Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4636
Revised: November 29, 2013
Accepted: January 2, 2014
Published online: April 28, 2014
Processing time: 194 Days and 10.1 Hours
AIM: To investigate the protective effects of combinations of probiotic (Bifico) on interleukin (IL)-10-gene-deficient (IL-10 KO) mice and Caco-2 cell monolayers.
METHODS: IL-10 KO mice were used to assess the benefits of Bifico in vivo. IL-10 KO and control mice received approximately 1.5 × 108 cfu/d of Bifico for 4 wk. Colons were then removed and analyzed for epithelial barrier function by Ussing Chamber, while an ELISA was used to evaluate proinflammatory cytokines. The colon epithelial cell line, Caco-2, was used to test the benefit of Bifico in vitro. Enteroinvasive Escherichia coli (EIEC) and the probiotic mixture Bifico, or single probiotic strains, were applied to cultured Caco-2 monolayers. Barrier function was determined by measuring transepithelial electrical resistance and tight junction protein expression.
RESULTS: Treatment of IL-10 KO mice with Bifico partially restored body weight, colon length, and epithelial barrier integrity to wild-type levels. In addition, IL-10 KO mice receiving Bifico treatment had reduced mucosal secretion of tumor necrosis factor-α and interferon-γ, and attenuated colonic disease. Moreover, treatment of Caco-2 monolayers with Bifico or single-strain probiotics in vitro inhibited EIEC invasion and reduced the secretion of proinflammatory cytokines.
CONCLUSION: Bifico reduced colon inflammation in IL-10 KO mice, and promoted and improved epithelial-barrier function, enhanced resistance to EIEC invasion, and decreased proinflammatory cytokine secretion.
Core tip: We investigated the protective effects of combinations of probiotic bacteria (Bifico) on interleukin (IL)-10 gene-deficient (IL-10 KO) mice and Caco-2 cell monolayers. Treatment of IL-10 KO mice with Bifico partially restored body weight, colon length, and epithelial barrier integrity to wild-type levels. Treatment of Caco-2 monolayers with Bifico or single-strain probiotics inhibited enteroinvasive Escherichia coli (EIEC) invasion and reduced secretion of proinflammatory cytokines. Oral administration of Bifico reduced colon inflammation, and directly promoted epithelial barrier function. In addition, Bifico improved epithelial barrier function, and enhanced resistance to EIEC invasion in vitro.
- Citation: Shi CZ, Chen HQ, Liang Y, Xia Y, Yang YZ, Yang J, Zhang JD, Wang SH, Liu J, Qin HL. Combined probiotic bacteria promotes intestinal epithelial barrier function in interleukin-10-gene-deficient mice. World J Gastroenterol 2014; 20(16): 4636-4647
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4636.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4636
The human gut is colonized with a wide variety of microorganisms, including pathogenic, probiotic and commensal bacteria. Bifidobacterium, Lactobacillus and Enterococcus faecalis are probiotics with beneficial effects on maintenance therapy of human intestinal diseases[1-3]. For example, oral treatment with specific probiotic bacteria can ameliorate inflammatory bowel disease (IBD). In addition, multiple studies have demonstrated that colonization strategies using defined commensals or exogenous specific probiotic treatment may prevent host intestinal inflammation and ameliorate intestinal epithelial barrier function[3-9].
The intestinal barrier prevents microbial contamination of interstitial tissues. Tight junctions play an important role in modulating intestinal epithelial paracellular permeability[10] and promoting defenses against harmful molecules and microorganisms. Tight junctions are common targets of enteric pathogens, and their disruption occurs with IBD and/or diarrhea[11]. For example, many virulence genes of bacteria encode toxins and other proteins that either directly disassemble tight junction proteins[12,13] or modulate intracellular pathways that lead to tight junction redistribution[14,15]. Enteroinvasive Escherichia coli (EIEC)[12,13], Salmonella typhimurium[16], Shigella flexneri[17,18], and Campylobacter jejuni[14,15] all infect host cells by targeting the paracellular pathway.
Past studies have demonstrated that IBD patients have reduced bifidobacteria and lactobacilli in their gut microbiota[19,20] suggesting these patients may benefit from probiotic treatment. Indeed, recent clinical trials have confirmed the therapeutic effects of probiotics in virus-, bacterium-induced intestinal infections and antibiotic-induced diarrhea[21-23]. Among the most distinctive benefits of probiotic bacteria are modulation of host defense responses, and protection against infectious diseases[24,25]. However, the molecular mechanisms underlying these effects have not fully been elucidated.
The probiotic compound, Bifico (Bifico Pharmaceuticals, Sine, Shanghai, China), contains about 1.0 × 109 cfu/g of viable lyophilized bifidobacteria (Bifidobacterium longum), 1.0 × 109 cfu/g lactobacilli (Lactobacillus acidophilus), and 1.0 × 109 cfu/g Ent. faecalis. This probiotic combination has been effective in the maintenance therapy of diarrhea induced by intestinal flora disturbance or enteritis. However, the use of Bifico as a primary therapy for IBD has not yet been investigated. To address this issue, we treated interleukin (IL)-10-gene-deficient (IL-10 KO) with Bifico and monitored the presence of IBD, which spontaneously develops in these mice. In addition, Caco-2 cells were cultured in vitro with EIEC with or without Bifico pretreatment to monitor EIEC invasion. Bifico had a direct effect on epithelial barrier function in vivo by reducing mucosal secretion of tumor necrosis factor (TNF)-α and interferon (IFN)-γ, and altered the expression and distribution of tight junction proteins. Bifico exposure in vitro reduced bacterial invasion. Moreover, the effects of combined probiotics were more pronounced than single-strain probiotics.
Homozygous IL-10 KO mice, generated on a 129 Sv/Ev background, and normal 129 Sv/Ev controls (Jackson Laboratory, Bar Harbor, ME, United States) were housed under specific-pathogen-free conditions in Shanghai Jiao Tong University Medical School. Mice were fed a standard sterile diet and filtered water ad libitum under a 12-h light-dark cycle. Animal studies were approved by the Ethical Committee of the Affiliated Sixth People’s Hospital of Shanghai Jiao Tong University.
Ten-week-old female IL-10 KO mice (n = 12) and wild-type (WT) controls (n = 12) were randomized and divided into two groups each. Mice then either received a daily oral gavage of Bifico dissolved in 0.5 mL physiological saline at 3.0 × 108 cfu/mL or saline alone for 4 wk.
Mice were sacrificed following Bifico therapy, and a segment of the colon was removed for mucosa isolation from the muscular layer. Mucosal cells were mounted in Lucite Chambers, exposing mucosal and submucosal surfaces to 10 mL oxygenated Krebs Buffer (EasyMount-CSYS-8 Using Chamber Systems; San Diego, CA, United States). The buffers were maintained at 37 °C by a heated water jacket and circulated in CO2/O2[26]. The nonabsorbable tracer molecule inulin-FITC (2000-5000 kDa, Sigma-Aldrich, St Louis, MO, United States) (1.0 mg/mL) was then added to the mucosal side of the Lucite Chambers. At 0, 30, 60, 90 and 120 min following addition of inulin-FITC, 100 μL buffer from the submucosal side of the Lucite Chambers was collected and analyzed for fluorescence in black-walled 96-well plates (Costar, Corning, NY, United States) using a Spectral Scanning Multimode Reader (Thermo Scientific Varioskan Flash, Vantaa, Finland) at an excitation wavelength of 485 nm and emission at 530 nm[27,28]. Standard curves were obtained by diluting inulin-FITC in Krebs Buffer. The barrier function of the intestinal epithelium was also determined by measuring transepithelial electrical resistance (TER) (multi-channel voltage current clamp, VCC MC8, San Diego, CA, United States).
Mice were sacrificed after 4 wk Bifico treatment. Colons were harvested and fixed in 10% phosphate-buffered formalin. The samples were paraffin-embedded, sectioned at 5 µm, and stained with hematoxylin and eosin (HE) for microscopic examination and imaging (Nikon Eclipse 80i, Tokyo, Japan). The slides were reviewed in a blinded fashion by two pathologists and were assigned a histological score for intestinal inflammation based on previously described criteria[29,30]. No inflammation was scored as 0; modest numbers of infiltrating cells in the lamina propria as 1; infiltration of mononuclear cells leading to separation of crypts and mild mucosal hyperplasia as 2; massive infiltration with inflammatory cells accompanied by disrupted mucosal architecture, loss of goblet cells, and marked mucosal hyperplasia as 3; these issues plus crypt abscesses or ulceration as 4; with a total score from 0 to 15.
To observe the ultrastructural changes of tight junctions, colonic segments were prepared for transmission electron microscopy (JEM1230 Electro-microscope; JEOL, Tokyo, Japan) as previously described[8].
The serum levels of TNF-α and IFN-γ were measured using ELISA kits (BD Pharmingen, Oxford, United Kingdom) as previously described[8]. The levels of the cytokines TNF-α, macrophage inflammatory protein (MIP)-1α, IL-6 and IL-8 in the supernatant of cultured Caco-2 monolayers were measured using Sandwich ELISA kits (R&D, Minneapolis, MN, United States) according to manufacturer’s instructions.
Caco-2 cells (Shanghai Institute of Cell Biology, Chinese Academy of Science) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Invitrogen, Carlsbad, CA, United States) supplemented with 100 mL/L heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were cultured in 25-cm2 flat-bottom culture flasks (Corning, Corning, NY, United States) and seeded onto Transwell semipermeable filters (filter grown; 1.12 cm2 polyester membranes, 3.0 µm pore size, 12 well) in Transwell units (Costar; Corning). The barrier function of tight junctions in Caco-2 monolayers was determined by measuring TER.
B. longum, L. acidophilus, and Ent. faecalis were obtained from Shanghai Sine Pharmaceutical Co. Ltd. EIEC (O124: NM, ATCC 43893) was obtained from the Shanghai Municipal Center for Disease Control and Prevention. B. longum was cultured in tryptone polypeptone yeast extract broth agar (Shanghai Sine Pharmaceutical Co. Ltd) at 37 °C. Ent. faecalis was cultured in Slanetz and Bartley agar (Shanghai Sine Pharmaceutical Co. Ltd.) at 37 °C. L. acidophilus was cultured in MRS agar (Merck, Darmstadt, Germany) at 37 °C. EIEC was cultured in LB agar (Oxoid, Hampshire, United Kingdom) at 37 °C. Bacteria were added to DMEM and then subjected to photoelectric colorimeter to measure consistency.
Caco-2 cells were washed three times in Hank’s Solution to remove antibiotics. The inoculation ratio of EIEC to Caco-2 cells was approximately 100:1. Prior to EIEC infection, four groups of Caco-2 monolayers were incubated with B. longum (B), Ent. faecalis (F), L. acidophilus (L) or the triple bacteria (BFL) for 30 min. The ratio of probiotics to EIEC was 10:1. EIEC was allowed to infect Caco-2 monolayers for 24 h. TER of Caco-2 monolayers was measured with a voltmeter (Millicell-ERS; Millipore, CA, United States) for 6 h. Untreated Caco-2 monolayers served as a control (C). Caco-2 cells infected with EIEC alone served as the EIEC (E) group. Incubation medium was collected and processed for cytokine analysis using Sandwich ELISA kits (R&D).
Tissue total RNA (SLNco, Shanghai, China) was extracted and analyzed by qRT-PCR (Funglyn, Ontario, Canada) as previously described[8,13]. Rabbit polyclonal antibodies against zonula occludentes 1 (ZO-1, Invitrogen), claudin-1 (Invitrogen) and occludin (Invitrogen) were used in western blotting and immunofluorescence assays according to the manufacturer’s instructions.
Data were analyzed using the GraphPad Prism 5 software (San Diego, CA, United States) and expressed as mean ± SEM. Differences in parametric data were evaluated by Student’s two-tailed unpaired t test. Differences with P < 0.05 were considered statistically significant.
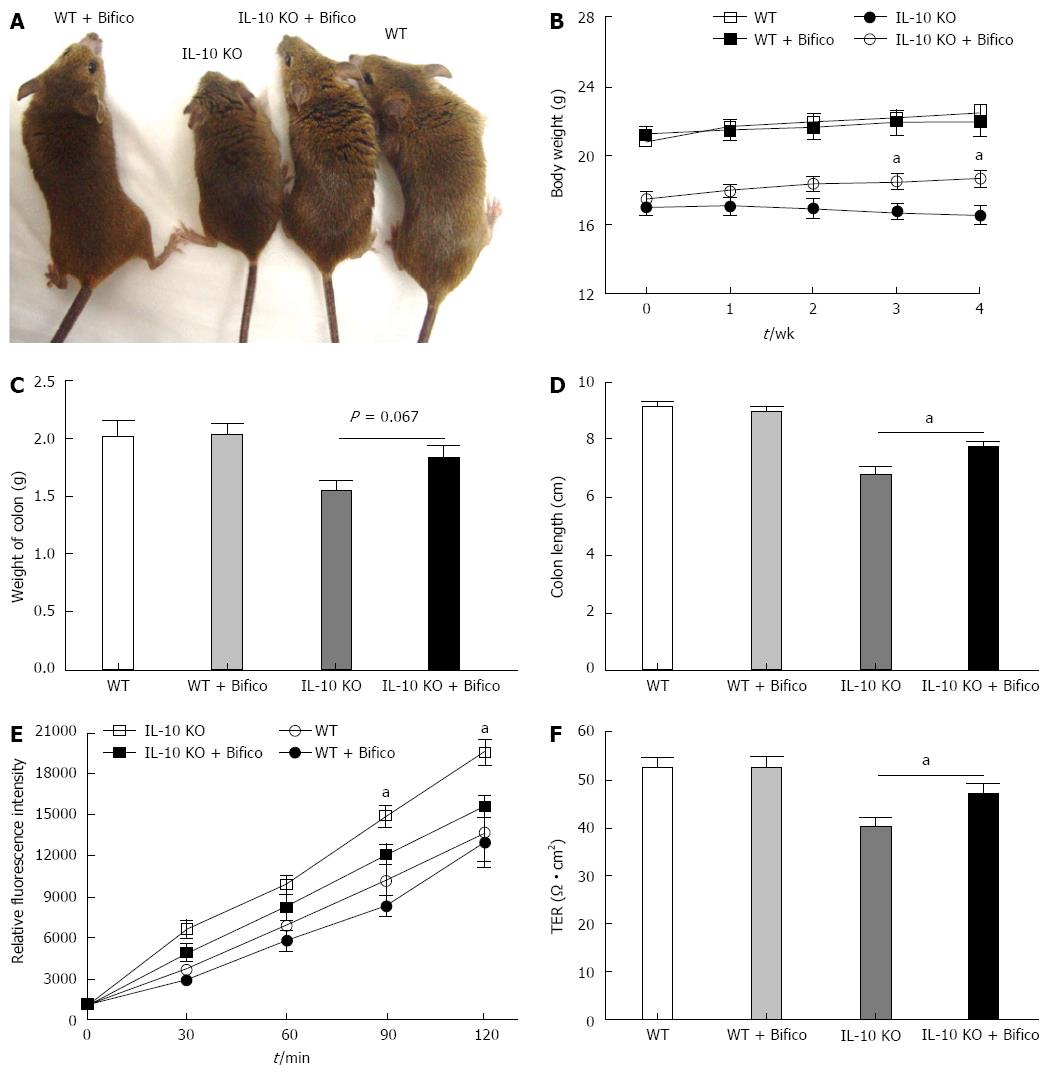
Bifico therapy in IL-10 KO mice was used to assess the effects of probiotics in reducing IBD. As seen in the representative photomicrographs of Figure 1, the morbidity in IL-10 KO mice was greater than in IL-10 KO mice treated with Bifico (Figure 1A). During the 4-wk observation, IL-10 KO mice displayed a significant reduction in body weight compared to WT mice. However, Bifico treatment restored the body weight in IL-10 KO mice (Figure 1B). In addition, the presence of diarrhea was greater in IL-10 KO mice than in mice receiving Bifico treatment. The colonic weight did not differ significantly between Bifico-treated or untreated IL-10 KO mice (Figure 1C); however, the colons of IL-10 KO mice were significantly shorter than those of IL-10 KO receiving Bifico treatment (Figure 1D).
To assess the effects of Bifico on colon function, we measured colonic permeability by Ussing Chamber. IL-10 KO mice exhibited a significant increase in the cumulative permeation of inulin-FITC through the colonic mucosa compared with WT and Bifico-treated WT mice (Figure 1E). In accordance with increased inulin-FITC permeability, a significant decrease of TER was observed in IL-10 KO mice (Figure 1F). However, Bifico treatment restored colon function in IL-10 KO mice (Figure 1E and F).
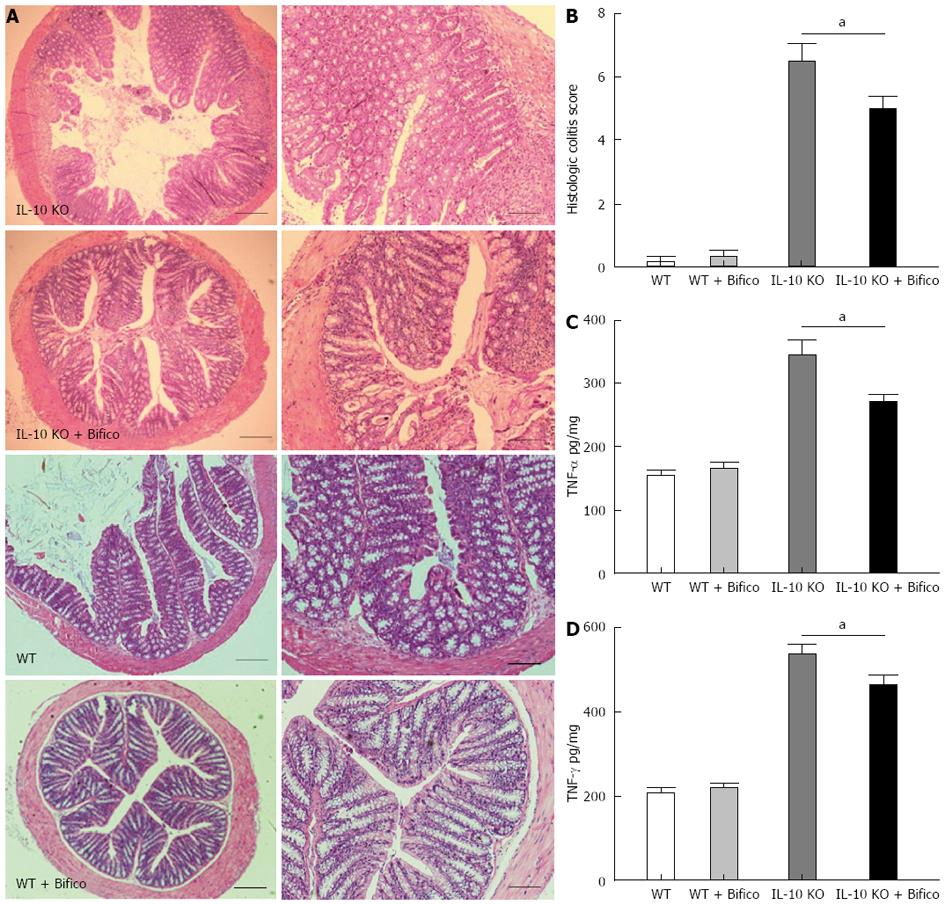
To evaluate further the effects of Bifico in vivo, mice were sacrificed following Bifico therapy and mucosal cells were evaluated by histology. HE staining revealed a large amount of mucosal damage and inflammatory cell infiltration in the lamina propria of IL-10 KO mice (Figure 2A). In addition, all IL-10 KO mice developed colitis, and the inflammatory score was significantly greater in IL-10 KO than WT mice. However, IL-10 KO mice receiving Bifico treatment had reduced inflammatory scores with only mild cell infiltration and mucosal damage (Figure 2B).
We next evaluated the secretion of proinflammatory cytokines from colonic tissue ex vivo. Expression of TNF-α and IFN-γ in the colonic mucosa was significantly increased in IL-10 KO mice (Figure 2C and D). However, 4 wk Bifico treatment significantly reduced the levels of TNF-α and IFN-γ (Figure 2C and D). These data suggest that Bifico treatment reduces the induction of IBD in IL-10 KO mice.
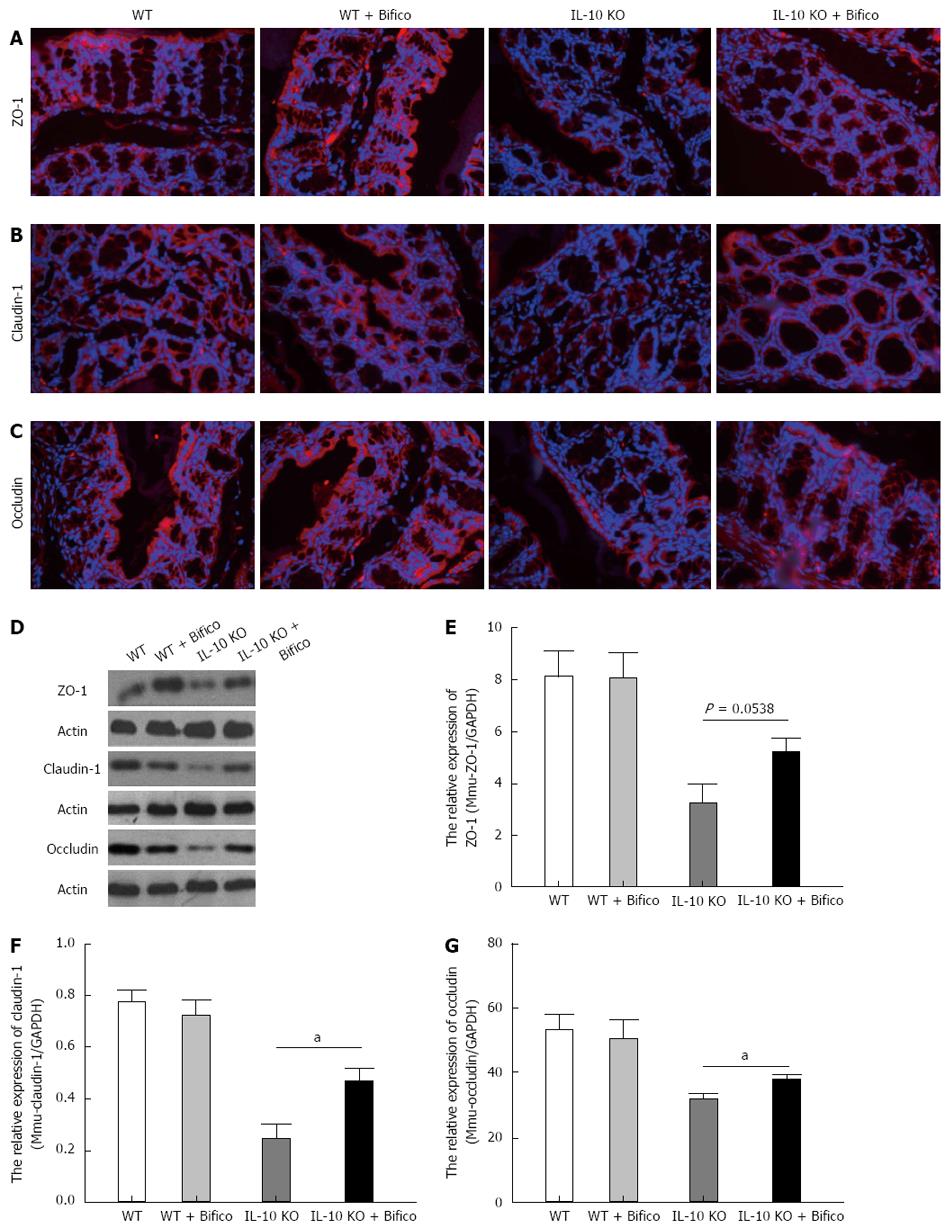
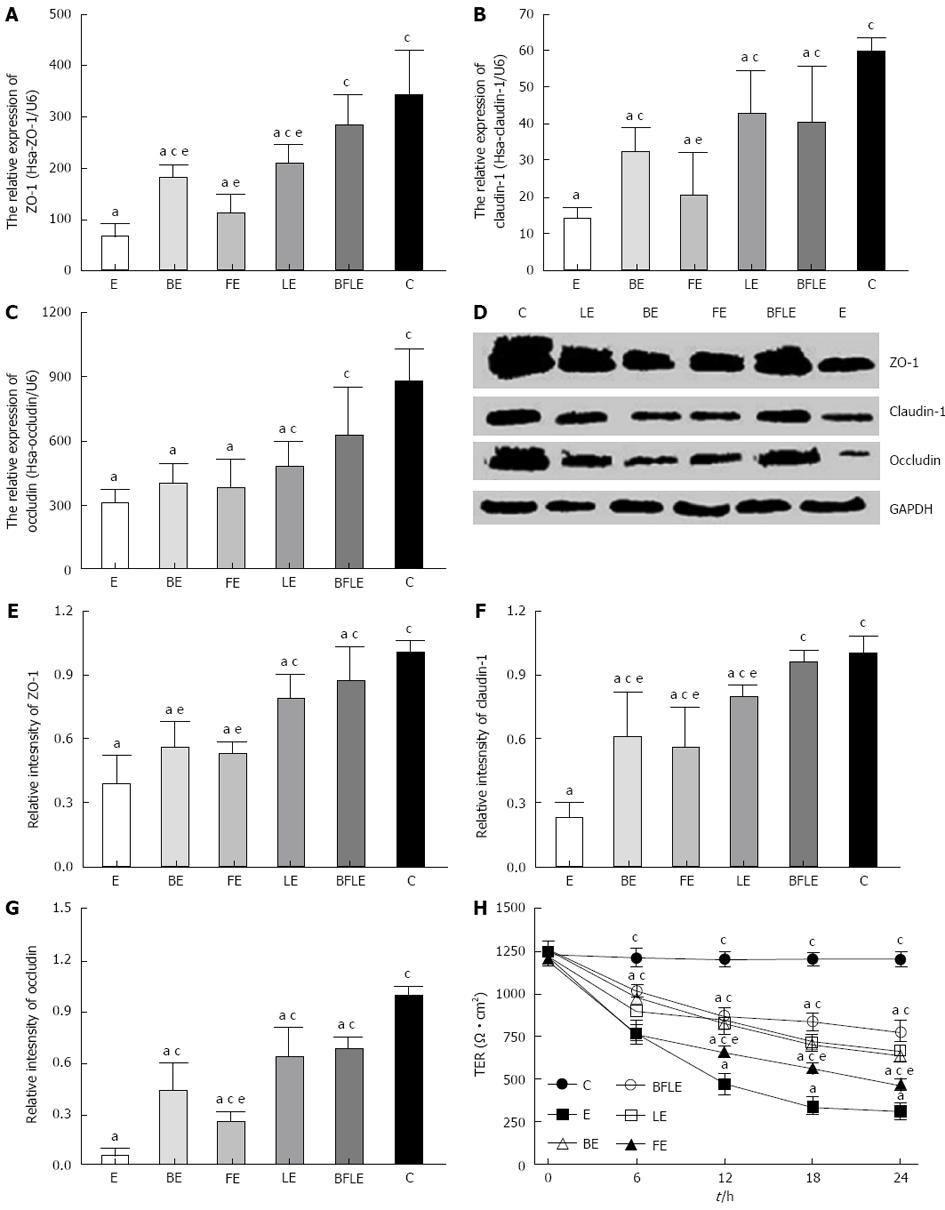
To identify whether there were changes in apical junction protein expression and distribution following Bifico treatment, immunofluorescence, western blotting and real-time qRT-PCR assays of ZO-1, claudin-1 and occludin were performed on the colonic tissue. There were significantly decreased in the expression of protein and mRNA of ZO-1, claudin-1 and occludin. As expected, the network of ZO-1, claudin-1 and occludin was predominantly intact and localized along the apical cellular border in WT mice. In IL-10 KO mice, the expression of ZO-1, claudin-1 and occludin at the apical cellular border was decreased, discontinuous, and redistributed. Bifico treatment ameliorated these changes in IL-10 KO mice (Figure 3A-C).
Western blotting and RT-PCR showed that the protein and mRNA levels of ZO-1, claudin-1 and occludin were reduced in IL-10 KO mice when compared with WT mice (Figure 3D). Four weeks of Bifico therapy restored the protein and mRNA levels of ZO-1, claudin-1 and occludin in IL-10 KO mice (Figure 3E-G). These data suggest that Bifico treatment promotes proper tight junction protein expression and distribution.
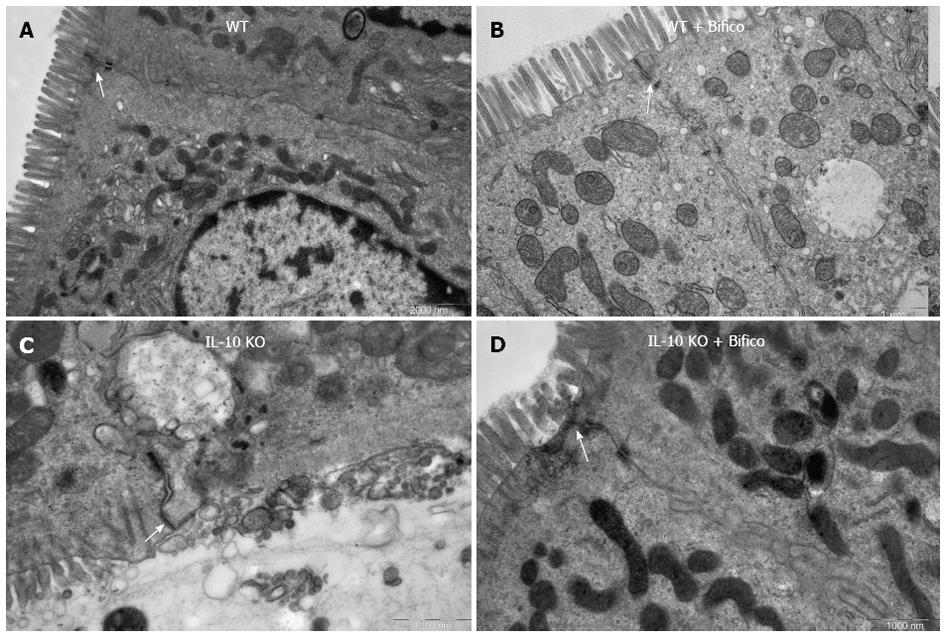
Colonic epithelial paracellular permeability is controlled mainly by tight junction proteins. Given that Bifico treatment restored tight junction distribution, we examined the ultrastructural changes of intercellular tight junction proteins by transmission electron microscopy. Compared with WT controls (Figure 4A and B), the colonic epithelium of IL-10 KO mice displayed significant ultrastructural changes in tight junction proteins and microvilli, along with marked increases in intercellular gaps and intracellular vacuolization (Figure 4C). However, Bifico therapy increased intact intercellular tight junctions and microvilli and decreased intercellular gaps and intracellular vacuolization (Figure 4D).
Caco-2 monolayers were incubated with EIEC alone, or were pretreated with Bifico or single-strain probiotics for 24 h. Real-time qRT-PCR was then performed to analyze the expression of mRNA of ZO-1, claudin-1 and occludin in treated and untreated Caco-2 monolayers. Expression of ZO-1, claudin-1 and occludin mRNA was significantly decreased after treatment with EIEC in comparison to untreated controls. The decrease in tight junction proteins was inhibited by pretreatment with probiotics, with Bifico being the most effective (Figure 5A-C).
We next used western blotting to determine the relative proteins levels of ZO-1, claudin-1 and occludin in control and EIEC-treated Caco-2 monolayers incubated with or without various probiotics. Levels of tight junction proteins were markedly decreased after treatment with EIEC compared to untreated controls (Figure 5D-G). However, preincubation of Caco-2 cells with Bifico or single-strain probiotics significantly inhibited the reduction of tight junction proteins after EIEC infection, with Bifico being the most effective (Figure 5D-G). These data suggest that Bifico therapy can prevent EIEC infection by promoting tight junction protein expression.
To investigate the effect of probiotics on EIEC-treated Caco-2 monolayers, we measured the TER of untreated and treated Caco-2 monolayers. Caco-2 monolayers were incubated with EIEC alone, or were pretreated with Bifico or single-strain probiotics for 30 min and then incubated with EIEC for 24 h. TER was measured for 6 h. Infection with EIEC resulted in an about 80% decrease in TER when compared with the untreated group. However, preincubation of Caco-2 monolayers with Bifico or single-strain probiotics attenuated TER reduction, with Bifico treatment being the most effective (Figure 5H).
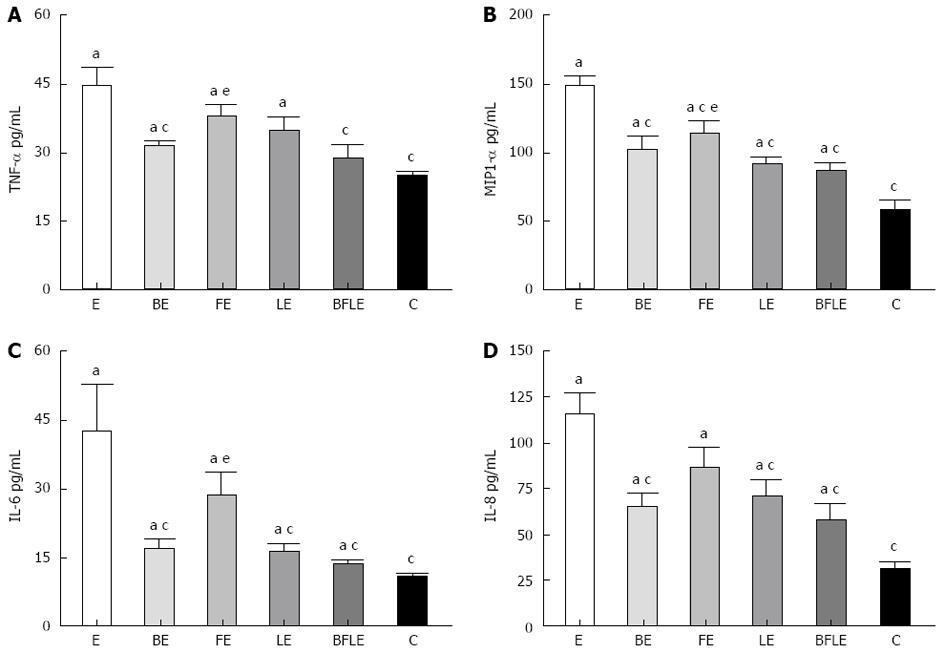
To address further the effects of probiotics on EIEC invasion, we measured the cytokine response in EIEC-treated Caco-2 monolayers. Caco-2 monolayers were incubated as described above, and the cytokines in the cell culture supernatant were evaluated by ELISA. The proinflammatory cytokine release from EIEC-treated Caco-2 monolayers was significantly inhibited by pretreatment with Bifico or single-strain probiotics (Figure 6). In addition, Bifico treatment was the most effective.
In the present study, we demonstrated that treating IL-10 KO mice with the probiotic mixture, Bifico, partly recovered colonic barrier integrity, reduced mucosal secretion of proinflammatory cytokines, and attenuated histopathological changes. Furthermore, in vitro studies revealed that epithelial barrier function and resistance to EIEC invasion was enhanced following exposure to Bifico.
The intestinal luminal microflora and their products are important initiating and modulating factors in the pathogenesis of IBD and some diarrhea diseases in humans and animals[31-34]. IL-10 KO mice spontaneously develop IBD, and this is associated with altered colonic microflora colonization[35]. These data suggest that probiotic therapy could reduce IBD in theses mice. Indeed, inoculation of Lactobacillus sp. reduced IBD in IL-10 KO mice. In the present study, we demonstrated that IBD was reduced in IL-10 KO mice by the probiotic mixture Bifico, and this reduction was greater than with the use of single-strain probiotics. These data are consistent with other in vivo experiments using VSL#3 (VSL Pharmaceuticals, Gaithersburg, MD, contains 9 × 1010 cfu/g of viable, lyophilized bifidobacteria (B. longum, Bifidobacterium infantis, and Bifidobacterium breve), 8 × 1010 lactobacilli (L. acidophilus, Lactobacillus casei, Lactobacillus delbrueckii subsp. L. bulgaricus, and Lactobacillus plantarum), and 20 × 1010Streptococcus salivarius subsp. Thermophilus) compound strains, which are more effective than the use of a single Lactobacillus in improving colitis and normalizing epithelial function[3]. These data suggest that combinations of adherent probiotic strains can influence the adhesion and activity of other strains in the human intestinal tract[36]. Our study is believed to be the first to show that Bifico therapy is effective in ameliorating epithelial damage and restoring epithelial function in both in vivo and in vitro models.
In IL-10 KO mice, colonic inflammation is related to high levels of mucosal TNF-α and IFN-γ[32]. Recently, multiple studies have characterized the ability of various strains of probiotics to alter the activity and cytokine expression in the colons of IL-10 KO mice[3,8,37,38]. TNF-α and IFN-γ produced by colonic mucosa are both normalized when IL-10 KO mice are raised under germ-free conditions[32], suggesting that colitis in IL-10 KO mice occurs as a consequence of a Th1-predominant intestinal inflammation in the presence of the gut flora[3,8,37,38]. In present study, treating IL-10 KO mice with the probiotic mixture Bifico attenuated TNF-α and IFN-γ secretion. In addition, pretreatment of Caco-2 cells with Bifico significantly inhibited the secretion of TNF-α, MIP-1α, IL-6 and IL-8 following EIEC infection. These results indicate that the gut is able to discriminate and define selective responses to different bacteria.
To reduce inflammation, the normal intestinal barrier is impermeable to bacteria, bacterial products, and dietary antigens that are present within the lumen. Thus, probiotic bacteria may exert protection through enhancing intestinal barrier function, including decreasing intestinal permeability and maintaining normal expression and distribution of tight junction proteins[6,8]. IL-10 KO mice demonstrated increased colonic permeability that is absent in mice raised under germ-free conditions[32]. Bifico treatment, however, reduced epithelial permeability in IL-10 KO mice, and increased expression of tight junction proteins in both colonic epithelia in vivo and in EIEC-treated Caco-2 monolayers. Moreover, the use of the Bifico therapy was more effective than the use of a single-strain probiotics in preventing disruption and normalizing function of Caco-2 monolayers following EIEC infection.
Although Bifico therapy was the most effective, single-strain probiotics were also effective in restoring Caco-2 cells function following EIEC infection. These data suggest that epithelial cells may respond directly to certain probiotic bacteria. Certain strains of Lactobacillus, for example, release surface-active components, which inhibit adhesion of pathogenic bacteria[38]. Thus, probiotic bacteria may protect epithelium by receptor competition, whereby probiotics compete with microbial pathogens for a limited number of receptors present on the surface epithelium.
In conclusion, the probiotic mixture, Bifico, is highly effective in reducing colitis in IL-10 KO mice. Furthermore, the present study revealed that Bifico treatment reduced colonic epithelial permeability, recovered normal expression and distribution of tight junction proteins, and protected against pathogenic bacterial invasion both in vivo and in vitro.
The human gut is colonized with a wide variety of microorganisms, including pathogenic, probiotic and commensal bacteria. Bifidobacterium, Lactobacillus and Enterococcus faecalis are probiotics with beneficial effects on maintenance therapy of human intestinal diseases. For example, oral treatment with specific probiotic bacteria can ameliorate inflammatory bowel disease. The colonization strategies using defined commensals or exogenous specific probiotic treatment may prevent host intestinal inflammation and ameliorate intestinal epithelial barrier function.
The probiotic mixture, Bifico, is highly effective in the reducing colitis in interleukin (IL)-10 KO mice. Furthermore, Bifico treatment reduced colonic epithelial permeability, recovered normal expression and distribution of tight junction proteins, and protected against pathogenic bacterial invasion both in vivo and in vitro.
The probiotic mixture, Bifico had a direct effect on epithelial barrier function in vivo by reducing mucosal secretion of tumor necrosis factor-α and interferon-γ, and altering expression and distribution of tight junction proteins. Bifico exposure in vitro reduced bacterial invasion. Moreover, the effects of combined probiotics were more pronounced than those with single-strain probiotics.
By understanding the mechanism and effects of Bifico on the intestinal mucosal barrier, this study may represent a future strategy in the treatment of patients with colitis and increased intestinal permeability, such as ulcerative colitis, Crohn’s disease and irritable bowel syndrome.
The authors have performed a very good study of improving gastrointestinal mucosal biology in a colitis-like model and evaluating the effect of Bifico on the intestinal mucosal barrier, the expression and distribution of epithelial tight junction proteins, and secretion of inflammatory cytokines in vivo and in vitro.
P- Reviewers: Keshavarzian A, Mullin JM S- Editor: Cui XM L- Editor: Kerr C E- Editor: Wang CH
| 1. | Ventura M, O’Flaherty S, Claesson MJ, Turroni F, Klaenhammer TR, van Sinderen D, O’Toole PW. Genome-scale analyses of health-promoting bacteria: probiogenomics. Nat Rev Microbiol. 2009;7:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 348] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 2. | Jia W, Li H, Zhao L, Nicholson JK. Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov. 2008;7:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 3. | Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580-591. [PubMed] |
| 4. | Natividad JM, Petit V, Huang X, de Palma G, Jury J, Sanz Y, Philpott D, Garcia Rodenas CL, McCoy KD, Verdu EF. Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1-/-; Nod2-/- mice. Inflamm Bowel Dis. 2012;18:1434-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | von Schillde MA, Hörmannsperger G, Weiher M, Alpert CA, Hahne H, Bäuerl C, van Huynegem K, Steidler L, Hrncir T, Pérez-Martínez G. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe. 2012;11:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140-G1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (1)] |
| 7. | Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1691] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 8. | Chen HQ, Yang J, Zhang M, Zhou YK, Shen TY, Chu ZX, Zhang M, Hang XM, Jiang YQ, Qin HL. Lactobacillus plantarum ameliorates colonic epithelial barrier dysfunction by modulating the apical junctional complex and PepT1 in IL-10 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1287-G1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Fujiya M, Musch MW, Nakagawa Y, Hu S, Alverdy J, Kohgo Y, Schneewind O, Jabri B, Chang EB. The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe. 2007;1:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Tash BR, Bewley MC, Russo M, Keil JM, Griffin KA, Sundstrom JM, Antonetti DA, Tian F, Flanagan JM. The occludin and ZO-1 complex, defined by small angle X-ray scattering and NMR, has implications for modulating tight junction permeability. Proc Natl Acad Sci USA. 2012;109:10855-10860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 472] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 12. | Li Q, Zhang Q, Wang C, Li N, Li J. Invasion of enteropathogenic Escherichia coli into host cells through epithelial tight junctions. FEBS J. 2008;275:6022-6032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Liu Z, Shen T, Zhang P, Ma Y, Qin H. Lactobacillus plantarum surface layer adhesive protein protects intestinal epithelial cells against tight junction injury induced by enteropathogenic Escherichia coli. Mol Biol Rep. 2011;38:3471-3480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Zhang Q, Li Q, Wang C, Liu X, Li N, Li J. Enteropathogenic Escherichia coli changes distribution of occludin and ZO-1 in tight junction membrane microdomains in vivo. Microb Pathog. 2010;48:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Babbin BA, Sasaki M, Gerner-Schmidt KW, Nusrat A, Klapproth JM. The bacterial virulence factor lymphostatin compromises intestinal epithelial barrier function by modulating rho GTPases. Am J Pathol. 2009;174:1347-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Chen ML, Ge Z, Fox JG, Schauer DB. Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni. Infect Immun. 2006;74:6581-6589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Viswanathan VK, Koutsouris A, Lukic S, Pilkinton M, Simonovic I, Simonovic M, Hecht G. Comparative analysis of EspF from enteropathogenic and enterohemorrhagic Escherichia coli in alteration of epithelial barrier function. Infect Immun. 2004;72:3218-3227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 584] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 19. | Gueimonde M, Ouwehand A, Huhtinen H, Salminen E, Salminen S. Qualitative and quantitative analyses of the bifidobacterial microbiota in the colonic mucosa of patients with colorectal cancer, diverticulitis and inflammatory bowel disease. World J Gastroenterol. 2007;13:3985-3989. [PubMed] |
| 20. | Ewaschuk JB, Tejpar QZ, Soo I, Madsen K, Fedorak RN. The role of antibiotic and probiotic therapies in current and future management of inflammatory bowel disease. Curr Gastroenterol Rep. 2006;8:486-498. [PubMed] |
| 21. | Gorbach SL, Chang TW, Goldin B. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet. 1987;2:1519. [PubMed] |
| 22. | Eser A, Thalhammer F, Burghuber F, Högenauer C, Stockenhuber F, Wenisch C, Widhalm K, Reinisch W. Probiotics for the prevention of antibiotic-induced diarrhea. Z Gastroenterol. 2012;50:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Kamiya T, Shikano M, Wada T, Sasaki M, Joh T. The efficacy of probiotics in gastrointestinal disease. Nihon Rinsho. 2008;66:1385-1390. [PubMed] |
| 24. | Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 2006;6:849-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Saulnier DM, Spinler JK, Gibson GR, Versalovic J. Mechanisms of probiosis and prebiosis: considerations for enhanced functional foods. Curr Opin Biotechnol. 2009;20:135-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 27. | Baker NT, Graham LL. Campylobacter fetus translocation across Caco-2 cell monolayers. Microb Pathog. 2010;49:260-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1028-G1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Spencer DM, Veldman GM, Banerjee S, Willis J, Levine AD. Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology. 2002;122:94-105. [PubMed] |
| 30. | Vetrano S, Rescigno M, Cera MR, Correale C, Rumio C, Doni A, Fantini M, Sturm A, Borroni E, Repici A. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Scaldaferri F, Pizzoferrato M, Pecere S, Forte F, Gasbarrini A. Bacterial flora as a cause or treatment of chronic diarrhea. Gastroenterol Clin North Am. 2012;41:581-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262-270. [PubMed] |
| 33. | Rabizadeh S, Sears C. New horizons for the infectious diseases specialist: how gut microflora promote health and disease. Curr Infect Dis Rep. 2008;10:92-98. [PubMed] |
| 34. | Rigby RJ, Hunt MR, Scull BP, Simmons JG, Speck KE, Helmrath MA, Lund PK. A new animal model of postsurgical bowel inflammation and fibrosis: the effect of commensal microflora. Gut. 2009;58:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107-1114. [PubMed] |
| 36. | Ouwehand AC, Isolauri E, Kirjavainen PV, Tölkko S, Salminen SJ. The mucus binding of Bifidobacterium lactis Bb12 is enhanced in the presence of Lactobacillus GG and Lact. delbrueckii subsp. bulgaricus. Lett Appl Microbiol. 2000;30:10-13. [PubMed] |
| 37. | Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M, Versalovic J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One. 2012;7:e31951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 325] [Article Influence: 25.0] [Reference Citation Analysis (0)] |