitted by the gastro-oral route? Aliment Pharmacol Ther. 1995;9:585-588. [PubMed] |
| 8. | Calvet X, Ramírez Lázaro MJ, Lehours P, Mégraud F. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter. 2013;18 Suppl 1:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9 Suppl 2:33-39. [PubMed] |
| 10. | Hu LT, Foxall PA, Russell R, Mobley HL. Purification of recombinant Helicobacter pylori urease apoenzyme encoded by ureA and ureB. Infect Immun. 1992;60:2657-2666. [PubMed] |
| 11. | Logan RP. Adherence of Helicobacter pylori. Aliment Pharmacol Ther. 1996;10 Suppl 1:3-15. [PubMed] |
| 12. | Liu H, Semino-Mora C, Dubois A. Mechanism of H. pylori intracellular entry: an in vitro study. Front Cell Infect Microbiol. 2012;2:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 830] [Article Influence: 46.1] [Reference Citation Analysis (3)] |
| 14. | DuBois S, Kearney DJ. Iron-deficiency anemia and Helicobacter pylori infection: a review of the evidence. Am J Gastroenterol. 2005;100:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Asahi A, Nishimoto T, Okazaki Y, Suzuki H, Masaoka T, Kawakami Y, Ikeda Y, Kuwana M. Helicobacter pylori eradication shifts monocyte Fcgamma receptor balance toward inhibitory FcgammaRIIB in immune thrombocytopenic purpura patients. J Clin Invest. 2008;118:2939-2949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Rostami N, Keshtkar-Jahromi M, Rahnavardi M, Keshtkar-Jahromi M, Esfahani FS. Effect of eradication of Helicobacter pylori on platelet recovery in patients with chronic idiopathic thrombocytopenic purpura: a controlled trial. Am J Hematol. 2008;83:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Malfertheiner P, Sipponen P, Naumann M, Moayyedi P, Mégraud F, Xiao SD, Sugano K, Nyrén O. Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol. 2005;100:2100-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Voiosu TA, Giurcan R, Voiosu AM, Voiosu MR. Functional dyspepsia today. Maedica (Buchar). 2013;8:68-74. [PubMed] |
| 19. | Zhao B, Zhao J, Cheng WF, Shi WJ, Liu W, Pan XL, Zhang GX. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta-analysis of randomized controlled studies with 12-month follow-up. J Clin Gastroenterol. 2014;48:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 21. | Fallone CA, Barkun AN, Friedman G, Mayrand S, Loo V, Beech R, Best L, Joseph L. Is Helicobacter pylori eradication associated with gastroesophageal reflux disease? Am J Gastroenterol. 2000;95:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | den Hollander WJ, Sostres C, Kuipers EJ, Lanas A. Helicobacter pylori and nonmalignant diseases. Helicobacter. 2013;18 Suppl 1:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Graham DY, Alpert LC, Smith JL, Yoshimura HH. Iatrogenic Campylobacter pylori infection is a cause of epidemic achlorhydria. Am J Gastroenterol. 1988;83:974-980. [PubMed] |
| 24. | Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch’s postulates for pyloric Campylobacter. Med J Aust. 1985;142:436-439. [PubMed] |
| 25. | Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987;82:192-199. [PubMed] |
| 26. | Fox JG, Correa P, Taylor NS, Zavala D, Fontham E, Janney F, Rodriguez E, Hunter F, Diavolitsis S. Campylobacter pylori-associated gastritis and immune response in a population at increased risk of gastric carcinoma. Am J Gastroenterol. 1989;84:775-781. [PubMed] |
| 27. | Rosh JR, Kurfist LA, Benkov KJ, Toor AH, Bottone EJ, LeLeiko NS. Helicobacter pylori and gastric lymphonodular hyperplasia in children. Am J Gastroenterol. 1992;87:135-139. [PubMed] |
| 28. | Genta RM, Hamner HW. The significance of lymphoid follicles in the interpretation of gastric biopsy specimens. Arch Pathol Lab Med. 1994;118:740-743. [PubMed] |
| 29. | Sipponen P, Ranta P, Helske T, Kääriäinen I, Mäki T, Linnala A, Suovaniemi O, Alanko A, Härkönen M. Serum levels of amidated gastrin-17 and pepsinogen I in atrophic gastritis: an observational case-control study. Scand J Gastroenterol. 2002;37:785-791. [PubMed] |
| 30. | Väänänen H, Vauhkonen M, Helske T, Kääriäinen I, Rasmussen M, Tunturi-Hihnala H, Koskenpato J, Sotka M, Turunen M, Sandström R. Non-endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gastrin-17 and pepsinogen I: a multicentre study. Eur J Gastroenterol Hepatol. 2003;15:885-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Li Z, Zou D, Ma X, Chen J, Shi X, Gong Y, Man X, Gao L, Zhao Y, Wang R. Epidemiology of peptic ulcer disease: endoscopic results of the systematic investigation of gastrointestinal disease in China. Am J Gastroenterol. 2010;105:2570-2577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Ciociola AA, McSorley DJ, Turner K, Sykes D, Palmer JB. Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimated. Am J Gastroenterol. 1999;94:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Jyotheeswaran S, Shah AN, Jin HO, Potter GD, Ona FV, Chey WY. Prevalence of Helicobacter pylori in peptic ulcer patients in greater Rochester, NY: is empirical triple therapy justified? Am J Gastroenterol. 1998;93:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Chiorean MV, Locke GR, Zinsmeister AR, Schleck CD, Melton LJ. Changing rates of Helicobacter pylori testing and treatment in patients with peptic ulcer disease. Am J Gastroenterol. 2002;97:3015-3022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Nguyen TL, Uchida T, Tsukamoto Y, Trinh DT, Ta L, Mai BH, Le SH, Thai KD, Ho DD, Hoang HH. Helicobacter pylori infection and gastroduodenal diseases in Vietnam: a cross-sectional, hospital-based study. BMC Gastroenterol. 2010;10:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Hussein NR. The association of dupA and Helicobacter pylori-related gastroduodenal diseases. Eur J Clin Microbiol Infect Dis. 2010;29:817-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Zhang Z, Zheng Q, Chen X, Xiao S, Liu W, Lu H. The Helicobacter pylori duodenal ulcer promoting gene, dupA in China. BMC Gastroenterol. 2008;8:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev. 2006;CD003840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol. 2004;99:1833-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Taniyama K, Shimbo T, Iwase H, Tanaka S, Watanabe N, Uemura N. Evidence-based therapy according to the guideline for gastric ulcers is cost-effective in Japan. J Physiol Pharmacol. 2011;62:627-635. [PubMed] |
| 41. | Hsiao FY, Tsai YW, Wen YW, Kuo KN, Tsai CR, Huang WF. Effect of Helicobacter pylori eradication therapy on risk of hospitalization for a major ulcer event. Pharmacotherapy. 2011;31:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Gisbert JP, Calvet X, Cosme A, Almela P, Feu F, Bory F, Santolaria S, Aznárez R, Castro M, Fernández N. Long-term follow-up of 1,000 patients cured of Helicobacter pylori infection following an episode of peptic ulcer bleeding. Am J Gastroenterol. 2012;107:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Wong CS, Chia CF, Lee HC, Wei PL, Ma HP, Tsai SH, Wu CH, Tam KW. Eradication of Helicobacter pylori for prevention of ulcer recurrence after simple closure of perforated peptic ulcer: a meta-analysis of randomized controlled trials. J Surg Res. 2013;182:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37-S43. [PubMed] |
| 45. | Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169-1179. [PubMed] |
| 46. | Danesh J. Helicobacter pylori infection and gastric cancer: systematic review of the epidemiological studies. Aliment Pharmacol Ther. 1999;13:851-856. [PubMed] |
| 47. | Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, Grilli D, Bazzoli F. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151:121-128. [PubMed] |
| 48. | Take S, Mizuno M, Ishiki K, Yoshida T, Ohara N, Yokota K, Oguma K, Okada H, Yamamoto K. The long-term risk of gastric cancer after the successful eradication of Helicobacter pylori. J Gastroenterol. 2011;46:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 936] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 50. | Maehata Y, Nakamura S, Fujisawa K, Esaki M, Moriyama T, Asano K, Fuyuno Y, Yamaguchi K, Egashira I, Kim H. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012;75:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 51. | Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013;62:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 52. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 53. | Correa P, Fox J, Fontham E, Ruiz B, Lin YP, Zavala D, Taylor N, Mackinley D, de Lima E, Portilla H. Helicobacter pylori and gastric carcinoma. Serum antibody prevalence in populations with contrasting cancer risks. Cancer. 1990;66:2569-2574. [PubMed] |
| 54. | Sipponen P, Hyvärinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol Suppl. 1993;196:3-6. [PubMed] |
| 55. | Kikuchi S, Wada O, Nakajima T, Nishi T, Kobayashi O, Konishi T, Inaba Y. Serum anti-Helicobacter pylori antibody and gastric carcinoma among young adults. Research Group on Prevention of Gastric Carcinoma among Young Adults. Cancer. 1995;75:2789-2793. [PubMed] |
| 56. | Kokkola A, Valle J, Haapiainen R, Sipponen P, Kivilaakso E, Puolakkainen P. Helicobacter pylori infection in young patients with gastric carcinoma. Scand J Gastroenterol. 1996;31:643-647. [PubMed] |
| 57. | Tanaka A, Watari J, Tanabe H, Maemoto A, Fujiya M, Ashida T, KM D, Kohgo Y. Effect of eradication of Helicobacter pylori on genetic instabilities in gastric intestinal metaplasia. Aliment Pharmacol Ther. 2006;24 Suppl 4:194-202. |
| 58. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3187] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 59. | Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, Abnet CC, Albanes D, Virtamo J, Taylor PR. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 233] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 60. | Graham DY, Lu H, Yamaoka Y. African, Asian or Indian enigma, the East Asian Helicobacter pylori: facts or medical myths. J Dig Dis. 2009;10:77-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | Miwa H, Go MF, Sato N. H. pylori and gastric cancer: the Asian enigma. Am J Gastroenterol. 2002;97:1106-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 62. | Goh KL. Epidemiology of Helicobacter pylori infection in Malaysia--observations in a multiracial Asian population. Med J Malaysia. 2009;64:187-192. [PubMed] |
| 63. | Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636-1644. [PubMed] |
| 64. | Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 525] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 65. | Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710-1714. [PubMed] |
| 66. | Maeda S, Ogura K, Yoshida H, Kanai F, Ikenoue T, Kato N, Shiratori Y, Omata M. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut. 1998;42:338-343. [PubMed] |
| 67. | Yamaoka Y, Souchek J, Odenbreit S, Haas R, Arnqvist A, Borén T, Kodama T, Osato MS, Gutierrez O, Kim JG. Discrimination between cases of duodenal ulcer and gastritis on the basis of putative virulence factors of Helicobacter pylori. J Clin Microbiol. 2002;40:2244-2246. [PubMed] |
| 68. | González CA, Figueiredo C, Lic CB, Ferreira RM, Pardo ML, Ruiz Liso JM, Alonso P, Sala N, Capella G, Sanz-Anquela JM. Helicobacter pylori cagA and vacA genotypes as predictors of progression of gastric preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Am J Gastroenterol. 2011;106:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 69. | Wang HJ, Kuo CH, Yeh AA, Chang PC, Wang WC. Vacuolating toxin production in clinical isolates of Helicobacter pylori with different vacA genotypes. J Infect Dis. 1998;178:207-212. [PubMed] |
| 70. | Mukhopadhyay AK, Kersulyte D, Jeong JY, Datta S, Ito Y, Chowdhury A, Chowdhury S, Santra A, Bhattacharya SK, Azuma T. Distinctiveness of genotypes of Helicobacter pylori in Calcutta, India. J Bacteriol. 2000;182:3219-3227. [PubMed] |
| 71. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 72. | Lechago J, Correa P. Prolonged achlorhydria and gastric neoplasia: is there a causal relationship? Gastroenterology. 1993;104:1554-1557. [PubMed] |
| 73. | de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007;12:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 74. | Kim N, Park YS, Cho SI, Lee HS, Choe G, Kim IW, Won YD, Park JH, Kim JS, Jung HC. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia in a Korean population without significant gastroduodenal disease. Helicobacter. 2008;13:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 75. | Sipponen P, Kekki M, Haapakoski J, Ihamäki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173-177. [PubMed] |
| 76. | Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 77. | Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, Oguma K, Okada H, Shiratori Y. The effect of eradicating helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol. 2005;100:1037-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 78. | Miki K, Ichinose M, Shimizu A, Huang SC, Oka H, Furihata C, Matsushima T, Takahashi K. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn. 1987;22:133-141. [PubMed] |
| 79. | Ichinose M, Yahagi N, Oka M, Ikeda H, Miki K, Omata M. Screening for gastric cancer in Japan. Cancer screening for common malignancies. Totowa, New Jersey: Humana Press 2001; 87-102. |
| 80. | Watanabe Y, Kurata JH, Mizuno S, Mukai M, Inokuchi H, Miki K, Ozasa K, Kawai K. Helicobacter pylori infection and gastric cancer. A nested case-control study in a rural area of Japan. Dig Dis Sci. 1997;42:1383-1387. [PubMed] |
| 81. | Dinis-Ribeiro M, Yamaki G, Miki K, Costa-Pereira A, Matsukawa M, Kurihara M. Meta-analysis on the validity of pepsinogen test for gastric carcinoma, dysplasia or chronic atrophic gastritis screening. J Med Screen. 2004;11:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 82. | Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 373] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 83. | Yanaoka K, Oka M, Yoshimura N, Mukoubayashi C, Enomoto S, Iguchi M, Magari H, Utsunomiya H, Tamai H, Arii K. Risk of gastric cancer in asymptomatic, middle-aged Japanese subjects based on serum pepsinogen and Helicobacter pylori antibody levels. Int J Cancer. 2008;123:917-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Rugge M, Genta RM. Staging gastritis: an international proposal. Gastroenterology. 2005;129:1807-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005;36:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 86. | Rugge M, Meggio A, Pennelli G, Piscioli F, Giacomelli L, De Pretis G, Graham DY. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 87. | Satoh K, Osawa H, Yoshizawa M, Nakano H, Hirasawa T, Kihira K, Sugano K. Assessment of atrophic gastritis using the OLGA system. Helicobacter. 2008;13:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Rugge M, de Boni M, Pennelli G, de Bona M, Giacomelli L, Fassan M, Basso D, Plebani M, Graham DY. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinico-pathological follow-up study. Aliment Pharmacol Ther. 2010;31:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 89. | Sipponen P, Stolte M. Clinical impact of routine biopsies of the gastric antrum and body. Endoscopy. 1997;29:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Garcia SB, Park HS, Novelli M, Wright NA. Field cancerization, clonality, and epithelial stem cells: the spread of mutated clones in epithelial sheets. J Pathol. 1999;187:61-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 91. | Filipe MI, Muñoz N, Matko I, Kato I, Pompe-Kirn V, Jutersek A, Teuchmann S, Benz M, Prijon T. Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer. 1994;57:324-329. [PubMed] |
| 92. | Filipe MI, Jass J. Intestinal metaplasia subtypes and cancer risk. Gastric carcinoma. Edinburgh: Churchill Livingston 1986; 212-236. |
| 93. | González CA, Pardo ML, Liso JM, Alonso P, Bonet C, Garcia RM, Sala N, Capella G, Sanz-Anquela JM. Gastric cancer occurrence in preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Int J Cancer. 2010;127:2654-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 94. | Rokkas T, Filipe MI, Sladen GE. Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut. 1991;32:1110-1113. [PubMed] |
| 95. | Shiotani A, Haruma K, Uedo N, Iishi H, Ishihara R, Tatsuta M, Kumamoto M, Nakae Y, Ishiguro S, Graham DY. Histological risk markers for non-cardia early gastric cancer. Pattern of mucin expression and gastric cancer. Virchows Arch. 2006;449:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 96. | Ectors N, Dixon MF. The prognostic value of sulphomucin positive intestinal metaplasia in the development of gastric cancer. Histopathology. 1986;10:1271-1277. [PubMed] |
| 97. | Ramesar KC, Sanders DS, Hopwood D. Limited value of type III intestinal metaplasia in predicting risk of gastric carcinoma. J Clin Pathol. 1987;40:1287-1290. [PubMed] |
| 98. | El-Zimaity HM, Ramchatesingh J, Saeed MA, Graham DY. Gastric intestinal metaplasia: subtypes and natural history. J Clin Pathol. 2001;54:679-683. [PubMed] |
| 99. | Tosi P, Filipe MI, Luzi P, Miracco C, Santopietro R, Lio R, Sforza V, Barbini P. Gastric intestinal metaplasia type III cases are classified as low-grade dysplasia on the basis of morphometry. J Pathol. 1993;169:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Matsukuma A, Mori M, Enjoji M. Sulphomucin-secreting intestinal metaplasia in the human gastric mucosa. An association with intestinal-type gastric carcinoma. Cancer. 1990;66:689-694. [PubMed] |
| 101. | Antonioli DA. Precursors of gastric carcinoma: a critical review with a brief description of early (curable) gastric cancer. Hum Pathol. 1994;25:994-1005. [PubMed] |
| 102. | Sasaki I, Yao T, Nawata H, Tsuneyoshi M. Minute gastric carcinoma of differentiated type with special reference to the significance of intestinal metaplasia, proliferative zone, and p53 protein during tumor development. Cancer. 1999;85:1719-1729. [PubMed] |
| 103. | Leung WK, Sung JJ. Review article: intestinal metaplasia and gastric carcinogenesis. Aliment Pharmacol Ther. 2002;16:1209-1216. [PubMed] |
| 104. | Sun JH, Das KK, Amenta PS, Yokota K, Watari J, Sato T, Kohgo Y, Das KM. Preferential expression of cyclooxygenase-2 in colonic-phenotype of gastric intestinal metaplasia: association with helicobacter pylori and gastric carcinoma. J Clin Gastroenterol. 2006;40:122-128. [PubMed] |
| 105. | Das KM, Sakamaki S, Vecchi M, Diamond B. The production and characterization of monoclonal antibodies to a human colonic antigen associated with ulcerative colitis: cellular localization of the antigen by using the monoclonal antibody. J Immunol. 1987;139:77-84. [PubMed] |
| 106. | Mirza ZK, Das KK, Slate J, Mapitigama RN, Amenta PS, Griffel LH, Ramsundar L, Watari J, Yokota K, Tanabe H. Gastric intestinal metaplasia as detected by a monoclonal antibody is highly associated with gastric adenocarcinoma. Gut. 2003;52:807-812. [PubMed] |
| 107. | Watari J, Das KK, Amenta PS, Tanabe H, Tanaka A, Geng X, Lin JJ, Kohgo Y, Das KM. Effect of eradication of Helicobacter pylori on the histology and cellular phenotype of gastric intestinal metaplasia. Clin Gastroenterol Hepatol. 2008;6:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 108. | Leung WK, Kim JJ, Kim JG, Graham DY, Sepulveda AR. Microsatellite instability in gastric intestinal metaplasia in patients with and without gastric cancer. Am J Pathol. 2000;156:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 109. | Liu P, Zhang XY, Shao Y, Zhang DF. Microsatellite instability in gastric cancer and pre-cancerous lesions. World J Gastroenterol. 2005;11:4904-4907. [PubMed] |
| 110. | Kashiwagi K, Watanabe M, Ezaki T, Kanai T, Ishii H, Mukai M, Hibi T. Clinical usefulness of microsatellite instability for the prediction of gastric adenoma or adenocarcinoma in patients with chronic gastritis. Br J Cancer. 2000;82:1814-1818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 111. | Jin Z, Tamura G, Satoh M, Meguro T, Miura T, Hayashi M, Osakabe M, Ohmura K, Ogata S, Endoh Y. Absence of BAT-26 instability in gastric intestinal metaplasia. Pathol Int. 2001;51:473-475. [PubMed] |
| 112. | Zaky AH, Watari J, Tanabe H, Sato R, Moriichi K, Tanaka A, Maemoto A, Fujiya M, Ashida T, Kohgo Y. Clinicopathologic implications of genetic instability in intestinal-type gastric cancer and intestinal metaplasia as a precancerous lesion: proof of field cancerization in the stomach. Am J Clin Pathol. 2008;129:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 113. | Watari J, Moriichi K, Tanabe H, Kashima S, Nomura Y, Fujiya M, Tomita T, Oshima T, Fukui H, Miwa H. Biomarkers predicting development of metachronous gastric cancer after endoscopic resection: an analysis of molecular pathology of Helicobacter pylori eradication. Int J Cancer. 2012;130:2349-2358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 114. | Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16:619-625. [PubMed] |
| 115. | Soubeyran P, André F, Lissitzky JC, Mallo GV, Moucadel V, Roccabianca M, Rechreche H, Marvaldi J, Dikic I, Dagorn JC. Cdx1 promotes differentiation in a rat intestinal epithelial cell line. Gastroenterology. 1999;117:1326-1338. [PubMed] |
| 116. | Satoh K, Mutoh H, Eda A, Yanaka I, Osawa H, Honda S, Kawata H, Kihira K, Sugano K. Aberrant expression of CDX2 in the gastric mucosa with and without intestinal metaplasia: effect of eradication of Helicobacter pylori. Helicobacter. 2002;7:192-198. [PubMed] |
| 117. | Mutoh H, Hakamata Y, Sato K, Eda A, Yanaka I, Honda S, Osawa H, Kaneko Y, Sugano K. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun. 2002;294:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 118. | Mutoh H, Sakurai S, Satoh K, Tamada K, Kita H, Osawa H, Tomiyama T, Sato Y, Yamamoto H, Isoda N. Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice. Cancer Res. 2004;64:7740-7747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 119. | Gong C, Mera R, Bravo JC, Ruiz B, Diaz-Escamilla R, Fontham ET, Correa P, Hunt JD. KRAS mutations predict progression of preneoplastic gastric lesions. Cancer Epidemiol Biomarkers Prev. 1999;8:167-171. [PubMed] |
| 120. | Hiyama T, Haruma K, Kitadai Y, Masuda H, Miyamoto M, Tanaka S, Yoshihara M, Shimamoto F, Chayama K. K-ras mutation in helicobacter pylori-associated chronic gastritis in patients with and without gastric cancer. Int J Cancer. 2002;97:562-566. [PubMed] |
| 121. | Watari J, Tanaka A, Tanabe H, Sato R, Moriichi K, Zaky A, Okamoto K, Maemoto A, Fujiya M, Ashida T. K-ras mutations and cell kinetics in Helicobacter pylori associated gastric intestinal metaplasia: a comparison before and after eradication in patients with chronic gastritis and gastric cancer. J Clin Pathol. 2007;60:921-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 122. | Ushijima T, Sasako M. Focus on gastric cancer. Cancer Cell. 2004;5:121-125. [PubMed] |
| 123. | Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA. 1998;95:6870-6875. [PubMed] |
| 124. | Fleisher AS, Esteller M, Tamura G, Rashid A, Stine OC, Yin J, Zou TT, Abraham JM, Kong D, Nishizuka S. Hypermethylation of the hMLH1 gene promoter is associated with microsatellite instability in early human gastric neoplasia. Oncogene. 2001;20:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 125. | Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 126. | Enomoto S, Maekita T, Tsukamoto T, Nakajima T, Nakazawa K, Tatematsu M, Ichinose M, Ushijima T. Lack of association between CpG island methylator phenotype in human gastric cancers and methylation in their background non-cancerous gastric mucosae. Cancer Sci. 2007;98:1853-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 127. | Nakajima T, Yamashita S, Maekita T, Niwa T, Nakazawa K, Ushijima T. The presence of a methylation fingerprint of Helicobacter pylori infection in human gastric mucosae. Int J Cancer. 2009;124:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 128. | Chan AO, Lam SK, Wong BC, Kwong YL, Rashid A. Gene methylation in non-neoplastic mucosa of gastric cancer: age or Helicobacter pylori related? Am J Pathol. 2003;163:370-31; author reply 370-31;. [PubMed] |
| 129. | Forbes GM, Warren JR, Glaser ME, Cullen DJ, Marshall BJ, Collins BJ. Long-term follow-up of gastric histology after Helicobacter pylori eradication. J Gastroenterol Hepatol. 1996;11:670-673. [PubMed] |
| 130. | Ito M, Haruma K, Kamada T, Mihara M, Kim S, Kitadai Y, Sumii M, Tanaka S, Yoshihara M, Chayama K. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther. 2002;16:1449-1456. [PubMed] |
| 131. | Zhou L, Sung JJ, Lin S, Jin Z, Ding S, Huang X, Xia Z, Guo H, Liu J, Chao W. A five-year follow-up study on the pathological changes of gastric mucosa after H. pylori eradication. Chin Med J (Engl). 2003;116:11-14. [PubMed] |
| 132. | Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, Lau JY, Sung JJ. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut. 2004;53:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 318] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 133. | Vannella L, Lahner E, Bordi C, Pilozzi E, Di Giulio E, Corleto VD, Osborn J, Delle Fave G, Annibale B. Reversal of atrophic body gastritis after H. pylori eradication at long-term follow-up. Dig Liver Dis. 2011;43:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 134. | Kodama M, Murakami K, Okimoto T, Sato R, Uchida M, Abe T, Shiota S, Nakagawa Y, Mizukami K, Fujioka T. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol. 2012;47:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 135. | Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. The long-term impact of Helicobacter pylori eradication on gastric histology: a systematic review and meta-analysis. Helicobacter. 2007;12 Suppl 2:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 136. | Wang J, Xu L, Shi R, Huang X, Li SW, Huang Z, Zhang G. Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion. 2011;83:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 137. | Lin JL, Geng X, Bhattacharya SD, Yu JR, Reiter RS, Sastri B, Glazier KD, Mirza ZK, Wang KK, Amenta PS. Isolation and sequencing of a novel tropomyosin isoform preferentially associated with colon cancer. Gastroenterology. 2002;123:152-162. [PubMed] |
| 138. | Chan AO, Peng JZ, Lam SK, Lai KC, Yuen MF, Cheung HK, Kwong YL, Rashid A, Chan CK, Wong BC. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut. 2006;55:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 139. | Leung WK, Man EP, Yu J, Go MY, To KF, Yamaoka Y, Cheng VY, Ng EK, Sung JJ. Effects of Helicobacter pylori eradication on methylation status of E-cadherin gene in noncancerous stomach. Clin Cancer Res. 2006;12:3216-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 140. | Nakajima T, Enomoto S, Yamashita S, Ando T, Nakanishi Y, Nakazawa K, Oda I, Gotoda T, Ushijima T. Persistence of a component of DNA methylation in gastric mucosae after Helicobacter pylori eradication. J Gastroenterol. 2010;45:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 141. | Enomoto S, Maekita T, Ohata H, Yanaoka K, Oka M, Ichinose M. Novel risk markers for gastric cancer screening: Present status and future prospects. World J Gastrointest Endosc. 2010;2:381-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 142. | Ito M, Takata S, Tatsugami M, Wada Y, Imagawa S, Matsumoto Y, Takamura A, Kitamura S, Matsuo T, Tanaka S. Clinical prevention of gastric cancer by Helicobacter pylori eradication therapy: a systematic review. J Gastroenterol. 2009;44:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 143. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1046] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 144. | Wright CL, Kelly JK. The use of routine special stains for upper gastrointestinal biopsies. Am J Surg Pathol. 2006;30:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 145. | Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, Sasaki N, Haruma K, Sumii K, Kajiyama G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639-642. [PubMed] |
| 146. | de Vries AC, Meijer GA, Looman CW, Casparie MK, Hansen BE, van Grieken NC, Kuipers EJ. Epidemiological trends of pre-malignant gastric lesions: a long-term nationwide study in the Netherlands. Gut. 2007;56:1665-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 147. | Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology. 2009;137:1641-8.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 148. | de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 586] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 149. | Areia M, Carvalho R, Cadime AT, Rocha Gonçalves F, Dinis-Ribeiro M. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter. 2013;18:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |