Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.3847
Revised: January 10, 2014
Accepted: February 26, 2014
Published online: April 14, 2014
Processing time: 184 Days and 17.3 Hours
Colorectal cancer (CRC) is one of the most prevalent cancers in developed countries. On the other hand, CRC is also one of the most curable cancers if it is detected in early stages through regular colonoscopy or sigmoidoscopy. Since CRC develops slowly from precancerous lesions, early detection can reduce both the incidence and mortality of the disease. Fecal occult blood test is a widely used non-invasive screening tool for CRC. Although fecal occult blood test is simple and cost-effective in screening CRC, there is room for improvement in terms of the accuracy of the test. Genetic dysregulations have been found to play an important role in CRC development. With better understanding of the molecular basis of CRC, there is a growing expectation on the development of diagnostic tests based on more sensitive and specific molecular markers and those tests may provide a breakthrough to the limitations of current screening tests for CRC. In this review, the molecular basis of CRC development, the characteristics and applications of different non-invasive molecular biomarkers, as well as the technologies available for the detection were discussed. This review intended to provide a summary on the current and future molecular diagnostics in CRC and its pre-malignant state, colorectal adenoma.
Core tip: In this review, the molecular basis of colorectal cancer (CRC) development, the characteristics and applications of different non-invasive molecular biomarkers, as well as the technologies available for the detection were discussed. This review intended to provide a summary on the current and future molecular diagnostics in CRC and its pre-malignant state, colorectal adenoma.
- Citation: Tsang AHF, Cheng KH, Wong ASP, Ng SSM, Ma BBY, Chan CML, Tsui NBY, Chan LWC, Yung BYM, Wong SCC. Current and future molecular diagnostics in colorectal cancer and colorectal adenoma. World J Gastroenterol 2014; 20(14): 3847-3857
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/3847.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.3847
Colorectal cancer (CRC) is one of the most prevalent cancers and cause of cancer mortality in developed countries[1]. Fortunately, CRC is also one of the most curable cancers if it is detected in early stage through regular colonoscopy[2]. Genetic mutations play important roles in CRC development[3]. Recently, different initiating genes have been found to be involved in different categories of CRC and most of the genetic mutations are somatic with no implication for future generations[4]. Nevertheless, studies on monozygotic twins have revealed that about 35% of CRC can be attributed to genetic susceptibility[5]. Based on the above findings, CRC development is most likely caused by genetic-environment interaction.
CRC can be broadly classified into two categories, one related to hereditary while another is non-hereditary (sporadic)[6,7]. Hereditary CRC can further be classified into two sub-groups, i.e., hereditary non-polyposis colorectal cancer (HNPCC) which comprises about one to six percent of all colorectal cancer[8] and multiple polyps CRC, which includes familial adenomatous polyposis (FAP), hamartomatous polyposis syndrome and MUTYH-associated adenomatous polyposis[6]. Each of the above mentioned CRC subtypes involve different genetic causes[6]. Conventionally, fecal occult blood testing (FOBT) was widely used as a non-invasive screening tool for CRC[9]. Although FOBT is simple and inexpensive, it is not an effective tool for screening CRC as false positive results might be yield by diet and medication. Immunological FOBT that detects human haemoglobin, although specific, shows low sensitivity at detecting adenomas and CRC[10]. Invasive screening tests such as colonoscopy are more effective and sensitive in screening CRC, however, high cost and inconvenience limits the diagnostic value since they require extensive bowel preparation and invasion of privacy[10]. Following the better understanding in the molecular basis of CRC, molecular markers testing may be an alternative to FOBT in non-invasive CRC screening.
Studies showed that the incidence rate of CRC was higher in men and the male-to-female incidence rate ratio has increased progressively[11]. However, the rate of incidence among races has not been frequently reported[12]. The peak incidence for CRC was found to be between ages of 61 and 70[11]. Approximately 6% of the incidence occurred before age of 30 is possibly hereditary CRC rather than sporadic CRC[13]. If CRC is found in young patients, pre-existing polyposis syndrome may be suspected[14]. The death rate of CRC is highest in the United States, Australia, New Zealand and Eastern European countries while comparatively lower in Mexico, South America and Africa[14]. Such a difference in the rate of incidence has been suggested to be related to their lifestyles. Studies also showed that dietary practices, obesity and physical inactivity are the risk factors for CRC[15,16].
According to the Hong Kong Cancer Registry, the incidence of CRC in Hong Kong was 4335 (16.6% of all cancers) in 2012 and it was the second most common cancer in Hong Kong[17]. There is a rising concern on the importance of the early diagnosis of CRC.
Many CRCs remain asymptomatic for years before diagnosis. However, as for cecal and right colonic cancers that cause fatigue, weakness and iron-deficiency anemia, early detection of the disease may be possible at early stage and these bulky lesions bleed readily. Concerning left-sided lesions, occult bleeding, changes in bowel habit, left quadrant discomfort and weight loss may develop. Furthermore, there is a higher chance for early discovery and hence successful removal of the left-sided lesions since patients have prominent disturbance in bowel function such as diarrhea and constipation. However, the localization of the bowel segments and the more infiltrative nature of rectum and sigmoid will reduce the chance of CRC detection. Colorectal tumors spread to other parts of the body by direct extension into adjacent structures and metastasis through the lymphatics and blood vessels. According to the previous studies, the favored metastatic sites of CRC are lymph nodes, liver, lung and bones[14]. Currently, the most commonly used system to describe the extent of CRC is the tumor-nodes-metastasis (TNM) classification and staging system released by the American Joint Commission on Cancer[18].
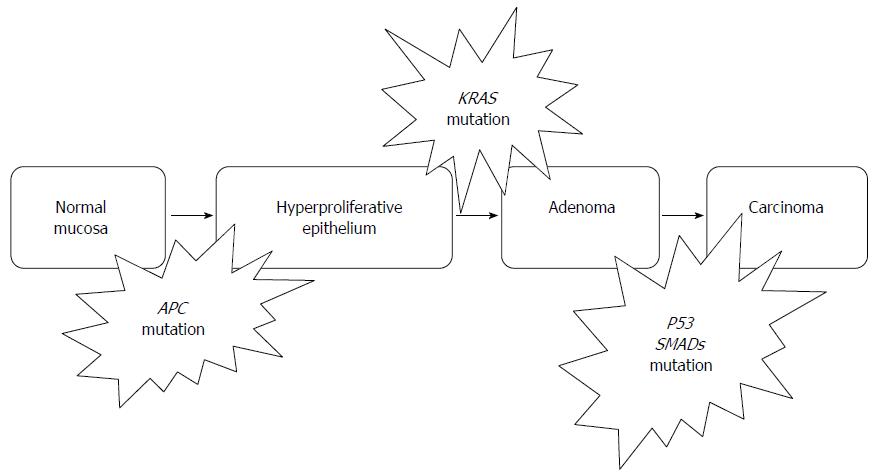
CRC is believed to be caused by a cascade of genetic mutations. The typical model for the carcinogenesis of CRC proposed by Fearon et al[19] can be described as adenoma-carcinoma sequence[6]. Adenoma-carcinoma sequence describes a gradual progression from normal epithelial mucosa to adenoma and then to carcinoma as a result of a series of genetic changes such as mutation and gene amplification[20] (Figure 1). The risk of recurrence and subsequent death due to CRC is closely related to the stage of the disease at the time of the first diagnosis[6]. Recent studies showed the risk of death from CRC could be reduced by shifting the detection of the disease to an earlier stage via mass screening and intervening[20]. Therefore, there is an urgent need for biomarkers for early detection of CRC[21].
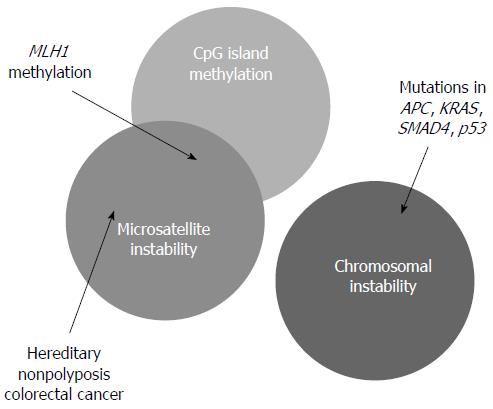
In the molecular aspects, CRC is caused by the loss of genomic stability that drives the development of CRC by facilitating the acquirement of tumor-associated mutations. In CRC or adenoma, several forms of genomic instability have been identified, including chromosomal instability (CIN), microsatellite instability (MSI), and epigenetic gene silencing.
Basically, CIN is characterized by any chromosomal copy number or structure change. CIN is the commonest genomic instability that encompasses 80% to 85% of all CRC and adenoma[6]. These types of genetic problem always result in abnormal karyotypes such as aneuploidy, chromosome rearrangement, oncogene activation and loss of heterozygosity of tumor suppressor genes[6]. It is suggested that CIN induces carcinoma through the loss or mutation of tumor suppressor genes such as APC, TP53 and also through activation of oncogenes such as KRAS[22]. CRC caused by CIN usually have poor prognosis[23].
MSI accounts for 15% of CRC[6]. It is characterized by altered length of gene with small deletions and insertions of short repetitive deoxyribonucleic acid (DNA) sequences (microsatellite) distributed throughout the genome[6,24]. Single MSI within the whole genome may have no significant effects but accumulation of the mutations can result in frame shifts within gene coding sequences and the subsequent inactivation of the genes would give rise to the progression of tumor[6]. Mutations are frequently found in the coding mononucleotide repeats of tumor suppressors such as transforming growth factor βR2 (69%) and activin receptor type 2 (83%). Unlike the tumor caused by chromosomal instability, these kinds of tumors are diploid or near-diploid[6].
The underlying cause of MSI can be explained by two mechanisms[6]. The first mechanism is the defective mismatch repair (MMR) system, in which both alleles of a MMR gene (MLH1, MSH2, MSH6, and PMS2) are nonfunctional. This results in the loss of ability to repair DNA replication mismatches in the affected cells[22]. The germline mutation of the MMR gene can lead to HNPCC[6]. The second mechanism is the hypermethylation of promoter in MMR genes that suppress the expression of the genes like MLH1[22].
Epigenetic silencing of genes is mostly caused by DNA methylation[6]. Cancers with high degrees of methylation can be considered as CpG island methylation phenotype (CIMP) positive[25], and CIMP encompasses 35%-40% of sporadic CRC[6].
DNA methylation is involved in normal cellular control of gene expression[6]. A vast majority of methylated cytosines in the human genome are found in the CpG dinucleotide sequences. In normal cells, dense regions of CpG sequences (CpG island) are usually found in the regions close to promoters[6]. The methylation patterns of these CpG sequences are gene-specific. Aberrant CpG hypermethylation can lead to silencing of tumor-suppressor genes in carcinogenesis since the expression of the genes is repressed[25]. For example, p16, p14, MGMT and hMLH1 are commonly silenced genes in CRC patients[26]. In some cases, the presence of epigenetic silencing is overlapped with MSI[22] (Figure 2). Some sporadic CRC with microsatellite instability is caused by DNA methylation. For example, DNA methylation of MLH1 gene promoter blocks its expression and destroys the ability of MMR system[27].
HNPCC: HNPCC is the most common hereditary colon cancer syndrome. It is autosomal dominant[28]. The presence of HNPCC is defined as the presence of germ-line mutation found in one of the four MMR genes, namely MLH1, MSH2, MSH6 and PMS2[29]. Bethesda guidelines can be used as a screening tool for HNPCC[30].
FAP: FAP is the most common polyposis syndrome. It is autosomal dominant and is caused by de novo germ-line mutations[31]. The presence of FAP can be diagnosed by direct sequencing of the germ-line mutations in APC gene on chromosome 5q21[32].
CRC develops slowly via accumulation of genetic mutations. Therefore, many CRCs remain asymptomatic for years before diagnosis. Carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) are the two most investigated gastrointestinal tumor markers of CRC. Although the high levels of the CEA and CA are associated with cancer progression in CRC, they may not be detected until the cancer is in advanced stages[10]. Moreover, the levels of those markers may also elevate in response to other diseases[10]. For example, high level of CEA may also be found in patients with inflammatory bowel disease. To conclude, CEA and CA are not effective in early detection of CRC. They should be used as prognostic markers rather than diagnostic markers.
Detection of CRC in early stages can reduce both the incidence and mortality of the disease. Molecular markers that detect gene mutation in the early stages of CRC can be used as non-invasive screening tests for early detection of CRC, followed by invasive confirmatory tests such as colonoscopy for individuals with positive results.
Since hereditary and sporadic forms of CRC are clinically different, the following discussions are focused on sporadic CRC.
CIN tumor marker: RAS family is a series of genes including HRAS, NRAS and KRAS[22]. The RAS family of oncogenes encode plasma membrane proteins at inner surface[6]. In RAS family, KRAS gene plays the most important role. KRAS gene encodes GTP (guanosine 5′-triphosphate)-binding proteins, which function as molecular switches and act as self-inactivating intracellular signal transducers for surface receptors such as epidermal growth factor receptor. The activation of RAS genes can promote cell survival and suppress apoptosis[22].
The oncogenic mutations of RAS family are believed to be an early event in CRC and they occur in 37% of CRCs and approximately 50% of adenomas > 1 cm (9% in adenomas < 1 cm)[23]. Most of KRAS mutations occur in codon 12 (70%-80%) and codon 13[23,33]. In clinical applications, KRAS mutation analysis is widely used as a prognostic and predictive biomarker of anti-EGFR monoclonal antibodies like cetuximab and panitumumab to predict the therapeutic effectiveness in CRC[34].
As well as KRAS, recent clinical studies start to focus on v-raf murine sarcoma viral oncogene homologue B1 (BRAF) and neuroblastoma-ras (NRAS). BRAF genes encode serine/threonine protein kinase that is regulated by KRA protein[35]. Mutation in BRAF gene occurs in approximately 12% of all CRC patients and it is mutually exclusive of KRAS mutation[35]. Recent studies try to investigate the clinical importance of BRAF mutation as a prognostic and predictive marker to predict resistance to anti-EGFR therapy[36]. Consideration of BRAF mutation is also recommended when KRAS mutation is not found[37]. NRAS is closely related to KRAS. NRAS mutation occurs in codon 61 and it is found in approximately 3%-5% of all CRC patients[38]. Similar to BRAF mutation, NRAS mutation is mutually exclusive of KRAS mutation[37,38].
Adenomatous polyposis coli (APC) gene plays a crucial role in the Wnt/Wingless pathway. APC gene is the most important gatekeeper of colonic epithelial cell proliferation and it is responsible for controlling the underlying oncoprotein called β-catenin. The loss of function in APC gene may lead to the transition of adenoma from normal colonic mucosa due to the up-regulation of β-catenin[22].
Germline APC mutations usually give rise to FAP. It is an inherited cancer-associated disorder in which more than 100 adenomatous polyps can be developed in mutant gene carriers. For patient with FAP, the risk of CRC by the age of 40 years is almost 100%. Somatic APC mutations are present in most sporadic colorectal adenomas and cancers. Similar to KRAS, APC mutations appear in the early stage of the progression from adenoma to carcinoma. Therefore, mutations in the APC gene are good biomarkers for identifying individuals at risk of CRC in patients’ families so as to guide the frequency of CRC surveillance and the recommendation of prophylactic surgery[22].
MSI markers: Both polymerase chain reaction (PCR) based methods and immunounhistochemistry can be used to detect MSI. Immunounhistochemistry staining detects DNA MMR system proteins such as MLH1 and MSH2. The loss in these markers is indicative of MSI. However, immunounhistochemistry staining is only best performed when the resection specimens are fixed promptly and properly since the quality of staining would be affected. Furthermore, the size of the specimens is also a concern in staining. Small specimens are not suitable for staining since the amount of tumor cells and internal staining controls are limited[6].
As PCR-based methods, currently, a 5 biomarkers panel MSI test, so-called Bethesda markers, has been developed to assess MSI status (Table 1). It consists of 2 mononucleotide loci (BAT25 and BAT26) and 3 dinucleotide loci (D2S123, D5S346, and D17S250)[39]. This panel is useful to detect about 15% of all CRC due to germline mutation in one of the mismatch repair genes (MLH1, MSH2, MSH6 and PMS2) or epigenetic slicing of MLH1. Among the five tested regions, when MSIs are present in two or more regions, the tumor is classified as MSI-High. Otherwise, the tumor is considered as MSI -Low (MSI in one region) or MSI-Stable (no MSI)[24].
| Microsatellite | Forward primer | Reverse primer |
| BAT25 | 5’-TCGCCTCCAAGAATGTAAGT-3’ | 5’-TCTGCATTTTAACTATGGCTC-3’ |
| BAT26 | 5’-TGACTACTTTTGACTTCAGCC-3’ | 5’-AACCATTCAACATTTTTAACCC-3’ |
| D2S123 | 5’-AAACAGGATGCCTGCCTTTA-3’ | 5’-GGACTTTCCACCTATGGGAC-3’ |
| D5S346 | 5’-AGCAGATAAGACAGTATTACTAGTT-3’ | 5’-ACTCACTCTAGTGATAAATCGGG-3’ |
| D17S250 | 5’-GGAAGAATCAAATAGACAAT-3’ | 5’-GCTGGCCATATATATATTTAAACC-3’ |
The above classification system is highly valuable in prognosis and therapy since standard chemotherapy using 5-fluorouracil is not effective in treating MSI-High tumor. Instead, irinotecan-containing regimens have improved response and better prognosis for MSI-High tumor[23].
Epigenetic markers: CpG regions that are hypermethylated in CRC patients when compared to normal individuals are valuable for biomarker development. Methylations on different regions of DNA promoter have been shown to be involved in the early event of CRC development. APC methylation is one of the examples. In addition, the methylation of MLH1 associated silencing is widely used as prognostic and predictive markers for CRC[6].
Septin 9 (SEPT9) gene encodes the septin 9 protein, which is a member of GTP-binding proteins that involves in many cellular processes such as cell cycle. Disruption of SEPT9 gene could result in tumor formation[40]. In CRC development, the promoter region of SEPT9 gene was found to be hypermethylated at the early stage of CRC[41]. In the blood samples of CRC patients, the level of circulating methylated SEPT9 DNA sequences was found to be increased when compared to those of normal controls. This elevation was possibly due to apoptotic release of DNA from tumor cells into the bloodstream. The methylated SEPT9 DNA has been reported to be an effective diagnostic marker for the non-invasive detection of CRC that is independent to the ages and genders of the patients[41-44].
Unfragmented long-form DNA: In normal colonocytes, DNA is digested during apoptosis and some DNA fragments of 180-200 bps long is released into stool. However, cancerous cells in CRC show a decreased rate of apoptosis and they are not affected by the normal action of nuclear endonuclease[9]. As a result, intact unfragmented DNA sequences with 1800-2400 bp are detected in stool samples when colonocytes are shed into the lumen and then fecal stream[9,45]. The detection of unfragmented long DNA molecules in stool samples could therefore be used for CRC screening in additional to FOBT[9].
Methods for detection of gene mutation: There are a number of molecular techniques available to detect gene mutation in CRC such as allele-specific PCR, also known as amplification refractory mutation system (ARMS) that detects specific known mutated form of a gene and real-time quantitative PCR.
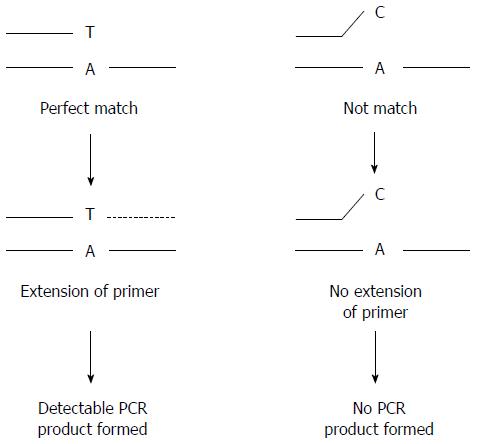
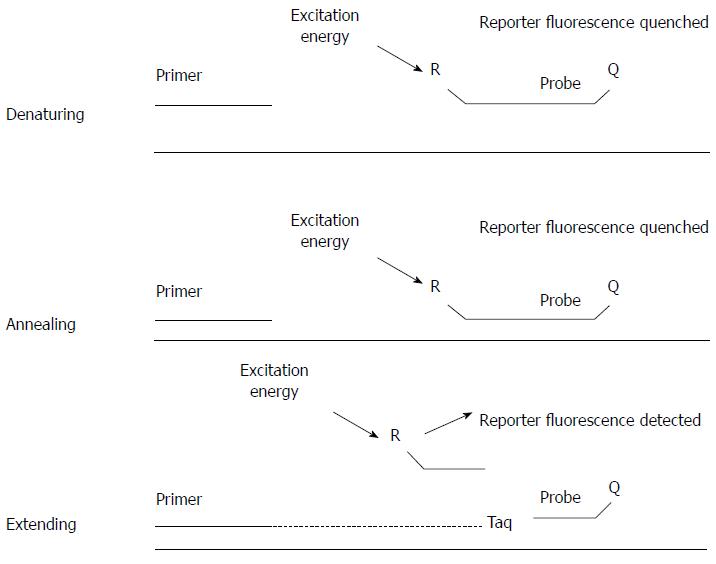
Both Sanger sequencing and allele-specific PCR can be used to detect the gene mutations mentioned above, such as APC and KRAS mutations. Sanger sequencing directly detects nucleotide sequences of regions of interest. Both known and novel nucleotide changes, including base substitution, insertion and deletion mutations, could be detected by this method. However, the long turnover time and high running cost make it not suitable for routine clinical uses. Allele-specific PCR is another choice for the detection of gene mutations with known positions and base changes (Figure 3). The method makes use of the allele-specific primers to selectively amplify the mutational allele sequences. Allele-specific PCR could be adopted to a real-time PCR platform in order to increase the speed and accuracy of the detection[46]. Real-time PCR employs a fluorogenic probe that is specific to the targeted DNA sequence. The probe consists of a fluorophore and a quencher attached covalently on both ends. When the probe is intact, the quencher masks any fluorescent signal emitted by the fluorophore. During PCR, the probe is first annealed to the target sequence, and then broken down by the 5’ to 3’ exonuclease activity of DNA polymerase. After the degradation of the probe, the fluorophore is released from the quencher and fluorescence is detected in real time (Figure 4). By detecting the change in fluorescent signal, the amount of target DNA molecules can be measured. With the help of real-time PCR, screening of genes mutations can be performed in batch mode and the turnover time is shorter than that of Sanger sequencing[6]. Compared to conventional PCR, real-time PCR is quantitative, fast and sensitive. As no post-PCR manipulation, such as gel or capillary electrophoresis, is required, the risk of laboratory cross-contamination can be minimized. However, real-time PCR is not suitable for studying microsatellite in which the analysis of amplification product size is required.
Microsatellite markers detection: PCR amplification of specific microsatellite repeats is used for MSI detection. The presence of MSI is determined by comparing the length of nucleotide repeats in tumor cells with that of normal cells from the same patient. Normal cells adjacent to the suspicious tumor cells should be collected for comparison. The region of interest is PCR amplified with fluorescent primers and the amplification product is detected by capillary electrophoresis[24].
DNA hypermethylation detection: DNA hypermethylation can be detected in primary colorectal carcinomas using bisulfite conversion of DNA samples followed by methylation-specific PCR. Both quantitative methylation-specific PCR and pyrosequencing techniques can be employed[41,43,47,48].
Choice of biomarkers is very important. In order to develop a new diagnostic method, suitable biomarkers, which are biological substances that can be used to indicate the biological state of a patient, must be identified[10]. A good marker helps the detection of disease at earlier stage so that diseases can be cured effectively. Regarding CRC detection, since CRC is believed to be developed slowly via accumulation of genetic mutations, detection of the disease at earlier stage is the key concern for developing new diagnostic methods.
The road to the development of novel biomarkers begins with the discovery of potential candidates. The number of potential candidates produced in the initial stage of development is usually large[44]. Therefore, well designed selection processes are critical to identify clinically significant biomarkers. In selection of novel biomarkers, in terms of CRC detection, a good marker must be capable of discriminating between CRC patients and healthy individuals significantly[44]. As a high-performing screening assay, it must be capable of detecting target analytes at extremely low level. In other words, the clinical sensitivity of the screening assay must be high enough to detect early stage CRC with adequately specificity to the disease[44]. Recommendations for biomarkers studies are given to the investigators by The National Cancer Institute Investigational Drug Steering Committee and United States Food and Drug Administration[49].
MicroRNAs (miRNAs) are small non-coding RNA that is usually 19-23 nucleotides in length. Due to their small sizes, miRNAs are more stable in blood and FFPE tissues than other nucleic acids such as DNA and RNA[50]. miRNAs are involved in post-transcriptional regulation of gene expression. Therefore, they are able to function as oncogenes or tumor suppressor genes, and dysregulation of miRNA would be associated to cancers. Recent studies showed that miRNA circulate in a stable and cell-free form in bloodstream[51]. Therefore, miRNAs that are specific to CRC in blood samples may be identified for the development of non-invasive prognostic and predictive markers of the diseases[52].
MiR-135a and miR-135b play important roles in the regulation of Wnt/Wingless pathway by down-regulating APC gene expression[53]. miR-17-3p and miR-92a have been found to be elevated in plasma and CRC tissues in CRC patients, and their levels decreased after removal of the cancer tissues[54]. In the plasma of CRC patients, circulating miR-92 and miR-17 concentrations have been reported to be elevated in the preoperative samples and the concentrations were markedly reduced in the postoperative samples[51]. Those results suggested that circulating miR-92 and miR-17 are potential non-invasive diagnostic markers for CRC. Apart from the miRNAs mentioned above, miR-211 is also believed to be a potential marker for the diagnosis and prognosis of CRC[55].
Currently, a number of techniques are available for the detection of miRNA expression. PCR based methods[56], microarrays and in situ hybridization are well developed platforms for miRNA profiling. Each technique has its own advantages and disadvantages. Therefore, a suitable technique that suits the requirements of the investigation and experimental conditions should be chosen.
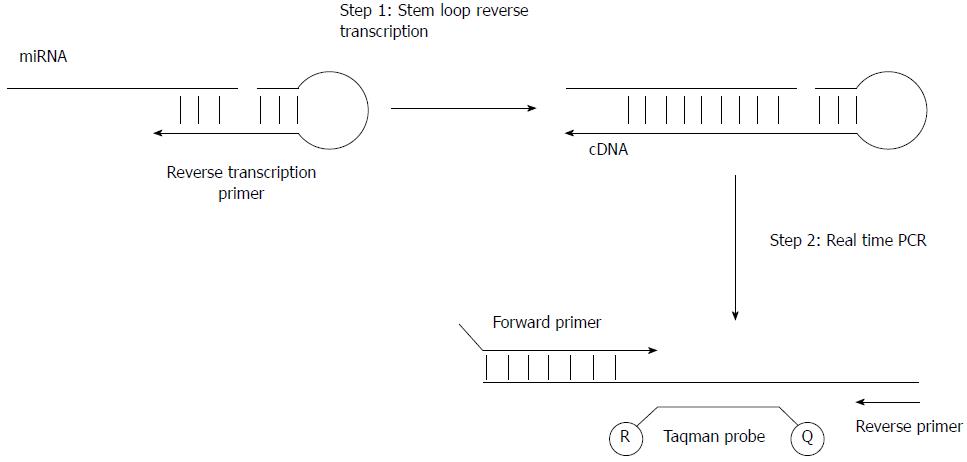
Quantitative reverse transcription PCR: The amount of miRNA in the specimen can be measured by real-time PCR. The first step of the measurement is to reverse transcribe miRNA into cDNA. A stem-loop primer, which enhances the miRNA reverse transcription efficiency by promoting the thermal stability of primer-miRNA complex, is usually employed. After reverse transcription, cDNA molecules are quantified by conventional real-time PCR[57] (Figure 5).
Microarray: MiRNA microarray is a technique based on the hybridization between target miRNAs and an array of predesigned detection probes that are covalently immobilized onto a glass slide. The isolated miRNAs are labeled with fluorescent dye and then hybridized to a miRNA microarray. Fluorescent signal that is emitted from the labeled miRNAs at different positions on the microarray is then detected. By evaluating and analyzing the fluorescence signal data, the identities and relative quantities of miRNAs can be determined[58].
Lateral flow nucleic acid assay: Molecular diagnostic techniques developed for miRNA detection can provide highly specific and sensitive diagnostic results. However, the current existing techniques are too expensive and resource-intensive for the clinics with poor settings to perform the test[59]. In addition, well trained personnel are also a must to carry out the test and analyze the results[60]. In order to put CRC related miRNA screening into routine health check up procedure, a simple and easy-to-use detection method is desired. The emergence of the lateral flow nucleic acid assay and gold nanoparticles (Au-NPs) may help in miRNA detection[60,61]. Lateral flow nucleic acid strip can give a simple, inexpensive, fast and sensitive assay which meets the needs of point-of-care in miRNA detection[59-61].
The principle of the assay is very straightforward. First of all, the specimen is mixed with gold nanoparticles conjugated detection probe (detection probe) and biotin-bridge probe (capture probe). If target miRNA exists in the specimen, after hybridization, both the detection probe and the target miRNA bind to the complementary capture probe. The miRNA-oligonucleotide complex flows down a test strip by capillary action. The complex is eventually captured at the detection zone containing anti-avidin antibody. Nuclease is used to degrade the nonbinding capture probe and detection probe[59]. Accumulation of gold nanoparticles causes the development of a red band which can be observed by naked eyes in the detection zone. As the strength of the signal generated is proportional to the amount of the target miRNA within the specimen, to assess the result quantitatively, the strips can be scanned and imaged by a quantitative detection platform[59-61]. In order to increase the sensitivity of the detection, silver enhancement can be used. Researchers have shown that the lateral flow nucleic acid test strips and gold nanoparticles were able to detect and quantify miRNA level in a specimen as low as 1 fmol and 5 amol without and with silver enhancement, respectively[59]. This technique provides a simple, convenient and fast detection for point-of-care detection[60,61].
Novel proteomic technologies have shown promise in identification of new protein markers and profiles for early detection of CRC[62-65]. Changes in protein patterns, either due to the secretion by the tumor tissues or the non-tumor cells in tumor microenvironment, in the bloodstream can serve as potential cancer markers for CRC detection. With the fast development of proteomics, CRC specific autoantibody patterns and proteomic expression profiles can now be identified by techniques such as matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) and surface-enhanced laser desorption/ionization time-of-flight[45]. Proteomic profiling of serum from CRC patients is a promising approach to discriminate CRC patients from healthy individuals[62].
In a previous study, blood samples from individuals diagnosed with CRC and normal healthy individuals were collected. Protein expression spectra were acquired using SELDI mass spectrometry. From the results, increased levels of transferrin, alpha-1-antitrypsin and complement C3a des-arg were identified and they are treated as potential biomarkers for CRC[65]. Similar works were also carried out using either SELDI[66] or MALDI-TOF[62-64].
Cancer biomarkers and characteristics of an ideal biomarker for CRC are discussed in this review, as well as the technologies available for their detection. This review aims to summarize the issues on the use of biomarkers for determination of prognosis as well as monitoring of response to therapy. Different molecular biomarkers including markers in stool and blood are discussed[21] (Table 2).
| Markers | Detection method | Specimen | Sensitivity | Specificity | Ref. | |
| KRAS | Mutation analysis | Stool | C | 55% | 100% | Puig et al[67], 2000 |
| A | 27% | 100% | ||||
| APC gene | Mutation analysis | Stool | C | 61% | 100% | Traverso et al[68], 2002 |
| A | 50% | 100% | ||||
| MSI markers | Digital-PCR based method | Stool | C | 37% | 100% | Traverso et al[69], 2002 |
| A | 0% | |||||
| Long form DNA | Quantitative fluorescence determination by PCR | Stool | C | 79% | 89% | Calistri et al[70], 2009 |
| Septin 9 methylated DNA | Real time PCR analysis | Plasma | C | 72% | 90% | Grützmann et al[42], 2008 |
The screening has been shown to be effective in terms of reduction of disease-related mortality and costs. Currently, FOBT is the only screening modality for CRC. DNA-based fecal markers are promising but are not widely used in clinical settings. Detecting abnormal DNA from the tumor cells, which are shed into the fecal stream, can give informative indication on the incidence of CRC. However, complicated environment in stool sample and the presence of PCR inhibitors such as bilirubin and bile acids limits the successful amplification of mutated DNA to a detectable quality[9].
In addition, insufficient sensitivity and specificity preclude the use of all existing serum markers such as carcinoembryonic antigen (CEA) for the early detection of CRC[10]. In the field of clinical research, oncology is expected to have the largest gains from biomarkers over the next five to ten years. Development of personalized medicine for cancer is closely linked to biomarkers, which may serve as the basis for diagnosis, drug discovery and monitoring of diseases. Early detection of CRC not only can help the patients but the healthcare system since expensive chemotherapies can be avoided.
A major challenge in the future development of cancer biomarkers will be the integration of proteomics with genomics and metabolomics data and their functional interpretation in conjunction with clinical data and epidemiology[21].
P- Reviewers: Casadesus D, Coleman HG, Mayol J, Soreide K, Yoshikawa R S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25543] [Article Influence: 1824.5] [Reference Citation Analysis (7)] |
| 2. | Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 373] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 3. | Nash GM, Gimbel M, Cohen AM, Zeng ZS, Ndubuisi MI, Nathanson DR, Ott J, Barany F, Paty PB. KRAS mutation and microsatellite instability: two genetic markers of early tumor development that influence the prognosis of colorectal cancer. Ann Surg Oncol. 2010;17:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer. 2009;9:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 507] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 5. | Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2809] [Cited by in RCA: 2769] [Article Influence: 110.8] [Reference Citation Analysis (1)] |
| 6. | Legolvan MP, Taliano RJ, Resnick MB. Application of molecular techniques in the diagnosis, prognosis and management of patients with colorectal cancer: a practical approach. Hum Pathol. 2012;43:1157-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Strate LL, Syngal S. Hereditary colorectal cancer syndromes. Cancer Causes Control. 2005;16:201-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Aaltonen LA, Salovaara R, Kristo P, Canzian F, Hemminki A, Peltomäki P, Chadwick RB, Kääriäinen H, Eskelinen M, Järvinen H. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 764] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 9. | Mak T, Hill J. A New Generation of Molecular Stool Testing. Int J Clin Rev. 2011;4:7. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 10. | Kim HJ, Yu MH, Kim H, Byun J, Lee C. Noninvasive molecular biomarkers for the detection of colorectal cancer. BMB Rep. 2008;41:685-692. [PubMed] |
| 11. | Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 566] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 12. | Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128:1668-1675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Xu AG, Yu ZJ, Jiang B, Wang XY, Zhong XH, Liu JH, Lou QY, Gan AH. Colorectal cancer in Guangdong Province of China: a demographic and anatomic survey. World J Gastroenterol. 2010;16:960-965. [PubMed] |
| 14. | Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Robbins and Cotran pathologic basis of disease. Philadelphia: Elsevier Saunders 2005; . |
| 15. | Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, Colditz GA. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 408] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 16. | Yusof AS, Isa ZM, Shah SA. Dietary patterns and risk of colorectal cancer: a systematic review of cohort studies (2000-2011). Asian Pac J Cancer Prev. 2012;13:4713-4717. [PubMed] |
| 17. | Hong Kong Cancer Registry. Top ten cancers in 2011. Available from: http://www3.ha.org.hk/cancereg/statistics.html. |
| 18. | Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 1004] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 19. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] |
| 20. | Cardoso J, Boer J, Morreau H, Fodde R. Expression and genomic profiling of colorectal cancer. Biochim Biophys Acta. 2007;1775:103-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Tanaka T, Tanaka M, Tanaka T, Ishigamori R. Biomarkers for colorectal cancer. Int J Mol Sci. 2010;11:3209-3225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1392] [Article Influence: 87.0] [Reference Citation Analysis (2)] |
| 23. | Al-Sohaily S, Biankin A, Leong R, Kohonen-Corish M, Warusavitarne J. Molecular pathways in colorectal cancer. J Gastroenterol Hepatol. 2012;27:1423-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 24. | Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 728] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 25. | Kanwal R, Gupta S. Epigenetic modifications in cancer. Clin Genet. 2012;81:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 26. | Kanthan R, Senger JL, Kanthan SC. Molecular events in primary and metastatic colorectal carcinoma: a review. Patholog Res Int. 2012;2012:597497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Curtin K, Slattery ML, Samowitz WS. CpG island methylation in colorectal cancer: past, present and future. Patholog Res Int. 2011;2011:902674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Clendenning M, Sotamaa K, Prior T, Westman JA. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 655] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 29. | Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum. 1991;34:424-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1278] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 30. | Peltomäki P. Lynch syndrome genes. Fam Cancer. 2005;4:227-232. [PubMed] |
| 31. | Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1271] [Article Influence: 90.8] [Reference Citation Analysis (1)] |
| 32. | Galiatsatos P, Foulkes WD. Familial adenomatous polyposis. Am J Gastroenterol. 2006;101:385-398. [PubMed] |
| 33. | Smith AJ, Stern HS, Penner M, Hay K, Mitri A, Bapat BV, Gallinger S. Somatic APC and K-ras codon 12 mutations in aberrant crypt foci from human colons. Cancer Res. 1994;54:5527-5530. [PubMed] |
| 34. | Heinemann V, Stintzing S, Kirchner T, Boeck S, Jung A. Clinical relevance of EGFR- and KRAS-status in colorectal cancer patients treated with monoclonal antibodies directed against the EGFR. Cancer Treat Rev. 2009;35:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Fransén K, Klintenäs M, Osterström A, Dimberg J, Monstein HJ, Söderkvist P. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004;25:527-533. [PubMed] |
| 36. | Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705-5712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1242] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 37. | NCCN clinical practice guidelines in oncology, colon cancer. Version 2, 2012,. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. |
| 38. | De Mattos-Arruda L, Dienstmann R, Tabernero J. Development of molecular biomarkers in individualized treatment of colorectal cancer. Clin Colorectal Cancer. 2011;10:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Losso GM, Moraes Rda S, Gentili AC, Messias-Reason IT. Microsatellite instability--MSI markers (BAT26, BAT25, D2S123, D5S346, D17S250) in rectal cancer. Arq Bras Cir Dig. 2012;25:240-244. [PubMed] |
| 40. | Hall PA, Russell SE. The pathobiology of the septin gene family. J Pathol. 2004;204:489-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 41. | Warren JD, Xiong W, Bunker AM, Vaughn CP, Furtado LV, Roberts WL, Fang JC, Samowitz WS, Heichman KA. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011;9:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 330] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 42. | Grützmann R, Molnar B, Pilarsky C, Habermann JK, Schlag PM, Saeger HD, Miehlke S, Stolz T, Model F, Roblick UJ. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS One. 2008;3:e3759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 307] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 43. | Tänzer M, Balluff B, Distler J, Hale K, Leodolter A, Röcken C, Molnar B, Schmid R, Lofton-Day C, Schuster T. Performance of epigenetic markers SEPT9 and ALX4 in plasma for detection of colorectal precancerous lesions. PLoS One. 2010;5:e9061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Lofton-Day C, Model F, Devos T, Tetzner R, Distler J, Schuster M, Song X, Lesche R, Liebenberg V, Ebert M. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54:414-423. [PubMed] |
| 45. | Bosch LJ, Carvalho B, Fijneman RJ, Jimenez CR, Pinedo HM, van Engeland M, Meijer GA. Molecular tests for colorectal cancer screening. Clin Colorectal Cancer. 2011;10:8-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Sundström M, Edlund K, Lindell M, Glimelius B, Birgisson H, Micke P, Botling J. KRAS analysis in colorectal carcinoma: analytical aspects of Pyrosequencing and allele-specific PCR in clinical practice. BMC Cancer. 2010;10:660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Herbst A, Kolligs FT. Detection of DNA hypermethylation in remote media of patients with colorectal cancer: new biomarkers for colorectal carcinoma. Tumour Biol. 2012;33:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | deVos T, Tetzner R, Model F, Weiss G, Schuster M, Distler J, Steiger KV, Grützmann R, Pilarsky C, Habermann JK. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem. 2009;55:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 404] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 49. | Dancey JE, Dobbin KK, Groshen S, Jessup JM, Hruszkewycz AH, Koehler M, Parchment R, Ratain MJ, Shankar LK, Stadler WM. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010;16:1745-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 50. | Rossi S, Di Narzo AF, Mestdagh P, Jacobs B, Bosman FT, Gustavsson B, Majoie B, Roth A, Vandesompele J, Rigoutsos I. microRNAs in colon cancer: a roadmap for discovery. FEBS Lett. 2012;586:3000-3007. [PubMed] |
| 51. | Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, Poon TC, Ng SS, Sung JJ. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 901] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 52. | Timoneda O, Balcells I, Córdoba S, Castelló A, Sánchez A. Determination of reference microRNAs for relative quantification in porcine tissues. PLoS One. 2012;7:e44413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Nagel R, le Sage C, Diosdado B, van der Waal M, Oude Vrielink JA, Bolijn A, Meijer GA, Agami R. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res. 2008;68:5795-5802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 376] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 54. | Dong Y, Wu WK, Wu CW, Sung JJ, Yu J, Ng SS. MicroRNA dysregulation in colorectal cancer: a clinical perspective. Br J Cancer. 2011;104:893-898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 55. | Pu XX, Huang GL, Guo HQ, Guo CC, Li H, Ye S, Ling S, Jiang L, Tian Y, Lin TY. Circulating miR-221 directly amplified from plasma is a potential diagnostic and prognostic marker of colorectal cancer and is correlated with p53 expression. J Gastroenterol Hepatol. 2010;25:1674-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 56. | Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods. 2010;50:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 932] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 57. | Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3676] [Cited by in RCA: 3907] [Article Influence: 195.4] [Reference Citation Analysis (0)] |
| 58. | Li W, Ruan K. MicroRNA detection by microarray. Anal Bioanal Chem. 2009;394:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 313] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 59. | Hou SY, Hsiao YL, Lin MS, Yen CC, Chang CS. MicroRNA detection using lateral flow nucleic acid strips with gold nanoparticles. Talanta. 2012;99:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Mao X, Xu H, Zeng Q, Zeng L, Liu G. Molecular beacon-functionalized gold nanoparticles as probes in dry-reagent strip biosensor for DNA analysis. Chem Commun (Camb). 2009;3065-3067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | He Y, Zhang S, Zhang X, Baloda M, Gurung AS, Xu H, Zhang X, Liu G. Ultrasensitive nucleic acid biosensor based on enzyme-gold nanoparticle dual label and lateral flow strip biosensor. Biosens Bioelectron. 2011;26:2018-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 62. | de Noo ME, Mertens BJ, Ozalp A, Bladergroen MR, van der Werff MP, van de Velde CJ, Deelder AM, Tollenaar RA. Detection of colorectal cancer using MALDI-TOF serum protein profiling. Eur J Cancer. 2006;42:1068-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Liao CC, Mehta A, Ward NJ, Marsh S, Arulampalam T, Norton JD. Analysis of post-operative changes in serum protein expression profiles from colorectal cancer patients by MALDI-TOF mass spectrometry: a pilot methodological study. World J Surg Oncol. 2010;8:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Karpova MA, Moshkovskii SA, Toropygin IY, Archakov AI. Cancer-specific MALDI-TOF profiles of blood serum and plasma: biological meaning and perspectives. J Proteomics. 2010;73:537-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Ward DG, Suggett N, Cheng Y, Wei W, Johnson H, Billingham LJ, Ismail T, Wakelam MJ, Johnson PJ, Martin A. Identification of serum biomarkers for colon cancer by proteomic analysis. Br J Cancer. 2006;94:1898-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 66. | Chen YD, Zheng S, Yu JK, Hu X. Artificial neural networks analysis of surface-enhanced laser desorption/ionization mass spectra of serum protein pattern distinguishes colorectal cancer from healthy population. Clin Cancer Res. 2004;10:8380-8385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 67. | Puig P, Urgell E, Capellá G, Sancho FJ, Pujol J, Boadas J, Farré A, Lluís F, González-Sastre F, Mora J. A highly sensitive method for K-ras mutation detection is useful in diagnosis of gastrointestinal cancer. Int J Cancer. 2000;85:73-77. [PubMed] |
| 68. | Traverso G, Shuber A, Levin B, Johnson C, Olsson L, Schoetz DJ, Hamilton SR, Boynton K, Kinzler KW, Vogelstein B. Detection of APC mutations in fecal DNA from patients with colorectal tumors. N Engl J Med. 2002;346:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 69. | Traverso G, Shuber A, Olsson L, Levin B, Johnson C, Hamilton SR, Boynton K, Kinzler KW, Vogelstein B. Detection of proximal colorectal cancers through analysis of faecal DNA. Lancet. 2002;359:403-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Calistri D, Rengucci C, Molinari C, Ricci E, Cavargini E, Scarpi E, Milandri GL, Fabbri C, Ravaioli A, Russo A. Quantitative fluorescence determination of long-fragment DNA in stool as a marker for the early detection of colorectal cancer. Cell Oncol. 2009;31:11-17. [PubMed] |