Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4503
Revised: January 23, 2014
Accepted: March 12, 2014
Published online: April 28, 2014
Processing time: 182 Days and 5.2 Hours
Latin America shows one of the highest incidence rates of gastric cancer in the world, with variations in mortality rates among nations or even within countries belonging to this region. Gastric cancer is the result of a multifactorial complex process, for which a multistep model of carcinogenesis is currently accepted. Additionally to the infection with Helicobacter pylori, that plays a major role, environmental factors as well as genetic susceptibility factors are significant players at different stages in the gastric cancer process. The differences in population origin, demographic structure, socio-economic development, and the impact of globalization lifestyles experienced in Latin America in the last decades, all together offer opportunities for studying in this context the influence of genetic polymorphisms in the susceptibility to gastric cancer. The aim of this article is to discuss current trends on gastric cancer in Latin American countries and to review the available published information about studies of association of gene polymorphisms involved in gastric cancer susceptibility from this region of the world. A total of 40 genes or genomic regions and 69 genetic variants, 58% representing markers involved in inflammatory response, have been used in a number of studies in which predominates a low number of individuals (cases and controls) included. Polymorphisms of IL-1B (-511 C/T, 14 studies; -31 T/C, 10 studies) and IL-1RN (variable number of tandem repeats, 17 studies) are the most represented ones in the reviewed studies. Other genetic variants recently evaluated in large meta-analyses and associated with gastric cancer risk were also analyzed in a few studies [e.g., prostate stem cell antigen (PSCA), CDH1, Survivin]. Further and better analysis centered in gene polymorphisms linked to other covariates, epidemiological studies and the information provided by meta-analyses and genome-wide association studies should help to improve our understanding of gastric cancer etiology in order to develop appropriate health programs in Latin America.
Core tip: This article is a review about the current state of art of studies carried out in Latin America using gene polymorphisms to assess gastric cancer susceptibility. Latin America shows one of the highest incidence rates of gastric cancer in the world, with variations in mortality rates among nations or even within countries belonging to this region. Moreover, Latin America is a region with a particular genetic background, high rates of Helicobacter pylori infection and lifestyles condition. This review also gives special emphasis on the importance of the studies conducted in gastric precancerous diseases.
- Citation: Chiurillo MA. Role of gene polymorphisms in gastric cancer and its precursor lesions: Current knowledge and perspectives in Latin American countries. World J Gastroenterol 2014; 20(16): 4503-4515
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4503.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4503
Gastric cancer is one of the most lethal types of cancer, accounting in 2008 for about 800000 deaths, but its incidence varies substantially worldwide[1]. There were approximately 870000 noncardia gastric cancer cases and 74.7% of them have been attributed to Helicobacter pylori (H. pylori) infection[1]. Although the rates of gastric cancer have been declining over the past 50 years in most Western countries, gastric cancer is still the fourth most common malignancy and the second leading cause of death due to cancer worldwide. The highest incidence (more than two-thirds) of gastric cancer is observed in East Asia, Eastern Europe, and the Andean region of South America, while North America, Northern Europe and North and East Africa show the lowest recorded rates[1].
Latin American countries display some of the highest mortality rates worldwide. For males the estimates age-standardized mortality rates (ASMR) are led by Honduras (25.9%), Ecuador (24.1%), Costa Rica (23.6%), Chile (23.1%) and Guatemala (22.3%), while for women the highest rates are found in Guatemala (22.0%), Honduras (19.0%), Ecuador (17.5%), Peru (17.1%) and Costa Rica (10.6%). In contrast, lower ASMR are observed for both sexes in Puerto Rico, Cuba, Dominican Republic, Mexico and Argentina[1,2].
Significant variations in the incidence of gastric cancer have been observed between different ethnic groups living in the same region; for example, African-Americans, Hispanics and Native Americans are affected more than Caucasians in the United States[3]. Moreover, in a comparison between Japanese migrants to the United States and Brazil, Japanese migrants to the United States show a significantly lower incidence rate than Japanese living in Japan, while Japanese migrants to Brazil show a similar rate to the latter group, suggesting that the geographical distribution of gastric cancer may not be solely attributable to ethnic differences[4].
Recently, Torres et al[5], with the apparent association between altitude and the incidence of gastric cancer in the countries of Western Latin America along the Pacific Rim, proposed that the altitude may be a surrogate for the clustering of host, bacterial, dietary, and environmental factors related to gastric cancer risk. The relation appears to be strongest in the mountainous regions of Central America and Andean South America, but it is absent in Chile, where risk is more strongly associated with the age of H. pylori acquisition and socio-economic determinants. Among possible explanations for this association could be that the host genetic background, as well as H. pylori genotypes, may cluster more readily in certain isolated mountainous communities[5].
It has been proposed a multistep cascade model for the development of intestinal-type gastric adenocarcinoma, which consists of a progression from chronic superficial (non atrophic) gastritis, to chronic atrophic gastritis to intestinal metaplasia, and finally, gastric adenocarcinoma[6]. This model hypothesizes the sequence of precancerous lesions as a dynamic process from an initial superficial inflammation caused by H. pylori infection to a fully malignant neoplasm of the stomach. Thus, the chronic infection of the gastric mucosa by the H. pylori is a major attributable risk factor of gastric cancer[7].
More than 50% of the population worldwide is infected with H. pylori with a higher prevalence in developing countries and in groups with poor socio-economic conditions. The improvements in living conditions in developed countries has determined a declining in the prevalence of infection, while remaining high, about 80%, in the developing world. In Latin American countries it has been reported a prevalence of H. pylori infection ranging from 70% to 90%[8]. However, less than 2% of H. pylori carriers develop gastric cancer[9]. Moreover, the incidence of gastric cancer in areas of Africa and South Asia with high prevalence of H. pylori infections is much lower than in other countries[10].
Consistent with the multifactorial pathogenesis, the observed differences in the clinical outcomes and gastric cancer prevalence worldwide may be due to environmental factors (mainly diet, smoking and alcohol use) often playing a dominant role. Moreover, the influence of host factors, especially those governing the severity of the immune response, is also relevant.
The identification and discrimination of host genetic variants influencing susceptibility in populations with high incidence of gastric cancer has been a major challenge. These genetic variants may modulate the effects of exposure to environmental factors by regulating multiple biological pathways during gastric carcinogenesis.
Common susceptibility genetic variants have been identified as significantly associated with gastric cancer risk by candidate-gene studies, such as inflammatory [interleukin (IL)-1β, IL-8 and tumor necrosis factor-α (TNF-α)] and anti-inflammatory cytokines (IL-10), DNA repair genes and metabolic enzymes (such as the glutathione S-transferase family, cytochrome P450 superfamily, and metabolism of folate and arachidonic acid)[11-20].
Moreover, recent genome-wide and large scale gene association studies have focused on analyzing regions of the genome in which have been detected candidate genes involved in cell proliferation, differentiation, and survival, such as MUC1[21], PLCE1[22], PTGER4, PRKAA1, ZBTB20[23,24], prostate stem cell antigen (PSCA)[25,26], genes participating in EGFR and FAS-mediated signaling pathway[27,28] and DNA repair pathways[29]. Chromosome 9p21.3 and 10q23 regions have been identified as genetic susceptibility loci for multiple disease phenotypes including gastric cancer[22,30].
The current understanding of host genetic polymorphisms and gastric cancer susceptibility is based largely on studies in Asians and Caucasians (from Europe and North America) populations. Moreover, ethnicity has been proposed as a factor modifying the risk of cancer[2].
The present-day population of Latin American countries is historically and anthropologically admixed, as the result of a mixing process between Native Americans, Europeans (mostly Spaniards, Portuguese and Italians) and Sub-Saharan Africans (mainly from Western Africa), whom came into contact for five centuries[31]. The populations of Latin America experienced different admixture processes with varying degrees of ancestral population proportions that came in different migration waves[32,33]. Therefore, in studies of genetic association to diseases, the addition of a population structure estimate could be very effective to identify and correct possible effects of the population substructure.
Genetic admixture studies have recently helped to identify variants associated with prostate and oral cancer in African-American and Hispanics populations, respectively[34,35]. A similar approach was applied recently by Pereira et al[36] using a panel of 103 ancestry informative markers (AIM) to test if individual Native American, European and African ancestries are risk factors for gastric cancer in an urban admixed sample in Peru. This work determined that Native American individual ancestry is associated with gastric cancer, but this was explained by the association of socioeconomic variables with both gastric cancer and Native American ancestry. Therefore, indicating that the high incidence of gastric cancer in the Peruvian population, with a very high Native American ancestry, does not seem to rely on a genetic basis. More recently, a study carried out in the Northern region of Brazil examined the effect of population substructure, by the analysis of a panel with 48 AIMs, on the association between five single nucleotide polymorphisms (SNP) of N-acetyltransferase 2 (NAT2) gene and the susceptibility to breast and gastric cancer[37]. They detected a higher African contribution in the study group with cancer, and a significant association of NAT2 282*T allele carriers with gastric cancer.
The present overview included studies carried out on humans that were found in the databases of PubMed/MEDLINE, LILACS and SciELO, published up to 25 October 2013 and with no restriction regarding language. This review includes the analysis of 61 articles reporting studies of association between genetic variants and the risk of gastric cancer and/or known precancerous lesions. All studies correspond to case-control comparisons, including healthy, non-cancer (counting precancerous lesions), asymptomatic and population-based controls[12,37-96].
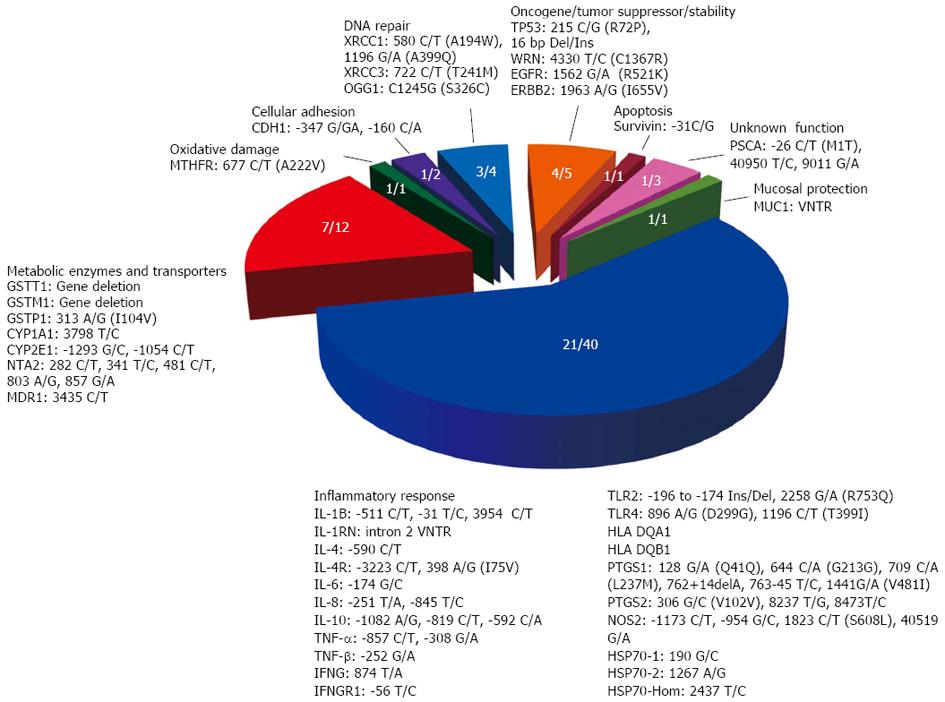
All reviewed studies were conducted with Latin American populations: Brazil (22), Chile (1), Colombia (6), Costa Rica (7), Honduras (1), Mexico (16), Peru (1) and Venezuela (7). Two-thirds of the articles considered the detection of H. pylori infection by urease test, culture, histology, serology or polymerase chain reaction (PCR). Some studies also included the typing of genetics variants of bacterial virulence factors by molecular methods. Regarding host genetic variants, studies evaluated 69 polymorphisms from 40 genes or genomic regions, including 2 microsatellites or variable number of tandem repeat (VNTR), 2 gene deletions, 3 insertion/deletions and 62 nucleotide substitutions (21 of them in protein coding regions, resulting in 3 synonymous and 18 nonsynonymous substitutions). Figure 1 shows a summary of gene polymorphisms included in the Latin American studies classified by functional categories: inflammatory response, mucosal protection, metabolic enzymes and transporters, oxidative damage, cellular adhesion, DNA repair, oncogene/tumor suppressor/stability genes, apoptosis.
Techniques used for detecting gene polymorphisms were dot blot hybridization, sequencing, conventional polymerase chain reaction (PCR), PCR-restriction fragment length polymorphism, PCR-single-strand conformation polymorphism, real-time PCR with fluorescent probes, PCR-sequence specific oligonucleotide probe, PCR-Sequence-Specific Primer, Amplification-refractory mutation system-PCR, and KASParTM SNP genotyping system.
The largest number of studies investigating the association between gene polymorphisms and gastric cancer (and premalignant lesions) risk in different countries of the region includes the evaluation of interleukin-1 family variants: IL-1B (IL-1B-511 C/T, 14 studies -31 T/C, 10 studies, +3954, 8 studies) and IL-1RN (VNTR, 17 studies). Table 1 shows the main characteristics of these studies.
| Year | Study population | Number of populations | Target genes and variants | Main findings associated with increased susceptibility |
| 2004 | Brazil Gatti et al[70] | 56 GC; 56 ChrG | IL-1B-511C/T; -31T/C IL-1RN intron 2 VNTR | There was no association |
| 2005 | Costa RicaAlpizar-Alpizar et al[40] | 58 GC; 41 nonneoplastic lesions; 58 cancer free patients; 41 healthy controls | IL-1B-511C/T; -31T/C; +3954C/TIL-10-1082G/A; -819C/T; -592C/A IL-1RN intron 2 VNTR | Carriers of the IL-1B+3954*T alle had an increased risk for developing GC (OR = 3.72, 95%CI: 1.34-10.2). IL-1RN heterozygote genotype (*2/*L) was associated with GC (OR = 2.942, 95%CI: 1.09-7.93). |
| 2005 | Mexico Garza-González et al[12] | 63 distal GC; 215 non-cancer lesions | IL-1B-31T/C IL-1RN intron 2 VNTRTNF-α-308G/A | Presence of IL-1B-31*C allele was associated with increased risk of distal GC (OR = 7.631, 95%CI: 1.73-46.94) |
| 2005 | Brazil Rocha et al[73] | 168 GC H. pylori +;541 asymptomatic controls | IL-1B-511C/T; -31T/CIL-1RN intron 2 VNTRTNF-α-308G/A | IL-1RN*2 was associated with noncardia GC (OR = 1.93, 95%CI: 1.06-3.49) |
| 2006 | Mexico Sicinschi et al[58] | 183 GC; 377 controls | IL-1B-31T/C; +3954 C/TIL-10-592C/A IL-1RN intron 2 VNTR | IL-10-592*C allele carrier was associated with intestinal-type of GC (OR = 2.081, 95%CI: 1.07-4.05). Subjects with IL-1B-31 CC genotype and H. pylori CagA positive serology had an increased risk of intestinal-type GC (OR = 3.192, 95%CI: 1.05-9.68) |
| 2006 | HondurasMorgan et al[45] | 170 GC; 162 healthy controls | IL-1B-511C/TIL-10-1082G/AIL-1RN intron 2 VNTRTNF-α-308 G/A | IL-1B-511 TT + IL-10-1082 AA combination increased risk of GC (OR = 2.6, 95%CI: 1.0-6.8) |
| 2007 | Costa Rica Con et al[43] | 58 AG; 31 corpus AG; 23 IM | IL-1B-511C/T; +3954C/TIL-10-1082G/A; -592C/AIL-1RN intron 2 VNTR | IL-1B+3954*T carrier and IL-1RN homozygous *2 allele were associated with IM (OR = 3.41, 95%CI: 1.2-10.00 and OR = 3.12, 95%CI: 1.1-9.00, respectively) |
| 2008 | Costa Rica Sierra et al[42] | 25 ABG; 76 AAG; 253 NAG; 21 Normal mucosa; 21 healthy controls | IL-1B+3954C/TIL-1RN intron 2 VNTR | No association was found |
| 2009 | Peru Gehmert et al[46] | 133 GC vs 133 NAG86 NAG vs 43 ChrAG | IL-1B-511C/TIL-1RN intron 2 VNTR | IL-1B-511*C allele carrier and CT and CC genotypes were associated with AG (OR = 5.61, 95%CI: 2.02-15.51; OR = 4.82, 95%CI: 1.65-13.83; OR = 11.22, 95%CI: 2.27-55.37, respectively) and GC (OR = 2.361, 95%CI: 1.34-4.11; OR = 2.172, 95%CI: 1.23-3.84; OR = 4.152, 95%CI: 1.33-12.93, respectively) |
| 2009 | Brazil Melo Barbosa et al[74] | 177 gastric benign pathologies; 100 asymptomatic controls | IL-1B-511C/T; -31T/C IL-1RN intron 2 VNTR TNF-α-308 G/A | Carriers of IL-1RN*2 allele with H. pylori CagA-positive serology had a greater risk of developing GU (OR = 8.82, 95%CI: 1.762-44.181) and GC (OR = 16.76, 95%CI: 1.99-140.71) |
| 2009 | Costa Rica Con et al[44] | 52 GC; 191 non-cancer H. pylori positive patients | IL-1B-511C/T; +3954C/T IL-10-1082G/A; -592C/A IL-1RN intron 2 VNTR | IL-1B+3954 TC (OR = 2.12, 95%CI: 1.0-4.3), IL-1RN *2/*L (OR = 3.52, 95%CI: 1.7-7.3), IL-10-592 AA (OR = 3.12, 95%CI: 1.2-8.2) and IL-10-592 CA (OR = 3.22, 95%CI: 1.5-6.8) genotypes, as well the IL-1B+3954 TC, IL-1RN *2/*L, IL-10-592 CA (OR = 4.7, 95%CI: 1.7-13.0) combination were associated with GC |
| 2009 | Venezuela Cañas et al[84] | 84 GC; 84 ChrG | IL-1B-511T/C; +3954C/TIL-10-592C/A IL-1RN intron 2 VNTR | IL-1B+3954*C carrier and IL-IRN *2/*2 genotype were associated with GC (OR = 6.21, 95%CI: 1.3-28.8 and OR = 7.02, 95%CI: 2.3-21.5, respectively). The IL-IRN *2/*2 genotype was also associated with a well/moderately-differentiated adenocarcinoma (OR = 8.12, 95%CI: 2.5-26.8) |
| 2010 | Mexico Martínez-Carrillo et al[56] | 100 ChrG; 28 GU102 healthy controls | IL-1B-511C/T; -31T/C | The IL-1B-511 TC genotype and the -511*C allele were associated with ChrG (OR = 3.12, 95%CI: 1.4-6.8 and OR = 3.01, 95%CI: 1.4-6.3, respectively). The subjects carrying -31*T were found to be at a higher risk of having ChrG (OR = 2.81, 95%CI: 1.3-5.8). The IL-1B-511*C/-31*T haplotype was associated with ChrG (OR = 2.1, 95%CI: 1.2-3.8). |
| 2010 | Venezuela Chiurillo et al[85] | 109 ChrG | IL-1B-511C/T; -31T/C; +3954C/T IL-1RN intron 2 VNTR | Carriage of IL-1B-511*T (OR = 5.4, 95%CI: 1.9–15.8) and -31*C (OR = 5.1, 95%CI: 1.8–14.7) alleles combined with iceA2+ H. pylori genotype increased the risk of ChrAG with severe histopathological changes. |
| 2011 | Colombia Martínez et al[88] | 46 GC; 99 NAG | IL-1B-511C/T IL-1RN intron 2 VNTR | IL-1B-511 TT carriers had increased risk of GC (OR = 11.312, 95%CI: 1.20-106.54) |
| 2011 | Colombia Martínez et al[91] | 58 GC; 89 DU (54 with precancerous lesions); 194 ChrG and normals | IL-1B-511C/T IL-1RN intron 2 VNTRIL-10-1082G/A; -819C/T;TNF-α-308G/A | Genotype IL-1B-511 TT was associated with GC (OR = 4.692, 95%CI: 1.22-18.09) |
| 2011 | Venezuela Chiurillo et al[86] | 121 ChrG | IL-1B-511C/T; -31T/C; +3954C/T | There was association with severe histological changes only considering H. pylori genotypes |
| 2012 | Mexico López-Carrillo et al[57] | 158 GC; 317 clinical controls | IL-1B-31T/C | IL-1B-31*C allele carriers who were both H. pylori CagA positive and with moderate to high Capsaicin consumption had increased risk of GC (OR = 3.411, 95%CI: 1.12-10.43) |
| 2013 | Brazil Mattar et al[94] | 19 GC; 71 clinical controls; 196 inflammation of the upper gastrointestinal tract; 28 GU; 76 DU | IL-1RN intron 2 VNTR | The carriage of IL-1RN *2/*2 was an independent risk factor for GC (OR = 5.81, 95%CI: 1.06-31.98). The carriage of allele *2 had an independent protective effect on DU (OR = 0.45, 95%CI: 0.22-0.91) |
| 2013 | Brazil de Oliveira et al[79] | 200 GC; 229 ChrG; 240 healthy individuals | IL-1RN intron 2 VNTR | Association with GC was observed for IL-1RN*2 (OR = 2.601, 95%CI: 1.65-4.10), TNF-α -857*T (OR = 1.701, 95%CI: 1.08-2.67), IL-8-845*C (OR = 3.461, 95 % CI: 1.69-7.07), IL-10-592*A (OR = 2.341, 95%CI: 1.47-3.70), TLR2 –196 to –174 *Del (OR = 2.201, 95%CI: 1.28-3.78) and TLR4+896*G (OR = 2.091, 95 % CI: 1.08-4.02) alleles. Association with ChrG was observed with IL-1RN*2 (OR = 1.881, 95%CI: 1.25-2.83) and IL-10-592*A (OR = 3.001, 95%CI: 1.99-4.50) alleles |
| TNF-α-857C/T | ||||
| TNF-α-308G/A | ||||
| TNF-β-252G/A | ||||
| IL-8-251T/A | ||||
| IL-8-845T/C | ||||
| IL-10-592C/A | ||||
| TLR2–196 to –174 Ins/Del | ||||
| TLR4+896A/G (D299G); +1196C/T (T399I) |
Genetic variants in inflammation-related genes, especially cytokines and their receptors are thought to influence the first stage of the precancerous cascade and are related to a more intense inflammatory response after gastritis associated to H. pylori infection[7]. The inflammatory-related genes that have been most frequently studied in relation to gastric cancer, sometimes with conflicting results, are the interleukin genes IL-1B, IL-1RN, IL-8 and IL-10. SNPs within these and other functional cytokine regions that markedly influence expression and secretion profiles may modify the intensity of the inflammatory response to infectious agents, thereby contributing to variations in gastric cancer risk[97].
Since the first study of El-Omar et al[98] in 2000, a significant number of studies have evaluated the association between genetic variations in the IL-1 gene cluster (IL-1B-511 C/T, IL-1B-31 T/C, IL-1B+3954 C/T and IL-1RN intron 2 VNTR) and gastric cancer. This association (significantly with noncardia or with intestinal type of gastric cancer) has a fundamental principle: alleles IL-1B-31*C, -511*T, and IL-1RN*2, lead to high-level expression of IL-1β, reduction of acid output, corpus-predominant colonization by H. pylori, pangastritis and atrophic gastritis, which are considered precursors as well as risk factors for gastric cancer[98]. Furthermore, H. pylori infection induces IL-1β production, and the consequent hypochlorydria favors further colonization by pH-sensitive H. pylori[99]. In addition, global meta-analyses have suggested race-specific associations of some cytokine variants in Caucasian and Asian populations[97,100-104].
A recent meta-analysis, showed that the profile of IL-1B risk alleles in Latin Americans mirrors that found in Asian populations with low or no associations with gastric cancer[105]. For example, in a high-incidence region of gastric cancer in Honduras, a sample of healthy (population-based) controls of Hispanic mestizo origin, had the IL-1B-511*T+ and IL-10-1082*A+ genotypes prevalence among the highest reported[45].
The meta-analysis of Xue et al[103] showed that IL-1B-511*T and IL-1RN*2 alleles were significantly associated with an increased risk of developing gastric carcinoma among Caucasians, but not in Asians or Hispanics. On the other hand, in the case-controls comparisons carried out in 2013 by Bonequi et al[105], in which were analyzed studies from Brazil, Colombia, Costa Rica, Honduras, Mexico, Peru and Venezuela, it was identified the IL-1RN*2 allele associated with a moderate increased risk for gastric cancer (overall OR = 1.51, 95%CI: 1.15-1.99), supporting its involvement in gastric carcinogenesis as has been previously reported in non-Asian populations[104].
Among pro-inflammatory cytokines, IL-8 acts as a potent chemoattractant and activator of neutrophils that may play a role in gastric cancer pathogenesis[14,106]. IL-8 exhibits several functional polymorphisms, among them the IL-8 -251 A/T SNP in the promoter region is associated with an increase in synthesis of that interleukin by gastric epithelial cells[14,106].
A study conducted in Mexico showed that the IL-8 -251*A allele is a risk factor for the development of noncardia gastric cancer[60]. Similarly, Vinagre et al[95] observed that the AA (P = 0.026) and AT (P = 0.005) genotypes were most frequent in the group of patients with gastric adenocarcinoma from the state of Pará, Brazil. Furthermore, they also found the IL-8 -251*A allele associated with the risk for developing gastric cancer. On the contrary, also in Brazil but in the state of São Paulo, Felipe et al[96] found the IL-8 -251 AT genotype and *T carriers associated with an increased risk of gastric cancer. These authors also observed that individuals with AA genotype may have protective effect for gastric cancer, while patients harboring the TT genotype presented a lower median survival time. Whereas Garcia de Oliveira et al[79], in another region of the state of São Paulo, found only the IL-8 -845 T/C SNP (P < 0.001) associated with risk for gastric cancer.
These results could suggest that the association between IL-8 -251 A/T polymorphism and gastric cancer is likely influenced by environmental factors, and even ethnicity, considering that the geographical conditions and the proportion of the genetic ancestral contributions differ between the northern and southeast regions of Brazil[107,108]. Moreover, a recent meta-analysis suggest the potential influence of ethnicity in the association of IL-8 -251 A/T polymorphism with gastric cancer, since it is generally stronger in Asian than in Caucasian population[109].
There are three functional promoter SNPs in the IL-10 locus: -1082 A/G, -819 C/T and -592 C/A. In this locus only the -592 C/A SNP was found associated with gastric cancer in Latin American studies. Con et al[44] in Costa Rica and Garcia de Oliveira et al in Brazil[79] found that IL-10-592 AA and CA genotypes were individually associated with gastric cancer. Contrary, Sicinschi et al[58] in Mexico identified the IL-10-592 CC genotype associated with more than double of the risk of the intestinal-type gastric cancer. A recent meta-analysis based on 12 previous studies concluded that the IL-10-592 C/A polymorphism was not a risk factor for gastric cancer. However, when stratifying the data by race, the IL-10-592 AA genotype was found to be a protective factor against the development of this neoplasm in Asians but not among Caucasians and Latinos[110].
Toll-like receptors (TLR2 and TLR4), involved in H. pylori recognition in gastric mucosa, also have polymorphic variants that modulate their functional pattern[111]. Hence, some reports have studied SNPs in TLRs that are associated with impaired immune response and induction of a potent inflammatory response in the gastric mucosa, being then associated with susceptibility of gastric diseases. In Mexico two studies evaluated the association of TLR4 +896A/G and +1196C/T SNPs with gastric cancer and precancerous diseases[60,93]. Although no association with gastric cancer was found in these studies, Trejo-de la et al[93], including also analysis of TLR2 +2258 G/A SNP, showed that patients with TLR4 polymorphisms expressed significantly lower levels of IL-1β, IL-6, IL-8 and GRO-α; and higher levels of TNF-α, IL-10, MCP-1 and MIP-1α. Moreover, Silva´s research group in two recent reports investigated TLR2 -196 to -174 del, TLR4 +896A/G and TLR4 +1196C/T polymorphisms at risk of chronic gastritis and gastric cancer in a Brazilian population in the state of São Paulo[78,79]. In both studies TLR2 -196 to -174*del and TLR4 +896*G alleles showed an association with increased risk for gastric cancer.
The study of Garcia de Oliveira et al[79] mentioned above evaluated ten inflammatory-related gene polymorphisms in 669 samples (200 of gastric cancer, 229 of chronic gastritis, and 240 of healthy individuals), of which IL-1RN L/2 (P < 0.001), TNF-α-857 C/T (P = 0.022), IL-8-845 T/C (P < 0.001), IL-10-592 C/A (P < 0.001), TLR2 ins/del (P < 0.001), and TLR4+896 A/G (P = 0.033) polymorphisms were observed associated with the risk of gastric cancer using a dominant model. In addition, a combined analysis of these six polymorphisms revealed a profile with two to four combined genotypes, which confers a higher risk of gastric carcinogenesis.
Regarding polymorphisms in inflammation-related genes, three genes encoding heat shock proteins (HSP) were also evaluated in two studies. Partida-Rodríguez et al[61] studied HSP70-1+190 G/C, HSP70-2+1267 A/G and HSP70-Hom+2437 T/C SNPs in 447 Mexican patients, including 228 with non-atrophic gastritis, 98 with intestinal metaplasia, 63 with gastric cancer, 58 with duodenal ulcer, and 132 asymptomatic individuals. They also evaluated in this analysis the TNF-α-308 G/A and TNF-β-252 G/A polymorphisms. Compared with the asymptomatic group, they found significant association of TNF-β-252*A and HSP70-1*C alleles with gastric cancer. More recently, Ferrer-Ferrer et al[41] in 2013 addressed the possible association between HSP70-2+1267 A/G and HSP70-Hom+2437 T/C polymorphisms and the risk of developing gastric cancer in a high-risk population in Costa Rica. These authors found that the GA genotype of HSP70-2+1267 was associated with increased risk of gastric cancer as compared to the GG genotype.
With regard to tumor-suppressor genes, seven studies conducted the analysis of the TP53 Arg72Pro polymorphism related to the risk of gastric cancer. In Mexican patients, Pérez-Pérez et al[55] identified association of the Arg/Arg genotype with the increased risk of distal gastric cancer. Similarly, in Venezuela, individuals carrying the Arg allele had an elevated risk of developing gastric cancer, while the Arg/Arg genotype was associated with poor-differentiated gastric cancer[83]. However, the association of gastric cancer with TP53 Arg72Pro polymorphism in Latin American countries was not consistent in the meta-analysis of Bonequi et al[105]. Differences in distribution of TP53 Arg72Pro genotypes could be associated with the location, stage, and histological differentiation of gastric cancer. Moreover, a meta-analysis suggests that the TP53 codon 72 polymorphism (Pro allele) may be associated with gastric cancer, particularly among Asians[112].
A Brazilian case-control study evaluated the effect of a functional SNP (-31C/G) of Survivin, which is involved in the regulation of apoptosis and cell cycle control[75]. Although this study included a small sample size, results suggest that the presence of the *C allele of Survivin gene promoter -31 C/G polymorphism in combination with D17S250 microsatellite instability (a marker of TP53 gene) may be used as risk factor for gastric cancer in this population. Involvement in gastric carcinogenesis of Survivin can be taken from the observation that overexpression of this protein in gastric cells reduces cell death after infection with H. pylori[113].
The CDH1 gene, encoding E-cadherin protein, is now established as a tumor suppressor in gastric cancer[114]. Polymorphisms at positions -347 G/GA and -160 C/A reduce the transcriptional activity of CDH1, although their association with susceptibility to gastric cancer is controversial[115,116]. Medina-Franco et al[62] analyzed a sample of 39 Mexican patients younger than 45 years old with diagnosis of diffuse gastric cancer and observed association with -160 CA and AA genotypes. Moreover, Borges et al[69] observed in Brazilian patients carrying CDH1 -160*A and -347*GA alleles an increased probability of developing gastric cancer, especially of the diffuse-type.
SNPs in the PSCA gene was found associated with gastric cancer risk in a Genome-wide association study (GWAS), and subsequently validated in other Asian and Caucasian populations[26,117,118]. Although its function remains unknown, the expression of PSCA has been observed downregulated in the gastric tissue with intestinal metaplasia[119]. Rizatto et al[82] analyzed 3 SNPs in the PSCA gene (rs2294008 C/T, rs9297976 T/C and rs12155758 G/A) in gastric biopsies of 2045 subjects with gastric precancerous lesions and 180 cases of gastric cancer from a high-risk region of Western Venezuela. In this study the *T and *A alleles of rs2294008 and rs12155758, respectively, were found to be associated with gastric cancer.
The role of gene polymorphisms in precancerous lesions remains poorly understood, even for those that have been identified as associated with increased risk of gastric cancer. Identification of biomarkers of the precancerous process is needed for development of screening programs to prevent gastric cancer, as this may contribute to the understanding of gastric carcinogenesis.
Association between cytokine gene polymorphisms and gastric precancerous lesions were identified in a work carried out in Costa Rica by Con et al[43], in which the IL-1B+3954*T and IL-1RN *2/*2 genotypes were associated with intestinal metaplasia. Whereas in Peruvians, Gehmert et al[46] revealed an increased risk of atrophic gastritis associated with IL-1B-511*C allele. A Brazilian study in the state of São Paulo demonstrated the existence of an association of the anti-inflammatory cytokine variant alleles IL-1RN*2 and IL-10-592*A with a higher risk of developing gastric cancer and chronic gastritis[79]. In a recent meta-analysis Peleteiro et al[120] showed an association of the IL-1RN *2/*2 genotype with the increased risk of gastric precancerous lesions, supporting a role for this polymorphism in the early stages of gastric carcinogenesis.
In the context of H. pylori infection, two studies of our group in Venezuela showed an association of chronic atrophic gastritis and severe histopathological changes with IL-1B-511*T, -31*C, +3954*C and IL-1RN*2 polymorphisms only in presence of specific bacterial virulence genotypes[85,86]. Similarly, Melo-Barbosa et al[74] in Brazil, found that carriers of IL-1RN*2 allele with H. pylori CagA-positive serology had a higher risk of developing gastric ulcer.
A research group have evaluated the prevalence of gastric precancerous lesions in a large number of Venezuelans in relation with several genetic polymorphisms, most of them mediators of inflammation, and their interactions with other environmental factors. The first of them, by Kato et al[81], studied IL-10, IL-4 and IL-4R SNPs in 2033 patients. Authors identified the IL-10-1082*A low activity allele associated with intestinal metaplasia and dysplasia, while homozygous of the low activity allele (GG) of the 398 A/G polymorphism in the IL-4R gene had a modest increase in the risk of atrophic gastritis.
This group of researchers also evaluated genetic polymorphisms in other mediators of inflammation: IFNG, IFNGR1, NOS2A, PTGS1, PTGS2[87]. A nonsynonymous substitution Ser608Leu of NOS2A gene (*A carriers) and the -56 C/T SNP located in the promoter of IFNGR1 (CC genotype) were associated with higher risk of atrophic gastritis. Additionally, two SNPs of PTGS2 were associated with risk of dysplasia (306 G/C -Val102Val- and 8473 T/C). More recently, in a further study of this group, the *T allele of the functional SNP rs2294008 in the PSCA gene was associated with atrophic gastritis and intestinal metaplasia[82].
SNPs of HSP70-1 (+190*C allele) and HSP70-2 (+1267 GA genotype) were associated with an increased risk of duodenal ulcer in patients of Mexico and Costa Rica, respectively[41,61]. Moreover, examination of TLR4 +896A/G SNP in a Southeastern Brazilian population showed that the heterozygous AG genotype and allele *G frequencies were significantly higher in chronic gastritis and gastric cancer groups than in controls[78].
Latin America is a territorial and cultural entity with a particular genetic complexity, but also characterized by wide socio-economic divergences and rapid changes in life styles throughout the continent with a strong trend towards urbanization of its population. Therefore, the study of the etiology of multifactorial diseases, such as gastric cancer, in this region appears to be a major challenge, but also an opportunity.
Given in Latin America the common scenario of a population with high rates of infection with H. pylori, persistent poverty, particular dietary habits, coupled with secular trends in environmental exposures and lifestyle, genetic can offer a useful tool to compare populations and assess gene-environment interactions that underline gastric cancer development.
Most studies here analyzed were conducted with samples from populations with high prevalence of H. pylori infection. Therefore, it is not surprising that most research in this region of the world have been carried out with gene variants involved in inducing a more intense inflammatory response after gastritis associated to H. pylori infection. Moreover, as has been raised in Asians, due to the distribution of IL-1B high-risk alleles in some Latin American populations shows an elevated prevalence, could be suggested that they do not influence gastric cancer susceptibility in these populations, or in any case, its effect cannot be demonstrated.
Some research groups have been investigating the genetic contribution to gastric cancer in subjects of different ethnic backgrounds (mainly in Asians and Caucasians from United States and Europe), using previous GWASs information or conducting parallel GWASs with a large number of genes and new candidate loci for gastric cancer, as well as employing innovative techniques for genotyping and statistical analysis[22,27-30]. Therefore, to the analysis of human genetic risk factors in our populations, it would be appropriate to exploit and replicate GWASs findings, since a simple extrapolation of results from these studies to the use of biomarkers in Latin American populations is not completely adequate.
Genetic studies in admixed populations are particularly susceptible to confusion due to population stratification resulting from the difference in ancestry between cases and controls. However, such confounding can be handled by estimating individuals’ genetic ancestry using AIMs and then adjusting the analysis for individual ancestry. If in this region the human genetic background influences the high incidence of gastric cancer then can be expected that genetic variants harbored in admixed population account for this high incidence. Therefore, it would be possible to apply the genome-wide strategy of admixture mapping to detect these variants.
Finally, it is necessary to advocate for multicenter studies involving several Latin American research groups and large number of samples for the analysis of genetic polymorphisms in relation to precancerous lesions and environmental variables (lifestyle, dietary habits, H. pylori infection), in order to contribute to the understanding of gastric carcinogenesis and for the development of screening programs to prevent gastric cancer.
P- Reviewers: Chung YJ, Xuei X, Yuzhalin A S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11835] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 2. | Ferlay J, Shin HR, Bray F, Mathers C, Parkin DM. Globocan 2008 v1. 2, Cancer incidence and mortality worldwide: IARC CancerBase No. 10. International Agency for Research on Cancer. Lyon, France; 2012; Available from: http://globocan.iarc.fr. |
| 3. | Haile RW, John EM, Levine AJ, Cortessis VK, Unger JB, Gonzales M, Ziv E, Thompson P, Spruijt-Metz D, Tucker KL. A review of cancer in U.S. Hispanic populations. Cancer Prev Res (Phila). 2012;5:150-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Tsugane S, de Souza JM, Costa ML, Mirra AP, Gotlieb SL, Laurenti R, Watanabe S. Cancer incidence rates among Japanese immigrants in the city of São Paulo, Brazil, 1969-78. Cancer Causes Control. 1990;1:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Torres J, Correa P, Ferreccio C, Hernandez-Suarez G, Herrero R, Cavazza-Porro M, Dominguez R, Morgan D. Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America. Cancer Causes Control. 2013;24:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Correa P. Chronic gastritis: a clinico-pathological classification. Am J Gastroenterol. 1988;83:504-509. [PubMed] |
| 7. | McNamara D, El-Omar E. Helicobacter pylori infection and the pathogenesis of gastric cancer: a paradigm for host-bacterial interactions. Dig Liver Dis. 2008;40:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Porras C, Nodora J, Sexton R, Ferreccio C, Jimenez S, Dominguez RL, Cook P, Anderson G, Morgan DR, Baker LH. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control. 2013;24:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 410] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 10. | Malaty HM. Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 676] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 12. | Garza-González E, Bosques-Padilla FJ, El-Omar E, Hold G, Tijerina-Menchaca R, Maldonado-Garza HJ, Pérez-Pérez GI. Role of the polymorphic IL-1B, IL-1RN and TNF-A genes in distal gastric cancer in Mexico. Int J Cancer. 2005;114:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Savage SA, Abnet CC, Mark SD, Qiao YL, Dong ZW, Dawsey SM, Taylor PR, Chanock SJ. Variants of the IL8 and IL8RB genes and risk for gastric cardia adenocarcinoma and esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:2251-2257. [PubMed] |
| 14. | Taguchi A, Ohmiya N, Shirai K, Mabuchi N, Itoh A, Hirooka Y, Niwa Y, Goto H. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev. 2005;14:2487-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Chang-Claude J, Popanda O, Tan XL, Kropp S, Helmbold I, von Fournier D, Haase W, Sautter-Bihl ML, Wenz F, Schmezer P. Association between polymorphisms in the DNA repair genes, XRCC1, APE1, and XPD and acute side effects of radiotherapy in breast cancer patients. Clin Cancer Res. 2005;11:4802-4809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Palli D, Polidoro S, D’Errico M, Saieva C, Guarrera S, Calcagnile AS, Sera F, Allione A, Gemma S, Zanna I. Polymorphic DNA repair and metabolic genes: a multigenic study on gastric cancer. Mutagenesis. 2010;25:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Boccia S, La Torre G, Gianfagna F, Mannocci A, Ricciardi G. Glutathione S-transferase T1 status and gastric cancer risk: a meta-analysis of the literature. Mutagenesis. 2006;21:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Zacho J, Yazdanyar S, Bojesen SE, Tybjærg-Hansen A, Nordestgaard BG. Hyperhomocysteinemia, methylenetetrahydrofolate reductase c.677C& gt; T polymorphism and risk of cancer: cross-sectional and prospective studies and meta-analyses of 75,000 cases and 93,000 controls. Int J Cancer. 2011;128:644-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Ke-Xiang Z, Yu-Min L, Xun L, Wen-Ce Z, Yong S, Tao L. Study on the association of COX-2 genetic polymorphisms with risk of gastric cancer in high incidence Hexi area of Gansu province in China. Mol Biol Rep. 2011;38:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Zhuo W, Zhang L, Wang Y, Ling J, Zhu B, Chen Z. CYP2E1 RsaI/PstI polymorphism and gastric cancer susceptibility: meta-analyses based on 24 case-control studies. PLoS One. 2012;7:e48265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Zheng L, Zhu C, Gu J, Xi P, Du J, Jin G. Functional polymorphism rs4072037 in MUC1 gene contributes to the susceptibility to gastric cancer: evidence from pooled 6,580 cases and 10,324 controls. Mol Biol Rep. 2013;40:5791-5796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Abnet CC, Freedman ND, Hu N, Wang Z, Yu K, Shu XO, Yuan JM, Zheng W, Dawsey SM, Dong LM. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet. 2010;42:764-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 421] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 23. | Shi Y, Hu Z, Wu C, Dai J, Li H, Dong J, Wang M, Miao X, Zhou Y, Lu F. A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13.31 and 5p13.1. Nat Genet. 2011;43:1215-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 24. | Song HR, Kim HN, Kweon SS, Choi JS, Shim HJ, Cho SH, Chung IJ, Park YK, Kim SH, Choi YD. Genetic variations in the PRKAA1 and ZBTB20 genes and gastric cancer susceptibility in a Korean population. Mol Carcinog. 2013;52 Suppl 1:E155-E160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Yoshida T, Ono H, Kuchiba A, Saeki N, Sakamoto H. Genome-wide germline analyses on cancer susceptibility and GeMDBJ database: Gastric cancer as an example. Cancer Sci. 2010;101:1582-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Shi D, Wang S, Gu D, Wu D, Wang M, Chu H, Tong N, Ma L, Zhong D, Zhang Z. The PSCA polymorphisms derived from genome-wide association study are associated with risk of gastric cancer: a meta-analysis. J Cancer Res Clin Oncol. 2012;138:1339-1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Li WQ, Hu N, Wang Z, Yu K, Su H, Wang L, Wang C, Chanock SJ, Burdett L, Ding T. Genetic variants in epidermal growth factor receptor pathway genes and risk of esophageal squamous cell carcinoma and gastric cancer in a Chinese population. PLoS One. 2013;8:e68999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Hyland PL, Lin SW, Hu N, Zhang H, Wang L, Su H, Wang C, Ding T, Tang ZZ, Fan JH. Genetic variants in fas signaling pathway genes and risk of gastric cancer. Int J Cancer. 2014;134:822-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Li WQ, Hu N, Hyland PL, Gao Y, Wang ZM, Yu K, Su H, Wang CY, Wang LM, Chanock SJ. Genetic variants in DNA repair pathway genes and risk of esophageal squamous cell carcinoma and gastric adenocarcinoma in a Chinese population. Carcinogenesis. 2013;34:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Gu F, Pfeiffer RM, Bhattacharjee S, Han SS, Taylor PR, Berndt S, Yang H, Sigurdson AJ, Toro J, Mirabello L. Common genetic variants in the 9p21 region and their associations with multiple tumours. Br J Cancer. 2013;108:1378-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Sans M. Admixture studies in Latin America: from the 20th to the 21st century. Hum Biol. 2000;72:155-177. [PubMed] |
| 32. | Wang S, Ray N, Rojas W, Parra MV, Bedoya G, Gallo C, Poletti G, Mazzotti G, Hill K, Hurtado AM. Geographic patterns of genome admixture in Latin American Mestizos. PLoS Genet. 2008;4:e1000037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 314] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 33. | Galanter JM, Fernandez-Lopez JC, Gignoux CR, Barnholtz-Sloan J, Fernandez-Rozadilla C, Via M, Hidalgo-Miranda A, Contreras AV, Figueroa LU, Raska P. Development of a panel of genome-wide ancestry informative markers to study admixture throughout the Americas. PLoS Genet. 2012;8:e1002554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 34. | Freedman ML, Haiman CA, Patterson N, McDonald GJ, Tandon A, Waliszewska A, Penney K, Steen RG, Ardlie K, John EM. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci USA. 2006;103:14068-14073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 490] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 35. | Erdei E, Sheng H, Maestas E, Mackey A, White KA, Li L, Dong Y, Taylor J, Berwick M, Morse DE. Self-reported ethnicity and genetic ancestry in relation to oral cancer and pre-cancer in Puerto Rico. PLoS One. 2011;6:e23950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Pereira L, Zamudio R, Soares-Souza G, Herrera P, Cabrera L, Hooper CC, Cok J, Combe JM, Vargas G, Prado WA. Socioeconomic and nutritional factors account for the association of gastric cancer with Amerindian ancestry in a Latin American admixed population. PLoS One. 2012;7:e41200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Fernandes MR, de Carvalho DC, dos Santos ÂK, dos Santos SE, de Assumpção PP, Burbano RM, dos Santos NP. Association of slow acetylation profile of NAT2 with breast and gastric cancer risk in Brazil. Anticancer Res. 2013;33:3683-3689. [PubMed] |
| 38. | Alpízar-Alpízar W, Sierra R, Cuenca P, Une C, Mena F, Pérez-Pérez GI. [Association of the p53 codon 72 polymorphism to gastric cancer risk in a hight risk population of Costa Rica]. Rev Biol Trop. 2005;53:317-324. [PubMed] |
| 39. | González A, Ramírez V, Cuenca P, Sierra R. [Polymorphisms in detoxification genes CYP1A1, CYP2E1, GSTT1 and GSTM1 in gastric cancer susceptibility]. Rev Biol Trop. 2004;52:591-600. [PubMed] |
| 40. | Alpízar-Alpízar W, Pérez-Pérez GI, Une C, Cuenca P, Sierra R. Association of interleukin-1B and interleukin-1RN polymorphisms with gastric cancer in a high-risk population of Costa Rica. Clin Exp Med. 2005;5:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Ferrer-Ferrer M, Malespín-Bendaña W, Ramírez V, González MI, Carvajal A, Une C. Polymorphisms in genes coding for HSP-70 are associated with gastric cancer and duodenal ulcer in a population at high risk of gastric cancer in Costa Rica. Arch Med Res. 2013;44:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Sierra R, Une C, Ramirez V, Alpizar-Alpizar W, Gonzalez MI, Ramirez JA, De Mascarel A, Cuenca P, Perez-Perez G, Megraud F. Relation of atrophic gastritis with Helicobacter pylori-CagA(+) and interleukin-1 gene polymorphisms. World J Gastroenterol. 2008;14:6481-6487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Con SA, Con-Wong R, Con-Chin GR, Con-Chin VG, Takeuchi H, Valerín AL, Echandi G, Mena F, Brenes F, Yasuda N. Serum pepsinogen levels, Helicobacter pylori CagA Status, and cytokine gene polymorphisms associated with gastric premalignant lesions in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2007;16:2631-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Con SA, Takeuchi H, Con-Chin GR, Con-Chin VG, Yasuda N, Con-Wong R. Role of bacterial and genetic factors in gastric cancer in Costa Rica. World J Gastroenterol. 2009;15:211-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Morgan DR, Dominguez RL, Keku TO, Heidt PE, Martin CF, Galanko JA, Omofoye OA, Sandler RS. Gastric cancer and the high combination prevalence of host cytokine genotypes and Helicobacter pylori in Honduras. Clin Gastroenterol Hepatol. 2006;4:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Gehmert S, Velapatiño B, Herrera P, Balqui J, Santivañez L, Cok J, Vargas G, Combe J, Passaro DJ, Wen S. Interleukin-1 beta single-nucleotide polymorphism’s C allele is associated with elevated risk of gastric cancer in Helicobacter pylori-infected Peruvians. Am J Trop Med Hyg. 2009;81:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Silva F, Carvalho F, Peixoto A, Teixeira A, Almeida R, Reis C, Bravo LE, Realpe L, Correa P, David L. MUC1 polymorphism confers increased risk for intestinal metaplasia in a Colombian population with chronic gastritis. Eur J Hum Genet. 2003;11:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Torres MM, Acosta CP, Sicard DM, Groot de Restrepo H. [Genetic susceptibility and risk of gastric cancer in a human population of Cauca, Colombia]. Biomedica. 2004;24:153-162. [PubMed] |
| 49. | Herrera-Goepfert R, Zúñiga J, Hernández-Guerrero A, Rodríguez-Reyna T, Osnalla N, Ruíz-Morales J, Vargas-Alarcón G, Yamamoto-Furusho JK, Mohar-Betancourt A, Hernández-Pando R. [Association of the HLA-DQB*0501, allele of the major histocompatibility complex with gastric cancer in Mexico]. Gac Med Mex. 2004;140:299-303. [PubMed] |
| 50. | Herrera-Goepfert R, Yamamoto-Furusho JK, Onate-Ocana LF, Camorlinga-Ponce M, Munoz L, Ruiz-Morales JA, Vargas-Alarcon G, Granados J. Role of the HLA-DQ locus in the development of chronic gastritis and gastric carcinoma in Mexican patients. World J Gastroenterol. 2006;12:7762-7767. [PubMed] |
| 51. | Garza-González E, Bosques-Padilla FJ, Pérez-Pérez GI, Flores-Gutiérrez JP, Tijerina-Menchaca R. Association of gastric cancer, HLA-DQA1, and infection with Helicobacter pylori CagA+ and VacA+ in a Mexican population. J Gastroenterol. 2004;39:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Zúñiga-Noriega JR, Velazco-Campos Mdel R, Aguirre-Rodríguez A, Villarreal LM, Garza-González E, Maldonado-Garza HJ, Bosques-Padilla FJ. [C677T polymorphism of the MTHFR gene and the risk of developing distal gastric cancer in a Mexican population]. Rev Gastroenterol Mex. 2007;72:355-358. [PubMed] |
| 53. | Lacasaña-Navarro M, Galván-Portillo M, Chen J, López-Cervantes M, López-Carrillo L. Methylenetetrahydrofolate reductase 677C& gt; T polymorphism and gastric cancer susceptibility in Mexico. Eur J Cancer. 2006;42:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Galván-Portillo MV, Cantoral A, Oñate-Ocaña LF, Chen J, Herrera-Goepfert R, Torres-Sanchez L, Hernandez-Ramirez RU, Palma-Coca O, López-Carrillo L. Gastric cancer in relation to the intake of nutrients involved in one-carbon metabolism among MTHFR 677 TT carriers. Eur J Nutr. 2009;48:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Pérez-Pérez GI, Bosques-Padilla FJ, Crosatti ML, Tijerina-Menchaca R, Garza-González E. Role of p53 codon 72 polymorphism in the risk of development of distal gastric cancer. Scand J Gastroenterol. 2005;40:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Martínez-Carrillo DN, Garza-González E, Betancourt-Linares R, Mónico-Manzano T, Antúnez-Rivera C, Román-Román A, Flores-Alfaro E, Illades-Aguiar B, Fernández-Tilapa G. Association of IL1B -511C/-31T haplotype and Helicobacter pylori vacA genotypes with gastric ulcer and chronic gastritis. BMC Gastroenterol. 2010;10:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | López-Carrillo L, Camargo MC, Schneider BG, Sicinschi LA, Hernández-Ramírez RU, Correa P, Cebrian ME. Capsaicin consumption, Helicobacter pylori CagA status and IL1B-31C& gt; T genotypes: a host and environment interaction in gastric cancer. Food Chem Toxicol. 2012;50:2118-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Sicinschi LA, Lopez-Carrillo L, Camargo MC, Correa P, Sierra RA, Henry RR, Chen J, Zabaleta J, Piazuelo MB, Schneider BG. Gastric cancer risk in a Mexican population: role of Helicobacter pylori CagA positive infection and polymorphisms in interleukin-1 and -10 genes. Int J Cancer. 2006;118:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Torres-Jasso JH, Bustos-Carpinteyro AR, Marín ME, Santiago E, Leoner C, Flores-Luna L, Torres J, Sánchez-Lopez JY. Analysis of the polymorphisms EGFR-r521K and ERBB2-I655V in Mexican patients with gastric cancer and premalignant gastric lesions. Rev Invest Clin. 2012;65:150-155. [PubMed] |
| 60. | Garza-Gonzalez E, Bosques-Padilla FJ, Mendoza-Ibarra SI, Flores-Gutierrez JP, Maldonado-Garza HJ, Perez-Perez GI. Assessment of the toll-like receptor 4 Asp299Gly, Thr399Ile and interleukin-8 -251 polymorphisms in the risk for the development of distal gastric cancer. BMC Cancer. 2007;7:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Partida-Rodríguez O, Torres J, Flores-Luna L, Camorlinga M, Nieves-Ramírez M, Lazcano E, Perez-Rodríguez M. Polymorphisms in TNF and HSP-70 show a significant association with gastric cancer and duodenal ulcer. Int J Cancer. 2010;126:1861-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Medina-Franco H, Ramos-De la Medina A, Vizcaino G, Medina-Franco JL. Single nucleotide polymorphisms in the promoter region of the E-cadherin gene in gastric cancer: case-control study in a young Mexican population. Ann Surg Oncol. 2007;14:2246-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Gomes de Souza L, Miranda de Lima J, Dale Cotrim Guerreiro da Silva I, Manoukian Forones N. P53 Arg72Pro polymorphism in gastric cancer patients. J Gastrointest Cancer. 2009;40:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Khayat AS, Lobo Gatti L, Moura Lima E, de Assumpção PP, Nascimento Motta FJ, Harada ML, Casartelli C, Marques Payão SL, Cardoso Smith MA, Burbano RR. Polymorphisms of the TP53 codon 72 and WRN codon 1367 in individuals from Northern Brazil with gastric adenocarcinoma. Clin Exp Med. 2005;5:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Neves Filho EH, Cordeiro DE, Vieira AP, Rabenhorst SH. TP53 codon 72 and intron 3 polymorphisms and mutational status in gastric cancer: an association with tumor onset and prognosis. Pathobiology. 2012;79:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Jorge YC, Duarte MC, Silva AE. Gastric cancer is associated with NOS2 -954G/C polymorphism and environmental factors in a Brazilian population. BMC Gastroenterol. 2010;10:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Nishimoto IN, Hanaoka T, Sugimura H, Nagura K, Ihara M, Li XJ, Arai T, Hamada GS, Kowalski LP, Tsugane S. Cytochrome P450 2E1 polymorphism in gastric cancer in Brazil: case-control studies of Japanese Brazilians and non-Japanese Brazilians. Cancer Epidemiol Biomarkers Prev. 2000;9:675-680. [PubMed] |
| 68. | Colombo J, Rossit AR, Caetano A, Borim AA, Wornrath D, Silva AE. GSTT1, GSTM1 and CYP2E1 genetic polymorphisms in gastric cancer and chronic gastritis in a Brazilian population. World J Gastroenterol. 2004;10:1240-1245. [PubMed] |
| 69. | Borges Bdo N, Santos Eda S, Bastos CE, Pinto LC, Anselmo NP, Quaresma JA, Calcagno DQ, Burbano RM, Harada ML. Promoter polymorphisms and methylation of E-cadherin (CDH1) and KIT in gastric cancer patients from northern Brazil. Anticancer Res. 2010;30:2225-2233. [PubMed] |
| 70. | Gatti LL, Burbano RR, de Assumpção PP, Smith Mde A, Payão SL. Interleukin-1beta polymorphisms, Helicobacter pylori infection in individuals from Northern Brazil with gastric adenocarcinoma. Clin Exp Med. 2004;4:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Lobo Gatti L, Zambaldi Tunes M, de Lábio RW, Silva LC, de Arruda Cardoso Smith M, Marques Payão SL. Interleukin-6 polymorphism and Helicobacter pylori infection in Brazilian adult patients with chronic gastritis. Clin Exp Med. 2005;5:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Gatti LL, Burbano RR, Zambaldi-Tunes M, de-Lábio RW, de Assumpção PP, de Arruda Cardoso-Smith M, Marques-Payão SL. Interleukin-6 polymorphisms, Helicobacter pylori infection in adult Brazilian patients with chronic gastritis and gastric adenocarcinoma. Arch Med Res. 2007;38:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Rocha GA, Guerra JB, Rocha AM, Saraiva IE, da Silva DA, de Oliveira CA, Queiroz DM. IL1RN polymorphic gene and cagA-positive status independently increase the risk of noncardia gastric carcinoma. Int J Cancer. 2005;115:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Melo Barbosa HP, Martins LC, Dos Santos SE, Demachki S, Assumpção MB, Aragão CD, de Oliveira Corvelo TC. Interleukin-1 and TNF-alpha polymorphisms and Helicobacter pylori in a Brazilian Amazon population. World J Gastroenterol. 2009;15:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (2)] |
| 75. | Borges Bdo N, Burbano RR, Harada ML. Survivin -31C/G polymorphism and gastric cancer risk in a Brazilian population. Clin Exp Med. 2011;11:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Hanaoka T, Sugimura H, Nagura K, Ihara M, Li XJ, Hamada GS, Nishimoto I, Kowalski LP, Yokota J, Tsugane S. hOGG1 exon7 polymorphism and gastric cancer in case-control studies of Japanese Brazilians and non-Japanese Brazilians. Cancer Lett. 2001;170:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Duarte MC, Colombo J, Rossit AR, Caetano A, Borim AA, Wornrath D, Silva AE. Polymorphisms of DNA repair genes XRCC1 and XRCC3, interaction with environmental exposure and risk of chronic gastritis and gastric cancer. World J Gastroenterol. 2005;11:6593-6600. [PubMed] |
| 78. | de Oliveira JG, Silva AE. Polymorphisms of the TLR2 and TLR4 genes are associated with risk of gastric cancer in a Brazilian population. World J Gastroenterol. 2012;18:1235-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 79. | de Oliveira JG, Rossi AF, Nizato DM, Miyasaki K, Silva AE. Profiles of gene polymorphisms in cytokines and Toll-like receptors with higher risk for gastric cancer. Dig Dis Sci. 2013;58:978-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Oliveira J, Felipe AV, Chang PY, Pimenta CA, Silva TD, Massud J, Forones NM. Association between the C3435T single-nucleotide polymorphism of multidrug resistance 1 gene and risk of gastric cancer. Mol Med Rep. 2012;6:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Kato I, Canzian F, Franceschi S, Plummer M, van Doorn LJ, Lu Y, Gioia-Patricola L, Vivas J, Lopez G, Severson RK. Genetic polymorphisms in anti-inflammatory cytokine signaling and the prevalence of gastric precancerous lesions in Venezuela. Cancer Causes Control. 2006;17:1183-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Rizzato C, Kato I, Plummer M, Muñoz N, Canzian F. Genetic variation in PSCA and risk of gastric advanced preneoplastic lesions and cancer in relation to Helicobacter pylori infection. PLoS One. 2013;8:e73100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Cañas M, Morán Y, Camargo ME, Rivero MB, Bohórquez A, Villegas V, Ramírez E, Rendón Y, Suárez A, Morales L. [TP53 codon 72 polymorphism and gastric cancer risk: a case-control study in individuals from the central-western region of Venezuela]. Invest Clin. 2009;50:153-161. [PubMed] |
| 84. | Cañas M, Morán Y, Rivero MB, Bohórquez A, Villegas V, Rendón Y, Ramírez E, Valderrama E, Briceño Z, Chiurillo MA. [Interleukin-1 genetic polymorphism: association with gastric cancer in the high-risk Central-Western population of Venezuela]. Rev Med Chil. 2009;137:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Chiurillo MA, Moran Y, Cañas M, Valderrama E, Alvarez A, Armanie E. Combination of Helicobacter pylori-iceA2 and proinflammatory interleukin-1 polymorphisms is associated with the severity of histological changes in Venezuelan chronic gastritis patients. FEMS Immunol Med Microbiol. 2010;59:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | Chiurillo MA, Moran YH, Cañas M, Valderrama EJ, Armanie E. Infection with specific Helicobacter pylori-cag pathogenicity island strains is associated with interleukin-1B gene polymorphisms in Venezuelan chronic gastritis patients. Dig Dis Sci. 2011;56:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Canzian F, Franceschi S, Plummer M, van Doorn LJ, Lu Y, Gioia-Patricola L, Vivas J, Lopez G, Severson RK, Schwartz AG. Genetic polymorphisms in mediators of inflammation and gastric precancerous lesions. Eur J Cancer Prev. 2008;17:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Martínez T, Hernández-Suárez G, Bravo MM, Trujillo E, Quiroga A, Albis R, Robayo JC, Bravo JC, Camorlinga M. [Association of interleukin-1 genetic polymorphism and CagA positive Helicobacter pylori with gastric cancer in Colombia]. Rev Med Chil. 2011;139:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Lee K, Cáceres D, Varela N, Csendes D A, Ríos R H, Quiñones S L. [Allelic variants of cytochrome P4501A1 (CYP1A1), glutathione S transferase M1 (GSTM1) polymorphisms and their association with smoking and alcohol consumption as gastric cancer susceptibility biomarkers]. Rev Med Chil. 2006;134:1107-1115. [PubMed] |
| 90. | Castaño-Molina E, Santacoloma M, Arango L, Camargo M. Gastric cancer and detoxifying genes in a Colombian Population. Rev Col Gastroenterol. 2010;25:252-260. |
| 91. | Martínez T, Hernández G, Bravo MM, Trujillo E, Quiroga A, Robayo JC, Pérez J, Bravo JC, Margarita Camorlinga M. [Genetic polymorphisms of IL-1B-511, IL-1RN, IL-10 interleukins, tumor necrosis α-308 and positive Helicobacter pylori CagA infection in gastric cáncer and duodenal ulcer in different populations in Colombia. Rev Col Cancerol. 2011;15:31-43. |
| 92. | Cardona-Rivas D, Castaño-Molina E, Marín-Marmolejo JC. [Gastric cancer, tobacco usage, alcohol consumption, socioeconomic stratification and polymorphism in codon 72 of gene p53 in a population of Manizales]. Biosalud. 2007;6:33-44. |
| 93. | Trejo-de la O A, Torres J, Pérez-Rodríguez M, Camorlinga-Ponce M, Luna LF, Abdo-Francis JM, Lazcano E, Maldonado-Bernal C. TLR4 single-nucleotide polymorphisms alter mucosal cytokine and chemokine patterns in Mexican patients with Helicobacter pylori-associated gastroduodenal diseases. Clin Immunol. 2008;129:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Mattar R, Marques SB, Dos Santos AF, do Socorro Monteiro M, Iriya K, Carrilho FJ. A possible role of IL-1RN gene polymorphism in the outcome of gastrointestinal diseases associated with H. pylori infection. Clin Exp Gastroenterol. 2013;6:35-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 95. | Vinagre RM, Corvelo TC, Arnaud VC, Leite AC, Barile KA, Martins LC. Determination of strains of Helicobacter pylori and of polymorphism in the interleukin-8 gene in patients with stomach cancer. Arq Gastroenterol. 2011;48:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Felipe AV, Silva TD, Pimenta CA, Kassab P, Forones NM. lnterleukin-8 gene polymorphism and susceptibility to gastric cancer in a Brazilian population. Biol Res. 2012;45:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Persson C, Canedo P, Machado JC, El-Omar EM, Forman D. Polymorphisms in inflammatory response genes and their association with gastric cancer: A HuGE systematic review and meta-analyses. Am J Epidemiol. 2011;173:259-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 98. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1675] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 99. | Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut. 2001;48:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Camargo MC, Mera R, Correa P, Peek RM, Fontham ET, Goodman KJ, Piazuelo MB, Sicinschi L, Zabaleta J, Schneider BG. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1674-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 101. | Kamangar F, Cheng C, Abnet CC, Rabkin CS. Interleukin-1B polymorphisms and gastric cancer risk--a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 102. | Loh M, Koh KX, Yeo BH, Song CM, Chia KS, Zhu F, Yeoh KG, Hill J, Iacopetta B, Soong R. Meta-analysis of genetic polymorphisms and gastric cancer risk: variability in associations according to race. Eur J Cancer. 2009;45:2562-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 103. | Xue H, Lin B, Ni P, Xu H, Huang G. Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta-analysis. J Gastroenterol Hepatol. 2010;25:1604-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 104. | He B, Zhang Y, Pan Y, Xu Y, Gu L, Chen L, Wang S. Interleukin 1 beta (IL1B) promoter polymorphism and cancer risk: evidence from 47 published studies. Mutagenesis. 2011;26:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 105. | Bonequi P, Meneses-González F, Correa P, Rabkin CS, Camargo MC. Risk factors for gastric cancer in Latin America: a meta-analysis. Cancer Causes Control. 2013;24:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 106. | Ye BD, Kim SG, Park JH, Kim JS, Jung HC, Song IS. The interleukin-8-251 A allele is associated with increased risk of noncardia gastric adenocarcinoma in Helicobacter pylori-infected Koreans. J Clin Gastroenterol. 2009;43:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Batista dos Santos SE, Rodrigues JD, Ribeiro-dos-Santos AK, Zago MA. Differential contribution of indigenous men and women to the formation of an urban population in the Amazon region as revealed by mtDNA and Y-DNA. Am J Phys Anthropol. 1999;109:175-180. [PubMed] |
| 108. | Lins TC, Vieira RG, Abreu BS, Grattapaglia D, Pereira RW. Genetic composition of Brazilian population samples based on a set of twenty-eight ancestry informative SNPs. Am J Hum Biol. 2010;22:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 109. | Cheng D, Hao Y, Zhou W, Ma Y. Positive association between Interleukin-8 -251A > T polymorphism and susceptibility to gastric carcinogenesis: a meta-analysis. Cancer Cell Int. 2013;13:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 110. | Zhu Y, Wang J, He Q, Zhang JQ. The association between interleukin-10-592 polymorphism and gastric cancer risk: a meta-analysis. Med Oncol. 2011;28:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 111. | El-Omar EM, Ng MT, Hold GL. Polymorphisms in Toll-like receptor genes and risk of cancer. Oncogene. 2008;27:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 112. | Tang W, Zhou X, Nie S, Yang Z, Zhu H, Wu X, Zhao R, Luo Y. Association of p53 Arg72Pro polymorphism with gastric cancer: a meta-analysis. Biomarkers. 2012;17:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 113. | Valenzuela M, Pérez-Pérez G, Corvalán AH, Carrasco G, Urra H, Bravo D, Toledo H, Quest AF. Helicobacter pylori-induced loss of the inhibitor-of-apoptosis protein survivin is linked to gastritis and death of human gastric cells. J Infect Dis. 2010;202:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Carneiro P, Fernandes MS, Figueiredo J, Caldeira J, Carvalho J, Pinheiro H, Leite M, Melo S, Oliveira P, Simões-Correia J. E-cadherin dysfunction in gastric cancer--cellular consequences, clinical applications and open questions. FEBS Lett. 2012;586:2981-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 115. | Cui Y, Xue H, Lin B, Ni P, Fang JY. A meta-analysis of CDH1 C-160A genetic polymorphism and gastric cancer risk. DNA Cell Biol. 2011;30:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 116. | Chen B, Zhou Y, Yang P, Liu L, Qin XP, Wu XT. CDH1 -160C& gt; A gene polymorphism is an ethnicity-dependent risk factor for gastric cancer. Cytokine. 2011;55:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Sala N, Muñoz X, Travier N, Agudo A, Duell EJ, Moreno V, Overvad K, Tjonneland A, Boutron-Ruault MC, Clavel-Chapelon F. Prostate stem-cell antigen gene is associated with diffuse and intestinal gastric cancer in Caucasians: results from the EPIC-EURGAST study. Int J Cancer. 2012;130:2417-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 118. | Qiao L, Feng Y. Genetic variations of prostate stem cell antigen (PSCA) contribute to the risk of gastric cancer for Eastern Asians: a meta-analysis based on 16792 individuals. Gene. 2012;493:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 119. | Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, Matsuno Y, Saito D, Sugimura H, Tanioka F, Kato S. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008;40:730-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 328] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 120. | Peleteiro B, Lunet N, Carrilho C, Durães C, Machado JC, La Vecchia C, Barros H. Association between cytokine gene polymorphisms and gastric precancerous lesions: systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:762-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |