Published online May 7, 2014. doi: 10.3748/wjg.v20.i17.5087
Revised: February 22, 2014
Accepted: March 4, 2014
Published online: May 7, 2014
Processing time: 159 Days and 10.7 Hours
AIM: To assess the value of a new test for the diagnosis of Helicobacter pylori (H. pylori) infection, Rapirun®H. pylori Antibody Stick (Rapirun® Stick), in a Vietnamese population.
METHODS: Eligible patients without previous history of H. pylori eradication were recruited. Rapid urease test (RUT) and histologic examination were used to diagnose the H. pylori infection. Patients were considered H. pylori positive when the RUT results were positive and/or the bacteria were detected histologically. Rapirun® Stick tests were performed using urine samples, and the results were compared with the other 2 methods.
RESULTS: We enrolled 200 patients with a mean age of 36 (range, 18-76) years. There were 116 females and 84 males. Of the 200 patients, 111 (55.5%) were diagnosed as being H. pylori positive. The sensitivity, specificity, and accuracy of the Stick test were 84.7%, 89.9%, and 87.0%, respectively. There were 17 (8.5%) false-negative patients and 9 (4.5%) false-positive patients.
CONCLUSION: The Rapirun® Stick test has high sensitivity, specificity, and accuracy for the diagnosis of H. pylori infection in the Vietnamese population. The test can be clinically applied in Vietnamese populations.
Core tip: The Rapirun®Helicobacter pylori (H. pylori) Antibody Stick (Rapirun® Stick) has recently been developed to detect anti-H. pylori antibody in urine. This test requires fewer processing steps and provides quicker results. This study attempted to assess the value of this new test for the diagnosis of H. pylori infection in a Vietnamese population. The sensitivity, specificity, and accuracy of the Stick test were 84.7%, 89.9%, and 87.0%, respectively. The Rapirun® Stick test has high sensitivity, specificity, and accuracy for the diagnosis of H. pylori infection in the Vietnamese population. The test can be clinically applied in Vietnamese populations.
-
Citation: Quach DT, Hiyama T, Shimamoto F, Le QD, Ho LX, Vu NH, Yoshihara M, Uemura N. Value of a new stick-type rapid urine test for the diagnosis of
Helicobacter pylori infection in the Vietnamese population. World J Gastroenterol 2014; 20(17): 5087-5091 - URL: https://www.wjgnet.com/1007-9327/full/v20/i17/5087.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i17.5087
Helicobacter pylori (H. pylori) infection plays an important role in the pathogenesis of chronic gastritis, peptic ulcer disease, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma[1]. Recent studies have demonstrated that a strategy to test and treat H. pylori in uninvestigated, dyspeptic patients in primary care is safe and reduces the need for endoscopy[2,3]. In addition, the indications to test and eradicate H. pylori have expanded even to subjects who do not have upper gastrointestinal symptoms, including first-class relatives of patients with gastric cancer and patients requiring long-term therapy with aspirin or non-steroidal anti-inflammatory drugs[4]. Therefore, there is an increasing need for non-invasive methods to diagnose H. pylori infection.
Several methods to diagnose H. pylori infection have been developed, among which the urea breath test (UBT) is currently regarded as the most accurate assay. However, the UBT is still expensive and not widely available in many countries, including Vietnam. An ideal non-invasive diagnostic test should be simple, inexpensive, rapid, and processed without special equipment and expertise but which delivers acceptably accuracy. A rapid urine test based on enzyme-linked immunosorbent assay (ELISA) has been developed for the detection of anti-H. pylori antibody in urine. One of these urine-based ELISA kits, the Rapirun®H. pylori Antibody Detection Kit (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan), has been reported to have high sensitivity and specificity in several trials in different geographic areas, including Vietnam[5-11]. Recently, a new stick-type rapid urine test, Rapirun®H. pylori Antibody Stick (Rapirun® Stick) (Otsuka Pharmaceutical Co., Ltd.), has been developed that requires less labour and which provides results more rapidly than the conventional Rapirun® kit. It takes 15 min to evaluate the result with the Rapirun® Stick, whereas 20 min is required for the conventional Rapirun® kit. This method was reported to have an agreement rate of 98.4% compared with the conventional method in a Japanese population[12]. However, it has not been evaluated in other populations. This study therefore aimed to assess the value of the Rapirun® Stick test for the diagnosis of H. pylori infection in a Vietnamese population.
From October 2012 to December 2012, patients undergoing upper gastrointestinal endoscopy at the Department of Endoscopy, University Medical Center in Ho Chi Minh, Vietnam, were recruited. Exclusion criteria for the patients included those with a past history of H. pylori eradication therapy or previous gastric surgery and patients taking any type of antibiotics, H2-receptor blockers, bismuth or proton pump inhibitors in the last 4 weeks before endoscopy. Informed written consent was obtained from all patients participating in the trial. This study was approved by the local ethics committee.
During upper gastrointestinal endoscopy, endoscopic lesions were recorded. Three biopsies were taken from each patient: 2 for histologic examination and 1 for rapid urease test (RUT). The 2 biopsies for histological examination were taken from the greater curvature, one in the antrum and the other in the corpus, and were sent for Haematoxylin and Eosin and Giemsa staining. Tissue specimens were examined by an experienced pathologist (FS) who was blind to all clinical information.
The biopsy for RUT was taken from the greater curvature of the corpus, about 2 cm above the atrophic border. This biopsy location has been reported to optimise the sensitivity of the RUT to detect H. pylori in a Vietnamese population[13]. PyloriTek® (Serim Research Co., Elkhart, IN, United States) was used and the colour change was read within 1 h after incubation. This RUT has been validated in several previous studies and has shown very high sensitivity and specificity (90%-98.5% and 97%-100%, respectively)[14-17].
After endoscopy, urine samples were collected and were processed within 1 h of collection for the detection of antibodies against H. pylori using the Rapirun® Stick. The test measures human immunoglobulin G (IgG) antibodies against H. pylori in urine using the principle of immunochromatography.
The antigen used is a crude extract from a clinically isolated H. pylori strain, the OHPC-040 strain, taken from a Japanese patient with chronic gastritis. A previous report demonstrated that OHPC-040 was the most suitable isolate to detect urinary antibodies to H. pylori among 20 clinical isolates extracted from patients with disorders of the upper digestive tract and that OHPC-040 was positive for the vacA, ureB, and cagA genes based on the results of DNA analysis[18]. The test stick contains colloidal gold-conjugated anti-human IgG (Fc) polyclonal antibody (goat). The test line and control line in the evaluation section of the stick are immobilised with H. pylori antigen and anti-human IgG polyclonal antibody, respectively.
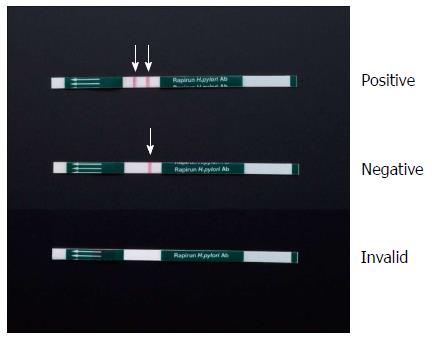
The Rapirun® Stick test procedure consisted of 2 steps: (1) taking approximately 0.3 mL of the urine sample, as indicated by the measurement guide on the pipette included in the kit, then adding it to a container holding the sample diluent (0.3 mL) and mixing them (an approximately 2-fold dilution); and (2) standing a test stick in the container that holds the mixture of urine and diluent (as described above) with the sample absorption section of the test stick submerged in the diluted sample. After leaving the kit undisturbed for 15 min at room temperature (25 °C-30 °C), we then confirmed visually whether any red lines appeared in the evaluation section. The appearance of 2 distinct red bands (one control and one test line) indicates a positive test (Figure 1). The appearance of the control line only indicates a negative result. The absence of a control line indicates an invalid result.
The results of the RUT and Rapirun® Stick test were read by different researchers who were not aware of the results of the other methods used to diagnose H. pylori infection. The definition of H. pylori infection in this study required at least one positive test of 2 tests, the RUT and histologic examination. Absence of H. pylori infection required both of these tests to be negative. Equivocal tests were excluded from the analysis.
Analysis to determine the sensitivity, specificity, positive and negative predictive values, and accuracy of the Rapirun® Stick test was performed with SPSS software for Windows, version 20 (SPSS Inc., Chicago, IL, United States).
We recruited 200 patients in this study. The quality of gastric biopsies for histologic examination to detect H. pylori was excellent in all patients. There were no invalid results with the Rapirun® Stick; therefore, we included data from all 200 patients in the analysis.
All patients were ethnic Vietnamese. The demographic characteristics of the patients are indicated in Table 1. The mean age of the patients was 36 (range, 18-76) years. There were 116 (58.0%) females and 84 (42.0%) males.
| Characteristics | H. pylori positive(n = 111) | H. pylori negative(n = 89) | Total(n = 200) |
| Mean age (range) (yr) | 35.5 (18-62) | 36.6 (18-76) | 36 (18-76) |
| Sex (female/male) | 59/52 | 57/32 | 116/84 |
| Diagnosis | |||
| Normal gastroduodenal tract | 1 | 6 | 7 |
| Gastritis and/or duodenitis | 83 | 66 | 149 |
| Gastric ulcer | 4 | 1 | 5 |
| Duodenal ulcer | 16 | 1 | 17 |
| Reflux esophagitis | 5 | 15 | 20 |
| Reflux esophagitis and peptic ulcer disease | 2 | 0 | 2 |
Of the 200 patients, 111 (55.5%) were diagnosed as being H. pylori positive: among them, 16 (14.4%) had duodenal ulcer, 5 (4.5%) had reflux esophagitis, 4 (3.6%) had gastric ulcer, and 2 (1.8%) had both gastro-duodenal ulcer and reflux esophagitis. Eighty-nine (44.5%) patients were H. pylori negative: among them, only one (1.1%) had gastric ulcer and one (1.1%) had duodenal ulcer, whereas 15 (16.9%) had reflux esophagitis. Of the 24 patients with gastro-duodenal ulcer, 22 (91.7%) had H. pylori infection. However, 7 of 22 (31.8%) patients with reflux esophagitis also had the infection.
The sensitivity, specificity, positive and negative predictive values, and accuracy of the Rapirun® Stick test were 84.7%, 89.9%, 91.2%, 82.5%, and 87.0%, respectively (Table 2). There were 17 (8.5%) false-negative patients including 3 with duodenal ulcer, 1 with reflux esophagitis, and 13 with gastritis/duodenitis. Among them, 14 had both a positive RUT and positive histologic examination, 2 had only a positive histologic result, and 1 had only a positive RUT result. There were 9 (4.5%) false-positive patients including 1 patient with reflux esophagitis and 8 with gastritis/duodenitis.
| Rapirun® Stick test | H. pylori infection status | |
| Positive | Negative | |
| Positive (103) | 94 | 9 |
| Negative (97) | 17 | 80 |
| Total | 111 | 89 |
To our knowledge, this study is the first to determine the validity of the Rapirun® Stick in a Vietnamese population. The assay is noninvasive, easy to handle, and the cost of using urine as a sample is low. The test can be clinically applied in populations of developing countries such as Vietnam.
Vietnam is one of the countries with a high prevalence of gastric cancer. The mortality rate for gastric cancer is 18.6/100000 for males and 8.4/100000 for females[19]. Among cancer deaths in Vietnam, gastric cancer is the second leading cause followed by lung cancer for males, and fourth, followed by breast, cervix, uterine, and colorectal cancer, for females during 2006 and 2007[20]. The reason of the high mortality from gastric cancer may mainly be the high prevalence of infection from H. pylori, a definite carcinogen of gastric cancer. H. pylori infection was detected in 65.6% of the hospital-based population (mean age, 42.5 years)[21].
To reduce the incidence of gastric cancer in Vietnam, a nationwide H. pylori eradication treatment may be recommendable because H. pylori has been regarded as a definite carcinogen, and several studies have shown that its eradication reduces the incidence of gastric cancer development[22,23].
To carry out H. pylori eradication treatment, a simple, low-cost, and accurate method is needed to diagnose the infection. There are various methods to detect the infection so far: RUT, bacteriologic culture, histologic examination, UBT, serum antibody assay, and detection of anti-H. pylori antibody in urine and H. pylori antigen in stool. RUT, bacteriologic culture, and histologic examination require endoscopic biopsy. UBT is regarded as the most accurate assay; however, it requires special apparatus and is expensive to perform. If sensitive screening for H. pylori infection were possible using urine samples, it would not only be more convenient in clinical practice but would also be very useful for mass screening. The Rapirun® Stick, a newly developed detection kit for anti-H. pylori antibody in urine, is very simple and requires only 15 min to complete. Furthermore, the test does not require technical expertise, special sample handling, or any additional equipment and thus allows considerable savings of diagnosis-related costs. The kit is a candidate test method that would be applicable for use with the Vietnamese population.
The sensitivity, specificity, and accuracy of the conventional Rapirun® kit in a Vietnamese population were reported to be 79.5%, 90.7%, and 84.5%, respectively[11]. In the present study, the sensitivity, specificity, and accuracy of the new Rapirun® Stick test, were 84.7%, 89.9%, and 87.0%, respectively. The values are relatively better in the present study compared with the study using the conventional Rapirun® kit. This may be due to the difference in the populations tested and the methods used to investigate H. pylori infection: bacterial culture, histologic examination, and serum ELISA in the study of the conventional Rapirun® kit, and RUT and histologic examination in the present study. Although the antigen used in the Rapirun® Stick is a crude extract from a clinically isolated H. pylori strain taken from a Japanese patient, our study clearly demonstrates the usefulness of the Rapirun® Stick in the Vietnamese population. This is truly the first report on the usefulness of the kit external to the Japanese population.
In the present study, 8.5% were false-negative patients. This may be due to the H. pylori polymorphism, the host factors in different geographic areas, and the extremely low level of anti-H. pylori-specific IgG in the urine of the patients. In contrast, 4.5% were false-positive patients. Graham et al[9] reported that 2 patients who had been treated for H. pylori infection more than 32 and 42 mo previously, respectively, had positive Rapirun® test results, suggesting that the urine test results may remain positive for an extended time after successful cure of the infection. H. pylori in our false-positive patients might have been eradicated intentionally or unintentionally. The reasons for the incidence of the false-positive and false-negative results should be investigated to improve the sensitivity, specificity, and accuracy of the kit.
Evaluation of the diagnostic performance of the conventional Rapirun® kit in various countries, including Japan, Taiwan, South Korea, Vietnam, United States, and European countries (Austria, France, Germany, and Italy), showed a sensitivity of 77.4%-96.7%, specificity of 83.3%-97.4%, and accuracy of 80.4%-96.1%[11,24]. The present study showed high sensitivity, specificity, and accuracy for the new Rapirun® Stick. In addition, the Rapirun® Stick has been reported to have an agreement rate of 98.4% compared with the conventional Rapirun® kit in a Japanese population[12]. Therefore, the Rapirun® Stick can be applicable in many countries, at least in the above-mentioned countries.
There are several limitations in the present study. First, the patients were enrolled in only one hospital in Ho Chi Minh, in southern Vietnam. There are reports showing differences in the prevalence of gastrointestinal diseases such as peptic ulcer and gastric cancer and of vacA-positive H. pylori between Hanoi, in northern Vietnam, and Ho Chi Minh[19]. Therefore, the study population may not be representative of the entire Vietnamese population. Second, RUT and histologic examination were used to diagnose the infection in the present study. In several patients, these methods produced false-negative or false-positive results, leading to the possible misdiagnosis of H. pylori infection.
In conclusion, we demonstrated the usefulness of the Rapirun® Stick test for the diagnosis of H. pylori infection in a Vietnamese population: the sensitivity, specificity, and accuracy of the Rapirun® Stick test were high. The test can be clinically applied in Vietnamese populations.
The Rapirun®Helicobacter pylori (H. pylori) Antibody Stick (Rapirun® Stick) has recently been developed to detect anti-H. pylori antibody in urine. This test requires fewer processing steps and provides quicker results. This study attempted to assess the value of this new test for the diagnosis of H. pylori infection in a Vietnamese population.
The Rapirun® Stick was reported to have an agreement rate of 98.4% compared with the conventional method in a Japanese population. However, it has not been evaluated in other populations.
This study is the first to determine the validity of the Rapirun® Stick in a Vietnamese population. The assay is noninvasive, easy to handle, and the cost of using urine as a sample is low.
The Rapirun® Stick can be clinically applied in populations of developing countries such as Vietnam.
Rapid urease test is a rapid test for diagnosis of H. pylori. The basis of the test is the ability of H. pylori to secrete the urease enzyme, which catalyzes the conversion of urea to ammonia and carbon dioxide.
The authors examined the value of new test for the diagnosis of H. pylori infection, Rapirun® Stick, in a Vietnamese population. The Stick test has high sensitivity, specificity, and accuracy for the diagnosis. The results are interesting, and suggest that the test can be clinically applied in Vietnamese populations.
P- Reviewers: Buzas GM, Huerta-Franco MR, Paulssen EJ S- Editor: Wen LL L- Editor: A E- Editor: Wang CH
| 1. | McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 550] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 2. | Miwa H, Ghoshal UC, Gonlachanvit S, Gwee KA, Ang TL, Chang FY, Fock KM, Hongo M, Hou X, Kachintorn U. Asian consensus report on functional dyspepsia. J Neurogastroenterol Motil. 2012;18:150-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Harmon RC, Peura DA. Evaluation and management of dyspepsia. Therap Adv Gastroenterol. 2010;3:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1589] [Article Influence: 122.2] [Reference Citation Analysis (5)] |
| 5. | Yamamoto S, Uemura N, Okamoto S, Yamaguchi S, Mashiba H, Tachikawa T. A new rapid test for detecting anti-Helicobacter pylori antibody excreted into urine. Helicobacter. 2000;5:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Yamamoto T, Ishii T, Kawakami T, Sase Y, Horikawa C, Aoki N, Sanaka M, Kuyama Y. Reliability of urinary tests for antibody to Helicobacter pylori in patients with pulmonary tuberculosis. World J Gastroenterol. 2005;11:412-414. [PubMed] |
| 7. | Wu DC, Kuo CH, Lu CY, Su YC, Yu FJ, Lee YC, Lin SR, Liu CS, Jan CM, Wang WM. Evaluation of an office-based urine test for detecting Helicobacter pylori: a Prospective Pilot Study. Hepatogastroenterology. 2001;48:614-617. [PubMed] |
| 8. | Fujisawa T, Kaneko T, Kumagai T, Akamatsu T, Katsuyama T, Kiyosawa K, Tachikawa T, Kosaka O, Machikawa F. Evaluation of urinary rapid test for Helicobacter pylori in general practice. J Clin Lab Anal. 2001;15:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Graham DY, Reddy S. Rapid detection of anti-Helicobacter pylori IgG in urine using immunochromatography. Aliment Pharmacol Ther. 2001;15:699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Hu HM, Kuo CH, Lo YC, Wu MT, Wu IC, Lu CY, Su YC, Yu FJ, Lee YC, Lin SR. Evaluation of the two immunochromatographic methods for detecting urine and serum IgG antibodies to Helicobacter pylori and comparison of accuracy and clinical utility. Hepatogastroenterology. 2007;54:119-123. [PubMed] |
| 11. | Nguyen LT, Uchida T, Tsukamoto Y, Trinh TD, Ta L, Ho DQ, Matsuhisa T, Uchida M, Takayama A, Hijiya N. Evaluation of rapid urine test for the detection of Helicobacter pylori infection in the Vietnamese population. Dig Dis Sci. 2010;55:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Murakami K, Kamada T, Ishikawa H, Imamura H, Matsumoto H, Fujita M, Tarumi K, Shiotani A, Mizukami K, Shiota S. An evaluation of the performance of a novel stick-type kit for rapid detection of Helicobacter pylori antibodies in urine. Clin Lab. 2011;57:481-487. [PubMed] |
| 13. | Quach DT. Optimal gastric biopsy site for Helicobacter pylori diagnosis by using rapid urease test. Helicobacter. 2006;11 Suppl 2:38. |
| 14. | Nishikawa K, Sugiyama T, Kato M, Ishizuka J, Kagaya H, Hokari K, Asaka M. A prospective evaluation of new rapid urease tests before and after eradication treatment of Helicobacter pylori, in comparison with histology, culture and 13C-urea breath test. Gastrointest Endosc. 2000;51:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Laine L, Lewin D, Naritoku W, Estrada R, Cohen H. Prospective comparison of commercially available rapid urease tests for the diagnosis of Helicobacter pylori. Gastrointest Endosc. 1996;44:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Yousfi MM, el-Zimaity HM, Genta RM, Graham DY. Evaluation of a new reagent strip rapid urease test for detection of Helicobacter pylori infection. Gastrointest Endosc. 1996;44:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Murata H, Kawano S, Tsuji S, Tsujii M, Sawaoka H, Iijima H, Kawai N, Hori M. Evaluation of the PyloriTek test for detection of Helicobacter pylori infection in cases with and without eradication therapy. Am J Gastroenterol. 1998;93:2102-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Katsuragi K, Noda A, Tachikawa T, Azuma A, Mukai F, Murakami K, Fujioka T, Kato M, Asaka M. Highly sensitive urine-based enzyme-linked immunosorbent assay for detection of antibody to Helicobacter pylori. Helicobacter. 1998;3:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 19. | Ngoan le T, Anh NT, Huong NT, Thu NT, Lua NT, Hang LT, Bich NN, Hieu NV, Quyet HV, Tai le T. Gastric and colo-rectal cancer mortality in Viet Nam in the years 2005-2006. Asian Pac J Cancer Prev. 2008;9:299-302. [PubMed] |
| 20. | Vuong DA, Velasco-Garrido M, Lai TD, Busse R. Temporal trends of cancer incidence in Vietnam, 1993-2007. Asian Pac J Cancer Prev. 2010;11:739-745. [PubMed] |
| 21. | Nguyen TL, Uchida T, Tsukamoto Y, Trinh DT, Ta L, Mai BH, Le SH, Thai KD, Ho DD, Hoang HH. Helicobacter pylori infection and gastroduodenal diseases in Vietnam: a cross-sectional, hospital-based study. BMC Gastroenterol. 2010;10:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3183] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 23. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 935] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 24. | Leodolter A, Vaira D, Bazzoli F, Schütze K, Hirschl A, Mégraud F, Malfertheiner P. European multicentre validation trial of two new non-invasive tests for the detection of Helicobacter pylori antibodies: urine-based ELISA and rapid urine test. Aliment Pharmacol Ther. 2003;18:927-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |