Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4648
Revised: January 13, 2014
Accepted: February 20, 2014
Published online: April 28, 2014
Processing time: 165 Days and 21.9 Hours
AIM: To investigate whether resveratrol (3,4,5-trihydroxy-trans-stilbene) inhibits collagen I synthesis induced by insulin growth factor-1 (IGF-1) in intestinal fibroblasts, and to explore the possible molecular mechanisms.
METHODS: Male Sprague-Dawley rats were randomly divided into two groups: a control group and a 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis group. After 21 d of TNBS administration, the degree of inflammation and fibrosis in colon was measured by HE staining and Masson’s trichrome staining. Western blotting was used to examine collagen I, IGF-1 and silent information regulator 1 (SIRT1) protein expression in colitis tissues. Western blotting and quantitative real-time polymerase chain reaction were used to characterize collagen I protein and col1a2 mRNA expression in mouse intestinal fibroblasts and CCD-18Co cells treated with IGF-1. A MEK inhibitor (U0126) was used to determine whether IGF-1-induced collagen I expression was mediated by extracellular signal-regulated kinase 1/2 (ERK1/2)-dependent mechanism. Effects of resveratrol on collagen I protein level, insulin growth factor-1 receptor (IGF-1R) and ERK1/2 phosphorylation levels were also examined after IGF-1 treatment in fibroblasts. To evaluate whether SIRT1 was necessary for the anti-fibrosis effect of resveratrol, cells were transfected with SIRT1-specific small interfering RNAs, wild-type SIRT1, and deacetylase-inactive mutant SIRT1.
RESULTS: Collagen I and IGF-1 expression was increased, and SIRT1 expression was decreased (0.67 ± 0.04 vs 1.05 ± 0.07, P < 0.001) in TNBS-induced colitis compared with the control group. In vitro, IGF-1 could induce collagen I expression, mainly through the ERK 1/2 signal pathway. Resveratrol reduced basal and IGF-1-induced collagen I gene and protein expression in intestinal fibroblasts. Overexpression of wild-type SIRT1, not deacetylase-inactive mutant SIRT1, decreased expression of collagen I induced by IGF-1. Moreover, silencing SIRT1 restored collagen I expression in fibroblasts challenged with resveratrol. However, disruption of SIRT1 did not influence the anti-fibrotic effects of resveratrol and IGF-1-induced collagen I expression. Further analysis revealed that resveratrol significantly decreased phosphorylation of IGF-1R and its downstream signaling molecules by inhibiting IGF-1 binding to its receptor.
CONCLUSION: Our data suggest that resveratrol effectively inhibits collagen I synthesis in IGF-1-stimulated fibroblasts, partly by inhibiting IGF-1R activation, and SIRT1 is also responsible for the process.
Core tip: This study showed that the expression of silent information regulator 1 (SIRT1) was decreased in 2,4,6-trinitrobenzenesulfonic acid-induced colitis tissues, and resveratrol down-regulated insulin growth factor (IGF)-1-induced collagen I synthesis by inhibiting IGF-1 receptor (IGF-1R) phosphorylation and its downstream extracellular signal-regulated kinase/mitogen-activated protein kinase signaling pathway in intestinal fibroblasts. Resveratrol alone suppressed collagen I synthesis through up-regulating activity of SIRT1. Our data highlight a previously unknown function of resveratrol on IGF-1R activation and provide novel insight of resveratrol as a therapeutic agent for intestinal fibrosis.
- Citation: Li P, Liang ML, Zhu Y, Gong YY, Wang Y, Heng D, Lin L. Resveratrol inhibits collagen I synthesis by suppressing IGF-1R activation in intestinal fibroblasts. World J Gastroenterol 2014; 20(16): 4648-4661
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4648.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4648
Intestinal fibrosis is a common and serious complication of Crohn’s disease (CD) and can increase the risk of intestinal stenosis or obstruction, and ultimately lead to surgical intervention at substantial personal and economic cost[1-3]. Until now, no effective therapy exists for averting such fibrogenic events, because the fibrotic process becomes autopropagative and fails to respond to antiinflammatory interventions[4,5].
The pathological process of intestinal fibrosis is characterized by mesenchymal cell proliferation and extracellular matrix (ECM) accumulation in interstitial space[6,7]. Collagens I is one of the major matrix molecules involved in intestinal fibrogenesis. The activation of extracellular signal-regulated kinase 1/2 (ERK1/2) is one of the major downstream signaling events that participate in collagen I synthesis[8,9]. Insulin-like growth factor 1 (IGF-1) is a potent profibrogenic agent involved in intestinal remodeling[10,11]. Laboratory- and population-based studies have shown that expression of IGF-1 in Crohn’s disease patients is significantly increased and IGF-1 receptor (IGF-1R) is also altered in the intestines of ulcerative colitis and Crohn’s disease patients[12-14]. IGF-1 binding to IGF-1R allows the β-subunits of IGF-1R to display intrinsic tyrosine kinase activity and activates downstream signals via phosphorylation of key proteins including phosphatidylinositol 3-kinase and mitogen-activated protein (MAP) kinase (MAPK)[15]. IGF-1 not only stimulates proliferation and inhibits apoptosis of fibroblasts and myofibroblasts, but also increases collagen expression and production in intestinal smooth muscle cells[16,17]. Collagen-producing fibroblasts and myofibroblasts are central cell types in intestinal fibrogenesis. There are no data showing the effect of IGF-1 on collagen I in intestinal fibroblasts.
Resveratrol (3,4,5-trihydroxy-trans-stilbene) is a polyphenol naturally occurring in grapes and red wine that exhibits beneficial health effects such as extending the life span and regulating tumor growth and oxidation. Resveratrol activates silent information regulator-1 (SIRT1), a nicotinamide adenine dinucleotide-dependent deacetylase, which has many biological functions by deacetylating a number of key transcription factors, including p53, nuclear factor-κB, and peroxisome proliferator-activated receptor gamma co-activator-1α[18]. In addition, resveratrol also has a dramatic antifibrotic effect in rodent models of renal fibrosis[19,20], cardiac fibrosis[21], and hepatic fibrosis[22,23], but the molecular mechanism(s) are currently unknown.
The protective role of resveratrol in colitis has been demonstrated in models of colitis induced by dextran sulfate sodium (DSS) and trinitrobenzene sulphonic acid (TNBS)[24-26]. Resveratrol may protect against colitis through up-regulation of SIRT1 in immune cells, which functions as an inverse regulator of NF-κB activation and inflammation in the colon[25]. Mounting evidence suggests that resveratrol also has an antifibrotic effect in the peptidoglycan-polysaccharide rat model of Crohn’s disease and can diminish IGF-1-stimulated collagen production in intestinal smooth muscle cells[27,28]. However, the mechanism for resveratrol to inhibit collagen synthesis and IGF-1-induced collagen production has not been established, and it is unclear whether resveratrol inhibits collagen expression through augmentation of SIRT1 activity in intestinal fibroblasts. The aim of this study was to investigate the effect of resveratrol on collagen I synthesis in intestinal fibroblasts and to explore the possible molecular mechanisms.
The technique for induction of colitis with TNBS (Sigma) was as described previously[29]. Male Sprague-Dawley rats (200-250 g) were purchased from and maintained in the Animal Center of Nanjing Medical University (Nanjing, China). To induce chronic fibrotic colitis, rats were fasted for 24 h, lightly anesthetized with diethyl ether, and TNBS solution [2.5% in 50 % ethanol (v/v)] was injected via a catheter advanced to 8 cm proximal to the anus. In order to distribute the TNBS within the colon, the rat was kept in a vertical position with the head downwards for 3 min after the injection. All rats were checked daily for loss of body weight, stool consistency, and the presence of gross bleeding. The disease activity index (DAI) was calculated as a sum of the scores of the three parameters according to the scoring system described by Murthy et al[30]. Animals were sacrificed after 21 d and body weight, colon weight, and colon length were recorded. HE staining and Masson’s trichrome staining were used to measure the degree of inflammation and fibrosis in the colon by microscopy.
Mouse intestinal fibroblasts (MIFs) were isolated and cultured as described previously[31,32]. The intestine tissue isolated from Balb/c mice (7 d) was cut into 1-mm pieces. Epithelial cells were removed in Hank’s Balanced Salt Solution without Ca2+and Mg2+ with 2 mmol/L EDTA. The remaining tissue was rinsed and then digested for 30 minutes at 37 °C with 1 mg/mL collagenase II and 0.3 mg/mL DNase I in PBS. The isolated cells were cultured in 25-cm2 culture flasks (Corning) with Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS, Hyclone) and 10 μg penicillin/streptomycin (Gibco). Nonadherent cells were removed by subsequent changes of medium after 2 h. The remaining cells were characterized by immunocytochemistry staining for vimentin (1:200) and α-smooth muscle action (1:200) as previously described[32]. For all experiments, fibroblasts were used between passages 3 and 8, and were treated with recombinant mouse IGF-1 (100 ng/mL, RD) or Resveratrol (50, 100 μmol/L, sigma).
CCD-18Co cells (CRL 1459) at passage 6 were obtained from American Type Culture Collection and used between passages 8 and 15. Cells were grown in DMEM supplemented with 10% FBS and 10 μg penicillin/streptomycin. Cells were maintained at 37 °C in a 5% CO2 incubator. Cells were treated with recombinant human IGF-1 (100 ng/mL, RD) or resveratrol (50, 100 μmol/L, Sigma) for 24 h as indicated.
WT and deacetylase-inactive mutant SIRT1 (H363Y) constructs were a gift from Dr. Yong Xu (Nanjing Medical University, Nanjing, China) and have been described previously[33]. Silencing of SIRT1 was mediated by small interfering RNAs (siRNAs) using the following sequences: for human SIRT1, 1: 5’-CGGGAAUCCAAAGG AUAAUTT-3’, 2: 5’-CCAUCUCUCUGUCACAAAUTT-3’ and 3: 5’-CCAAGCAGCUA AGAGUAAUTT-3’. CCD-18Co cells were transfected at 30%-40% confluency using either Lipofectamine 2000 or Lipofectamine RNAiMAX (Invitrogen). At 24 h post-transfection, cells were treated with IGF-1 followed by treatment with serum-free medium for 12 h. For siSIRT1 experiments, cells at 48 h post-transfection were treated for an additional 24 h with either 100 μmol/L resveratrol or 100 ng/mL IGF-1.
Whole cell lysates were obtained by re-suspending cell pellets in RIPA buffer (50 mmol/L Tris pH = 7.4, 150 mmol/L NaCl, 1%Triton X-100) with freshly added protease inhibitor and phosphatase inhibitor tablet (Roche). Cells lysates were subjected to SDS-PAGE and transferred onto PVDF membranes (Millipore) using a Semi-Phor system (Bio-Rad). After blocking in PBS containing 5% non-fat dry milk, blots were incubated with primary antisera for overnight at 4 °C, washed in PBS containing 0.05% Tween, and then incubated with peroxidase conjugated secondary antibodies for 30 min at RT. Immunoreactive proteins were identified using the ECL detection system. Antibodies against collagen type I were obtained from Rockland. IGF-1R, phosphorylated IGF-IR, ERK1/2, phosphorylated ERK1/2 and MEK inhibitor (U0126) were purchased from Cell Signaling Technology. β-actin (1:1000) and GAPDH were purchased from Bioworld, and SIRT1 monoclonal antibody (1:1000) was from Abcam.
Total RNA was extracted from cells grown in 60-mm tissue culture dishes (Corning) using TRIzol Reagent (Gibco), according to the manufacturer’s instructions. Reverse transcription reactions were performed using PrimeScript RT Master Mix and (Takara). Real-time PCR reactions were performed using SYBR-Green PREMIX EX TAQ (Takara) on an ABI Prism 7500 system. The primers used for real-time reactions were: (1) mouse col1a2, forward primer (5’-GGAGGGAACGGTCCACGAT-3’) and reverse primer (5’-GAGTCCGCGTATCCACAA-3’); (2) mouse col1a1, forward primer (5’-CCGGCTCCTGCTCCTCTTA-3’) and reverse primer (5’-CCATTGTGTATGCAGCTG ACTTC-3’); (3) mouse β-actin, forward primer (5’-CATCGTGGGCCGCTCTA-3’) and reverse primer (5’-CACCCACACATAGGAGTCCTTCTG-3’); (4) human col1a2, forward primer (5’-GCCCCCCAGGCAGAGA-3’) and reverse primer (5’-CCAACTCCTTTTCCAT CATACTGA-3’); (5) human col1a1, forward primer (5’-ACGAAGACATCCCACCA ATC-3’) and reverse primer (5’- GCACCATCCAAACCAC TGA-3’); (6) human sirt1, forward primer (5’-TGAGGCACTTCATGGGGTATGG-3’) and reverse primer (5’-TCCTAGGTTGCCCAGCTGATGAA-3’); and (7) human GAPDH, forward primer (5’-GAAATCCCATCACCATCTTCCAGG-3’) and reverse primer (5’-GAGCCCCAGCCT TCTCCATG-3’).
The results are expressed as mean ± SD. The SPSS statistical package (version 14.0; SPSS Inc, Chicago, IL, United States) was used for statistical analyses. The differences between the two groups were analyzed using Student’s t test. Unless otherwise specified, P values smaller than 0.05 were considered statistically significant.
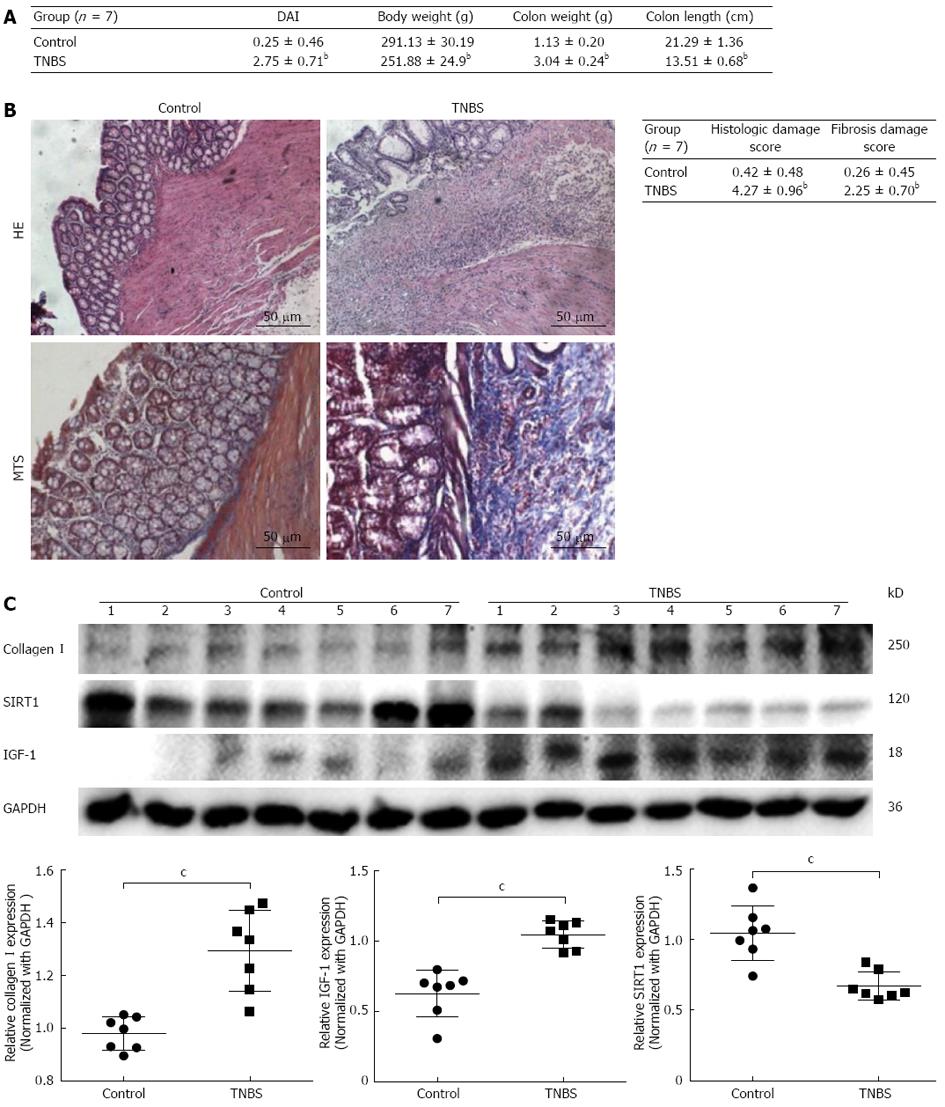
We initially tested the expression of collagen I and SIRT1 in colitis induced with TNBS. Compared with control normal rats, the DAI, body weight and colon weight significantly increased, and colon length decreased in TNBS-treated rats (Figure 1A). Paraffin-embedded colonic tissue samples from TNBS-treated and control rats were assessed for inflammation and fibrosis after HE and Masson’s trichrome staining (MTS). TNBS-treated rats showed significant colitis marked by submucosa thickening, epithelial layer destruction, and lymphocyte infiltration (Figure 1B). The sections stained with MTS displayed diffuse extracellular matrix deposition and fibrosis in the mucosa and submucosa (Figure 1B). Histologic scores for both inflammation and fibrosis were greater in TNBS-induced colitis rats than in normal rats (Figure 1B).
Furthermore, Western blotting analysis showed that collagen I protein level was increased in colitis rats compared to normal rats (Figure 1C). IGF-1 protein expression was also increased in colitis rats, whereas SIRT1 was decreased (Figure 1C). These data indicate that resveratrol may protect against intestinal fibrosis through stimulating SIRT1 expression.
Since earlier studies documented that IGF-1 attenuates inflammation and exacerbates intestinal fibrosis, we chose to investigate the possible mechanism underlying IGF-1 induced collagen I synthesis in intestinal fibroblasts.
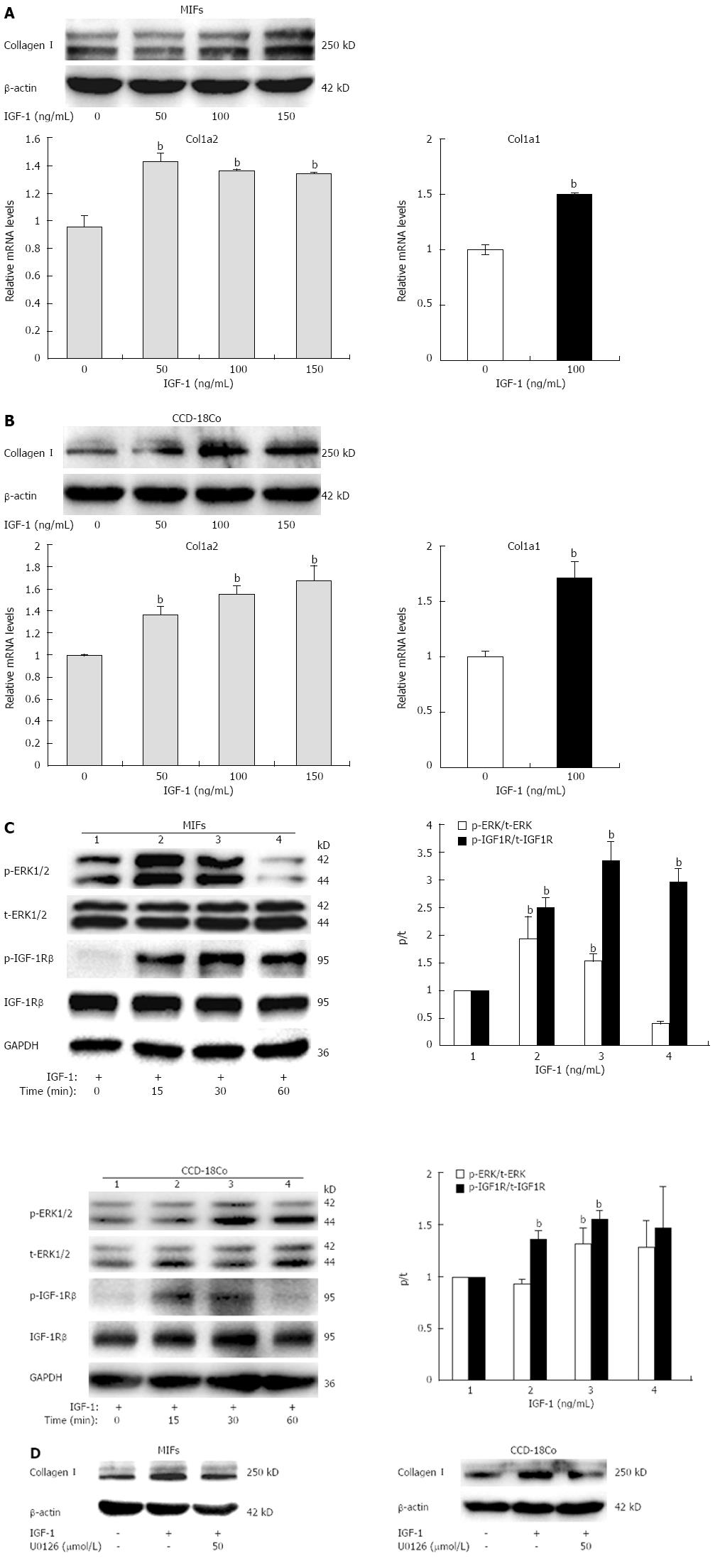
We first examined the expression of collagen I in both MIFs and CCD-18Co cells treated with increasing concentrations (50, 100 or 150 ng/mL) of IGF-1 for 24 h. IGF-1 potently increased both col1a2 mRNA levels (Figure 2A) and protein levels (Figure 2B) of collagen I in a dose-dependent manner. IGF-1 treatment for 24 h showed a maximal effect on collagen I expression. In addition, IGF-1 also induced col1a1 mRNA expression (Figure 2A and B).
To investigate the molecular mechanism underlying the induction of collagen I expression by IGF-1, we measured the phosphorylation of IGF-1R and ERK1/2 in response to IGF-1 treatment. IGF-1 significantly increased levels of phospho-IGF-1R and ERK1/2 in a time-dependent manner in both MIFs and CCD-18Co cells, and the most prominent effect was seen at 30 min (Figure 2C). Fibroblasts were pretreated with U0126 (50 μmol/L) for 1 h to block MEK1/2 phosphorylation and then coincubated with IGF-1 (100 ng/mL) for another 24 h. The ability of IGF-1 to increase collagen I expression was significantly inhibited by the MAP-kinase inhibitor (Figure 2D). Taken together, these data suggest that IGF-1 inhibited collagen I synthesis in fibroblasts through the IGF-1/IGF-1R/MAP-kinase pathway.
Since we observed that SIRT1 expression was decreased in colonic tissues of colitis rats, we evaluated the effect of the SIRT1 activator, resveratrol, on collagen I synthesis in intestinal fibroblasts and elucidated the underlying molecular mechanisms.
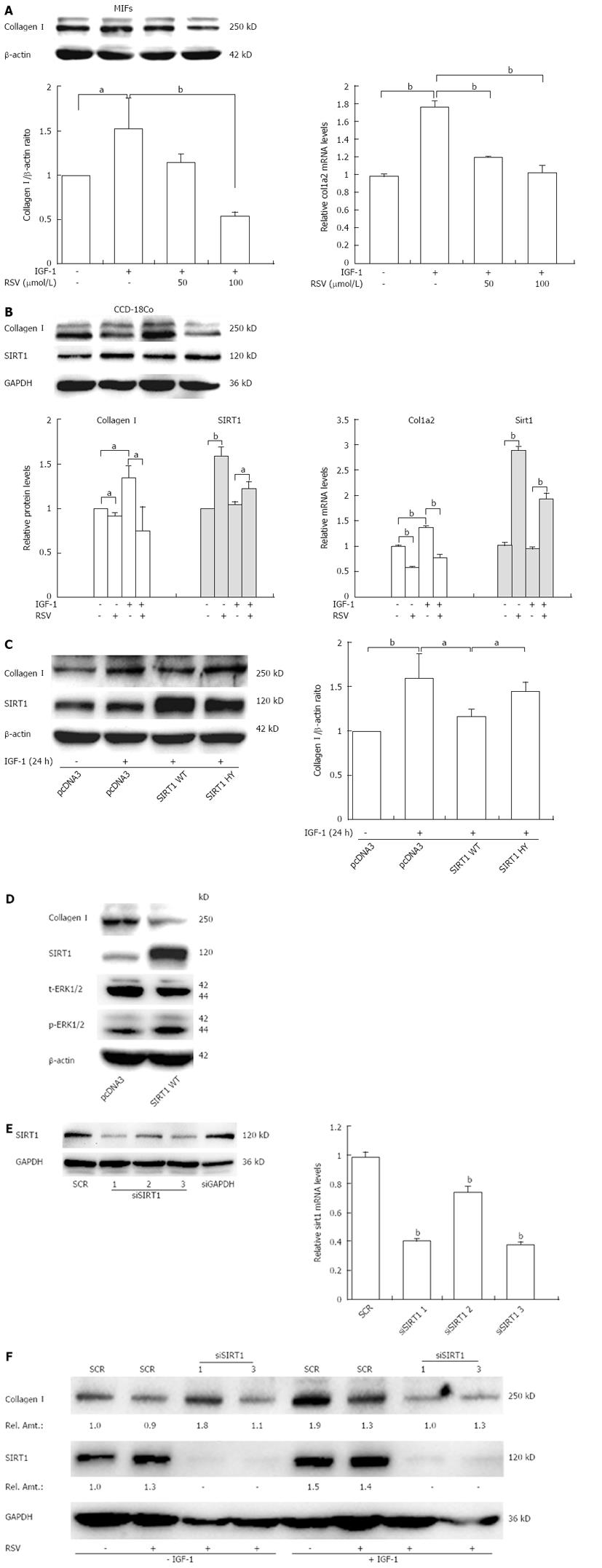
As shown in Figure 3A and B, resveratrol markedly decreased collagen I protein and mRNA levels induced by IGF-1 at a concentration of 100 μmol/L. In addition, resveratrol alone also inhibited collagen I synthesis (Figure 3B).
Next, to verify whether SIRT1 is required for resveratrol induced repression of collagen I, we performed the following experiments. First, fibroblasts were transfected with SIRT1 expression constructs (WT or HY) followed by IGF-1 treatment, and collagen I protein level was assessed. Overexpression of WT, but not enzyme deficient (HY) SIRT1, markedly decreased IGF-1-induced collagen I synthesis (Figure 3C). Overexpression of WT SIRT1 also led to the reduction of collagen I (Figure 3D). Second, we transfected specific siRNAs targeting SIRT1 into fibroblasts. To confirm the efficiency of siRNA-mediated SIRT1 knockdown, 293T cells were transiently transfected with the SIRT1 siRNA (siSIRT1 1, 2, 3) or scrambled siRNA with transfection reagents, and total cell lysates were prepared 48 h after transfection and used for Western blotting using anti-SIRT1 antibody or control anti-GAPDH antibody (Figure 3E). As shown in Figure 3F, depletion of SIRT1 by siRNA (1 and 3) in CCD-18Co cells blocked the reduction of collagen I expression induced by resveratrol. However, there was no effect of resveratrol on collagen I induced by IGF-1 (Figure 3F). Collectively, these results clearly documented that SIRT1 was partly involved in the resveratrol-dependent repression of collagen I.
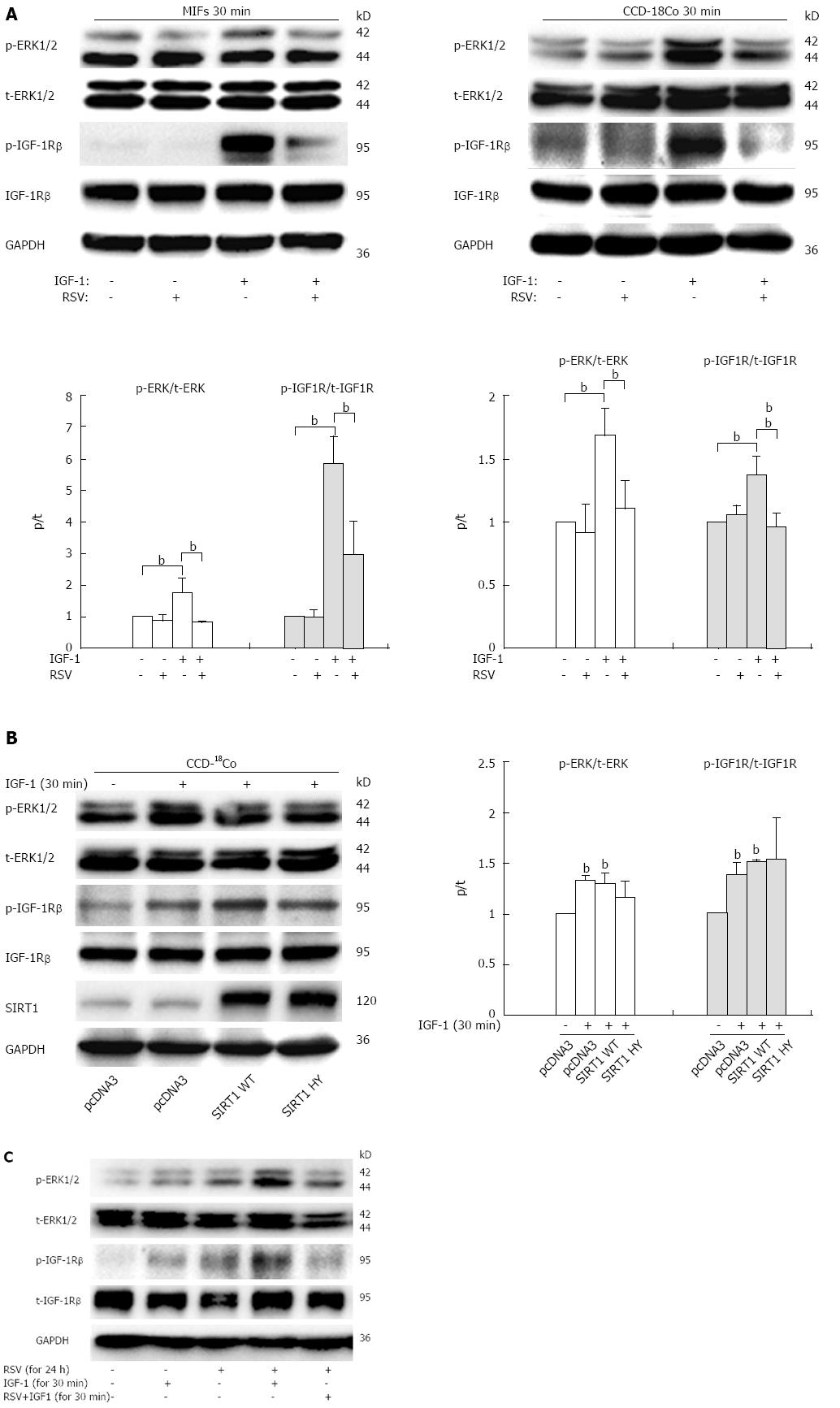
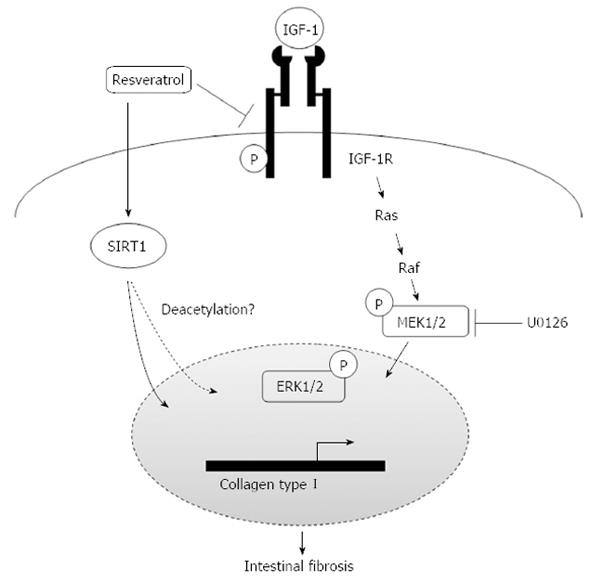
Our data so far demonstrate that IGF-1 promotes collagen I synthesis through the MAPK pathway, and resveratrol down-regulates collagen I expression induced by IGF-1. To investigate whether resveratrol suppressed the IGF-1/IGF-1R/ERK1/2 pathway, we next probed the effect of resveratrol on IGF-1R expression and phosphorylation with or without IGF-1 in both MIFs and CCD-18Co cells.
Resveratrol remarkably inhibited the phosphorylation of IGF-1R and ERK1/2 stimulated by IGF-1 for 30 min, and had no effect on the expression of total IGF-1R and ERK1/2 (Figure 4A), suggesting that resveratrol reduces IGF-1R activity and the intracellular ERK signaling cascade and thereby enhances the expression of collagen I. In addition, overexpression of WT or HY SIRT1 had no effect on phosphorylation of IGF1R (Figure 4B), and we did not observe any significant difference between SIRT1 WT and HY-transfected cells (Figure 4B). Since binding of IGF-1 to the IGF-1 receptor results in autophosphorylation of the receptor β subunits, and increased receptor tyrosine kinase activity, we further examined whether resveratrol affects the binding of IGF-1 to the IGF-1R. CCD-18Co cells were pretreated with resveratrol (100 μmol/L) for 24 h to induce SIRT1 expression, then removed and incubated with IGF-1 alone for another 30 min (Figure 4C, column 4) or IGF-1 and resveratrol for 30 min (Figure 4C, column 5), and the phosphorylation of IGF-1R and ERK1/2 was tested by immunoblot analysis. The results showed that phosphorylation levels of IGF-1R were down-regulated only after treatment with IGF-1 plus resveratrol for 30 min (column 5), compared with treatment with IGF-1 alone. In other words, resveratrol inhibited IGF-1R phosphorylation only when resveratrol was incubated together with IGF-1. Collectively, these data suggest that the repression of collagen I synthesis mediated by resveratrol can be partly attributed to the inhibition of IGF-1R/ERK1/2 signaling in a SIRT1 independent mechanism, and resveratrol may inhibited IGF-1 binding to its receptor.
Intestinal fibrosis in the form of fibrotic strictures is a well described complication of longstanding Crohn’s disease[34]. Currently, there are no inflammatory bowel disease therapies that have been shown to effectively decrease fibrosis, leaving surgery as the only treatment for symptomatic strictures[4]. It has long been documented that the accumulation of ECM in the intestinal wall contributes to intestinal remodeling and stricture formation. Since collagen I is one of the major matrix molecules involved in intestinal fibrogenesis, reduction and degradation of collagen I are a prominent treatment for CD intestinal fibrosis.
Resveratrol, a naturally occurring phytochemical, also known as SIRT1 activator, possesses anti-inflammatory and antioxidative effects[35,36]. In vivo studies have demonstrated the antifibrotic role of resveratrol in experimental colitis[28]. Increasing evidence has implicated the role of resveratrol in the regulation of inflammatory cytokines, profibrotic factors and procollagen[28]. These facts suggest that resveratrol could be utilized as a therapeutic against intestinal fibrosis. Results obtained by Susana Sánchez-Fidalgo et al[37] showed that dietary supplementation of resveratrol exerted a significant beneficial effect in chronic DSS-induced colitis. Larrosa et al[38] found that resveratrol pro-prodrugs prevented the rapid metabolism of resveratrol and delivered higher quantities of resveratrol to the colon in DSS-induced colitis. However, there are no preclinical and clinical studies that have shown that resveratrol can be used clinically in patients with intestinal fibrosis. Moreover, the rapid metabolism of resveratrol diminishes its effectiveness in the colon. According to previous studies, long-term epidemiologic studies and controlled clinical trials are also necessary for developing resveratrol to become a standard clinical agent.
According to previous studies, detailed investigations of the underlying mechanisms are limited. In this study, we reported several new findings that reveal the regulatory effect of resveratrol on collagen I synthesis and the mechanisms in intestinal fibroblasts. As a preliminary test, we initially induced intestinal fibrosis in rats with TNBS, and identified that collagen I and IGF-1 expression was significantly increased, but protein level of SIRT1 was decreased in colitis tissues. These data suggest that low expression of SIRT1 is related to intestinal fibrosis, and may explain the anti-fibrotic effect of resveratrol.
Based on these findings in vivo, we further researched the functions of resveratrol in vitro. We used intestinal fibroblasts for our subsequent experiments. Evidence suggests that the IGF system, including IGF-1, IGF-2, IGF-1R, and the IGF-binding proteins (IGFBPs, IGFBP1-6) play a crucial role in the gastrointestinal tract[15,39]. Since IGF-1 is regarded as a principal mediator of intestinal fibrosis, we investigated the effect of IGF-1 on collagen I synthesis in intestinal fibroblasts. Here, we reported that IGF-1 increased the protein and mRNA expression of collagen I in intestinal fibroblasts and that this upregulation was inhibited by pre-treatment with MEK1/2 inhibitor U0126. The phosphorylation of IGF-1R and ERK1/2 then gradually increased in a time-dependent manner after the incubation with IGF-1. Thus, these data suggest that IGF-1 stimulates collagen I synthesis in intestinal fibroblasts and that its action mechanism could be attributed to the IGF-1/IGF-1R/ERK1/2 pathway.
Previous in vitro studies have shown that resveratrol causes cell cycle arrest, decreased collagen synthesis, and apoptosis in rat intestinal smooth muscle cells[27]. We next explored the molecular mechanisms of anti-fibrosis effect of resveratrol in intestinal fibroblasts. Our experiments suggested that resveratrol significantly decreased collagen I expression induced by IGF-1 and resveratrol alone also inhibited collagen I synthesis. Resveratrol is known to activate deacetylase SIRT1, and this compound can also inhibit a number of other signaling pathways[40-42]. Several lines of evidence indicate that SIRT1 may play an important role in organ fibrosis. SIRT1 has been documented to inhibit tumor necrosis factor-α-induced inflammation in NIH/3T3 fibroblast cell line[43]. SIRT1 deacetylates smad3 and suppresses the transforming growth factor-β1-driven renal fibrosis[19]. Therefore, we used several approaches to examine whether the ability of resveratrol to inhibit collagen I operated via SIRT1. First, overexpression of WT but not HY SIRT1 provided a protective effect against IGF-1-induced collagen I synthesis in CCD-18Co. Second, knockdown of SIRT1 protein reversed the effect of resveratrol. These results indicate that resveratrol may protect against fibrosis through up-regulation of SIRT1 and enzymatic activity of SIRT1 may be responsible for its inhibitory effect on collagen I synthesis. Whether the mechanism underlying the effects is deacetylase activity of SIRT1 remains to be determined.
Since the IGF-1/IGF-1R/ERK1/2 signaling pathway has been previously described to be necessary and sufficient for the induction of collagen by IGF-1, we focused our investigation on the effect of resveratrol on IGF-1 signaling. Whether the expression and/or activity of IGF-1R were influenced by resveratrol? Our data demonstrated that treatment with resveratrol plus IGF-1 for 30 min inhibited the activation of IGF-1R and its downstream signaling molecules such as MAP-kinase (ERK1/2) in intestinal fibroblasts. Treatment with resveratrol for 24 h or overexpression of SIRT1 WT resulted in the down-regulation of collagen I expression in fibroblasts. However, overexpression of SIRT1 WT or HY had no effect on activation of IGF-1R. Thus, our findings suggest that resveratrol exerts its negative effect on the phosphorylation of IGF-1R independent of activating SIRT1, and resveratrol may regulate IGF-1R activity by directly inhibiting IGF-1 binding to its receptor. Further studies are needed to confirm these findings and elucidate the exact molecular mechanism underlying resveratrol-inhibited activation of IGF-1R.
In summary, our findings (Figure 5) allude to a scheme wherein upon challenge with IGF-1, collagen I expression is increased in intestinal fibroblasts, which can be repressed by resveratrol through either activating SIRT1 or inhibiting activation of IGF-1R. Collectively, these observations provide a new mechanistic framework to better understand the effects of SIRT1 activators on intestinal fibrosis, and will allow us to examine the possibility that dysregulation of the IGF-1/IGF-1R/ERK1/2 axis is involved in collagen synthesis in fibroblasts.
Intestinal fibrosis is an incurable complication of Crohn’s disease involving mesenchymal cell proliferation and extracellular matrix deposition. Until now, no effective therapy exists for averting such fibrogenic events. Insulin-like growth factor-1 (IGF-1), a potent profibrotic mediator, has been reported to be involved in gastrointestinal tract growth and tissue repair. Resveratrol is a polyphenol naturally occurring in grapes and its putative antifibrotic actions have been demonstrated in models of colitis.
Mounting evidence suggests that resveratrol has anti-inflammatory and antifibrotic effects in the animal models of colitis. Resveratrol diminishes IGF-1-stimulated collagen production in intestinal smooth muscle cells. However, the mechanism of resveratrol on collagen I synthesis in intestinal fibroblasts remains unclear.
In this study, the authors found that resveratrol down-regulated IGF-1-induced collagen I synthesis in intestinal fibroblasts by inhibiting IGF-1 receptor (IGF-1R) phosphorylation and its downstream extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinas signaling pathway. In addition, resveratrol alone suppressed collagen I synthesis through up-regulating activity of silent information regulator 1 (SIRT1).
These findings highlight a previously unknown function of resveratrol on IGF-1R activation and provide novel insight of resveratrol as a therapeutic agent for intestinal fibrosis.
SIRT1 is a nicotinamide adenine dinucleotide-dependent deacetylase which modulates metabolic homeostasis, stress resistance, cellular survival, cellular senescence/aging, inflammation-immune function, endothelial functions by deacetylating a number of key transcription factors.
The authors investigated the effect of resveratrol on collagen I synthesis in intestinal fibroblasts and explored the mechanism. Resveratrol effectively decreased collagen I expression in IGF-1-stimulated fibroblasts by inhibiting IGF-IR/ERK1/2 signaling in a SIRT1 independent manner. However, resveratrol alone inhibited collagen I synthesis by activating SIRT1. Overall, this manuscript is highly relevant and interesting.
P- Reviewers: Mihaila RG, Weng HL, Wong GLH S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 979] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 2. | Lichtenstein GR, Olson A, Travers S, Diamond RH, Chen DM, Pritchard ML, Feagan BG, Cohen RD, Salzberg BA, Hanauer SB. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn’s disease. Am J Gastroenterol. 2006;101:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255-281, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 189] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Froehlich F, Juillerat P, Mottet C, Pittet V, Felley C, Vader JP, Gonvers JJ, Michetti P. Fibrostenotic Crohn’s disease. Digestion. 2007;76:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis. 2004;10:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease - Current knowledge and future perspectives. J Crohns Colitis. 2008;2:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Pucilowska JB, Williams KL, Lund PK. Fibrogenesis. IV. Fibrosis and inflammatory bowel disease: cellular mediators and animal models. Am J Physiol Gastrointest Liver Physiol. 2000;279:G653-G659. [PubMed] |
| 8. | Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 248] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Papakrivopoulou J, Lindahl GE, Bishop JE, Laurent GJ. Differential roles of extracellular signal-regulated kinase 1/2 and p38MAPK in mechanical load-induced procollagen alpha1(I) gene expression in cardiac fibroblasts. Cardiovasc Res. 2004;61:736-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Lawrance IC, Maxwell L, Doe W. Inflammation location, but not type, determines the increase in TGF-beta1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis. 2001;7:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Szabò H, Fiorino G, Spinelli A, Rovida S, Repici A, Malesci AC, Danese S. Review article: anti-fibrotic agents for the treatment of Crohn’s disease - lessons learnt from other diseases. Aliment Pharmacol Ther. 2010;31:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Pucilowska JB, McNaughton KK, Mohapatra NK, Hoyt EC, Zimmermann EM, Sartor RB, Lund PK. IGF-I and procollagen alpha1(I) are coexpressed in a subset of mesenchymal cells in active Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1307-G1322. [PubMed] |
| 13. | Sipos F, Galamb O, Herszényi L, Molnár B, Solymosi N, Zágoni T, Berczi L, Tulassay Z. Elevated insulin-like growth factor 1 receptor, hepatocyte growth factor receptor and telomerase protein expression in mild ulcerative colitis. Scand J Gastroenterol. 2008;43:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | El Yafi F, Winkler R, Delvenne P, Boussif N, Belaiche J, Louis E. Altered expression of type I insulin-like growth factor receptor in Crohn’s disease. Clin Exp Immunol. 2005;139:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kuemmerle JF. Insulin-like growth factors in the gastrointestinal tract and liver. Endocrinol Metab Clin North Am. 2012;41:409-23, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Xin X, Hou YT, Li L, Schmiedlin-Ren P, Christman GM, Cheng HL, Bitar KN, Zimmermann EM. IGF-I increases IGFBP-5 and collagen alpha1(I) mRNAs by the MAPK pathway in rat intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G777-G783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Kuemmerle JF. Endogenous IGF-I protects human intestinal smooth muscle cells from apoptosis by regulation of GSK-3 beta activity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G101-G110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 528] [Cited by in RCA: 503] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 19. | Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol. 2010;177:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Kuruş M, Ugras M, Esrefoglu M. Effect of resveratrol on tubular damage and interstitial fibrosis in kidneys of rats exposed to cigarette smoke. Toxicol Ind Health. 2009;25:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Sutra T, Oiry C, Azay-Milhau J, Youl E, Magous R, Teissèdre PL, Cristol JP, Cros G. Preventive effects of nutritional doses of polyphenolic molecules on cardiac fibrosis associated with metabolic syndrome: involvement of osteopontin and oxidative stress. J Agric Food Chem. 2008;56:11683-11687. [PubMed] [DOI] [Full Text] |
| 22. | Lee ES, Shin MO, Yoon S, Moon JO. Resveratrol inhibits dimethylnitrosamine-induced hepatic fibrosis in rats. Arch Pharm Res. 2010;33:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Chávez E, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. Resveratrol prevents fibrosis, NF-kappaB activation and TGF-beta increases induced by chronic CCl4 treatment in rats. J Appl Toxicol. 2008;28:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Youn J, Lee JS, Na HK, Kundu JK, Surh YJ. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr Cancer. 2009;61:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS. Resveratrol (trans-3,5,4’-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332:829-839. [PubMed] |
| 26. | Martín AR, Villegas I, La Casa C, de la Lastra CA. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol. 2004;67:1399-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | Garcia P, Schmiedlin-Ren P, Mathias JS, Tang H, Christman GM, Zimmermann EM. Resveratrol causes cell cycle arrest, decreased collagen synthesis, and apoptosis in rat intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2012;302:G326-G335. [PubMed] |
| 28. | Rahal K, Schmiedlin-Ren P, Adler J, Dhanani M, Sultani V, Rittershaus AC, Reingold L, Zhu J, McKenna BJ, Christman GM. Resveratrol has antiinflammatory and antifibrotic effects in the peptidoglycan-polysaccharide rat model of Crohn’s disease. Inflamm Bowel Dis. 2012;18:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Hazelgrove KB, Flynn RS, Qiao LY, Grider JR, Kuemmerle JF. Endogenous IGF-I and alpha v beta3 integrin ligands regulate increased smooth muscle growth in TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1230-G1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722-1734. [PubMed] |
| 31. | Leeb SN, Vogl D, Falk W, Schölmerich J, Rogler G, Gelbmann CM. Regulation of migration of human colonic myofibroblasts. Growth Factors. 2002;20:81-91. [PubMed] |
| 32. | Leeb SN, Vogl D, Gunckel M, Kiessling S, Falk W, Göke M, Schölmerich J, Gelbmann CM, Rogler G. Reduced migration of fibroblasts in inflammatory bowel disease: role of inflammatory mediators and focal adhesion kinase. Gastroenterology. 2003;125:1341-1354. [PubMed] |
| 33. | Wu X, Kong X, Chen D, Li H, Zhao Y, Xia M, Fang M, Li P, Fang F, Sun L. SIRT1 links CIITA deacetylation to MHC II activation. Nucleic Acids Res. 2011;39:9549-9558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182-205. [PubMed] |
| 35. | Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020-1035. [PubMed] |
| 36. | de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005;49:405-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 508] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 37. | Sánchez-Fidalgo S, Cárdeno A, Villegas I, Talero E, de la Lastra CA. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur J Pharmacol. 2010;633:78-84. [PubMed] |
| 38. | Larrosa M, Tomé-Carneiro J, Yáñez-Gascón MJ, Alcántara D, Selma MV, Beltrán D, García-Conesa MT, Urbán C, Lucas R, Tomás-Barberán F. Preventive oral treatment with resveratrol pro-prodrugs drastically reduce colon inflammation in rodents. J Med Chem. 2010;53:7365-7376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Zimmermann EM, Li L, Hou YT, Cannon M, Christman GM, Bitar KN. IGF-I induces collagen and IGFBP-5 mRNA in rat intestinal smooth muscle. Am J Physiol. 1997;273:G875-G882. [PubMed] |
| 40. | Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340-8351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 711] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 41. | Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis. 2006;27:1465-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 42. | Stewart JR, O’Brian CA. Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKC alpha inhibition. Invest New Drugs. 2004;22:107-117. [PubMed] |
| 43. | Zhu X, Liu Q, Wang M, Liang M, Yang X, Xu X, Zou H, Qiu J. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS One. 2011;6:e27081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |