Published online Jan 7, 2014. doi: 10.3748/wjg.v20.i1.290
Revised: October 29, 2013
Accepted: November 12, 2013
Published online: January 7, 2014
Processing time: 115 Days and 22.8 Hours
AIM: To explore risk factors of lymphatic metastasis (LM) in gallbladder cancer, and their potential to complement unsatisfactory radiological detection.
METHODS: Radiological detection of LM by computed tomography (CT) was reported to fail in more than 60% of patients with pathological LM. In order to find risk factors highly suggestive of LM other than radiological manifestations, the documents of 63 patients were analyzed statistically. Except for 4 patients having T1a disease, in whom cholecystectomy is enough for radical resection, 59 patients underwent lymphadenectomy with at least 3 lymph nodes dissected. Fifty point eight percent (32/63) of patients were found to have LM during pathological examination. The median number of dissected lymph nodes was 6 (range 3-20).
RESULTS: Only 31.3% (10/32) of patients with LM were detected by CT. Through multivariate analysis, two risk factors of LM were discovered as age < 60 years (OR = 6.24; P < 0.01) and carbohydrate antigen (CA) 19-9 elevation (OR = 5.70; P < 0.05). By analysis of patients with pathological LM but failed to be detected by CT, 81.8% (18/22) of patients had at least one risk factor, including 31.3% (10/32) who had the risk factor of age < 60 years, and 37.5% (12/32) who had the risk factor of CA 19-9 elevation. Besides, among patients with LM (n = 32), those whose age were younger than 60 years (OR = 3.41; P < 0.05) were more likely to have 3 or more positive lymph nodes.
CONCLUSION: Age < 60 years and CA 19-9 elevation could complement radiological detection of LM. Patients aged < 60 years are at higher risk of multiple positive nodes.
Core tip: High-quality lymphadenectomy is an essential part of radical resection of gallbladder cancer. However, until now, no frequently used radiological modalities could satisfy the need of precise preoperative evaluation of lymphatic status, including ultrasonography, computed tomography (CT), magnetic resonance and positron emission tomography, and most patients with lymphatic metastasis (LM) could not be detected until postoperative pathology. This study discovered two risk factors of LM as age < 60 years and carbohydrate antigen 19-9 elevation. By combining these two factors to direct radiological detection of LM on CT, the total percentage of LM detected preoperatively could have a drastic increase from 31.3% to 81.8%.
- Citation: Yu TN, Shen B, Meng N, Yu H, Cai XJ. Risk factors of lymphatic metastasis complement poor radiological detection in gallbladder cancer. World J Gastroenterol 2014; 20(1): 290-295
- URL: https://www.wjgnet.com/1007-9327/full/v20/i1/290.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i1.290
Gallbladder cancer is the most aggressive biliary cancer, and is frequently accompanied with lymphatic metastasis (LM)[1]. Metastasis via the lymphatic route could happen when tumors extend to the muscular layer (T1b)[2], and deteriorate patients’ prognosis obviously.
In patients with LM, lymphadenectomy is an essential part of radical resection, and should ensure clearance of all positive lymph nodes. Although high-quality lymphadenectomy is becoming more and more popularized in some tertiary centers[3-7], according to the database of surveillance, epidemiology, and end results in United States, only 5.3% among a total of about 3000 patients underwent qualified lymphadenectomy based on the minimum standard set by American Joint Committee on Cancer (AJCC)[8]. Currently, there is no effective radiological method for preoperative detection of LM. Computed tomography (CT), which is the most frequently used radiological modality, could only detect 36% of patients with LM in N1 station, and 47% in N2 station[9]. Even worse, the poor detection of LM by CT could not be complemented by using advanced radiological modalities, such as positron emission tomography (PET)/CT and magnetic resonance imaging (MRI). In a study by Petrowsky et al[10], PET/CT detected 12% of LM in patients with biliary tract cancer. In a study by Corvera et al[11], PET/CT detected 29% of LM. Detection of LM was not obviously improved by using MRI, since according to a study by Kim et al[12], MR only detected 56% of LM.
In order to achieve effective preoperative detection of LM, this study aims to explore risk factors other than radiological manifestations. Besides, from data of patients with LM found through pathological examination, we also analyzed risk factors correlated with having 3 or more positive nodes in patients with LM. The phenomenon of having more than 1 positive node is not rare in patients with LM, which suggests poor prognosis even after radical resection[13,14].
Documents of patients with gallbladder cancer treated at Sir Run Run Shaw Hospital in China from January 2005 to May 2013 were reviewed. In our center, radical resection contained three parts: (1) cholecytectomy; (2) resection of partial liver with or without resection of other adjacent organ; and (3) lymphadenectomy.
During lymphadenectomy, the portal triad was routinely dissected. If there was suspicious metastasis, lymph nodes in other groups such as the peripancreatic, superior mesenteric, celiac and periaortic groups were selectively dissected.
According to the AJCC staging system[15], accurate pathological diagnosis of lymphatic status should be based on at least 3 dissected nodes. Patients with 0-2 dissected lymph nodes had unclear lymphatic status. Therefore, our study did not include these patients, unless they were staged as T1a (n = 4), for whom lymphadenectomy was not necessary[2,16]. Patients with incidental gallbladder cancer were not included, since most of them did not undergo preoperative staging by CT. In total, documents of 63 patients were analyzed in this study.
All patients had tests of tumor biomarkers before operation. Elevation of carbohydrate antigen 19-9 (CA 19-9), carcinoembryonic antigen (CEA) and CA-125 was defined as > 37 IU/L, > 5 ng/mL and > 35 IU/L respectively.
All the patients received CT within two weeks before operation. The instrument used was a Sensation 16 scanner (Siemens, Erlanger, Germany). CT scan included three phases as plain, arterial and venous. The multidetector thin-section was 7 mm. At CT imaging, none of the patients had unresectable invasions, such as metastasis to the peritoneum, superior mesenteric artery lymph nodes or the aortic lymphatic group.
At CT imaging, LM was defined as having lymph nodes with a dimension of at least 1.0 cm; adjacent organ invasion was defined as a loss of fat planes between gallbladder wall and adjacent organ; and dilatation of the common bile duct was defined as its diameter wider than 1.0 cm.
Independent samples were compared by using the Pearson’s χ2 test, continuity (Yate’s) correction or Fisher’s exact test, and related samples were compared by using sign tests. For candidate predictors in multivariate analysis (logistic regression), the removal limit was P < 0.10, and entry limit was P < 0.05. All analyses were performed using SPSS, version 16. P < 0.05 was considered statistically significant.
All patients (n = 63) in this study underwent radical resection. Among them, there were 23 males and 40 females. The median age was 62 years (range 39 to 81 years). The most commonly used operation (n = 38) was a combination of cholecystectomy and hepatic wedge resection. Besides, 3 patients had additional resection of the transverse colon, 3 patients had right hemihepatectomy and 6 patients had hepatopancreaticoduodenectomy (Table 1).
| Characteristic | Value |
| Median age (range) | 62 (39-81) |
| Gender | |
| Male/female | 23/40 |
| Types of radical resection1 | |
| Normal radical resection | |
| C alone | 61 |
| C + WR + N | 38 |
| C + WR + N + BD | 7 |
| Extensive resection | |
| C + WR + N + resection of the transverse colon | 3 |
| C + right hemihepatectomy + N | 3 |
| HPD | 6 |
Except 4 patients staged as T1aN0M0, 59 patients underwent lymphadenectomy. The median number of dissected lymph nodes was 6 (range 3-20). Fifty point eight (32/63) of patients were found to have LM during pathological examination. Among them, 12 patients had 3 or more positive lymph nodes, and the median number of positive lymph node was 2 (range 1 to 7) (Table 2).
| Characteristic | Value |
| AJCC stage1 | |
| Stage I | 10 |
| Stage II | 14 |
| Stage IIIA | 7 |
| Stage IIIB | 30 |
| Stage IVA | 2 |
| Histologic grade | |
| Grade 1 | 22 |
| Grade 2 | 22 |
| Grade 3 | 17 |
| Grade 4 | 2 |
| Lymph node dissection | |
| Median number of dissected lymph nodes (range) | 6 (0-20) |
| Patients with LM | 32 |
| Median number of positive lymph nodes in patients with LM (range) | 2 (1-7) |
Preoperative factors including age, gender, jaundice, CA 19-9, CEA, CA 125, adjacent organ invasion at CT imaging and dilatation of the common bile duct at CT imaging were analyzed for their correlations with occurrence of LM. Through univariate and multivariate analysis, only age < 60 years (OR = 6.24; P < 0.01) and CA 19-9 elevation (OR = 5.70; P < 0.05) were found to be independent factors correlated with a higher risk of LM (P < 0.05) (Table 3).
| Variable | No-LM (n = 31) | LM (n = 32) | Univariate | Multivariate | ||
| P value | OR | 95%CI | Pvalue | |||
| Age | 0.015a | 6.240 | 1.66-22.41 | 0.007a | ||
| < 60 yr (n = 28) | 9 | 19 | ||||
| ≥ 60 yr (n = 35) | 22 | 13 | ||||
| Gender | 0.082 | 2.532 | 0.70-9.28 | 0.155 | ||
| Male (n = 23) | 8 | 15 | ||||
| Female (n = 40) | 23 | 17 | ||||
| Jaundice | 0.062 | 3.100 | 0.59-16.32 | 0.182 | ||
| Present (n = 12) | 3 | 9 | ||||
| Absent (n = 51) | 28 | 23 | ||||
| CA 19-9 elevation | 0.014a | 5.700 | 1.48-22.00 | 0.011a | ||
| Present (n = 26) | 8 | 18 | ||||
| Absent (n = 37) | 23 | 14 | ||||
| CA 125 elevation | 0.165 | |||||
| Present (n = 9) | 2 | 7 | ||||
| Absent (n = 54) | 29 | 25 | ||||
| CEA elevation | 0.805 | |||||
| Present (n = 13) | 6 | 7 | ||||
| Absent (n = 50) | 25 | 25 | ||||
| Adjacent organ invasion at CT imaging | 0.476 | |||||
| Present (n = 21) | 9 | 12 | ||||
| Absent (n = 42) | 22 | 20 | ||||
| Dilatation of the common bile duct at CT imaging | 0.784 | |||||
| Present (n = 11) | 5 | 6 | ||||
| Absent (n = 52) | 26 | 26 | ||||
We also explored risk factors predictive of having 3 or more positive nodes in patients with LM (n = 32). By univariate analysis, age < 60 years and female gender were found to be factors with statistical significance (P < 0.05). But by multivariate analysis, only age < 60 years (OR = 3.41) was found to be an independent factor correlated with a higher risk of having 3 or more positive lymph nodes (P < 0.05) (Table 4).
| Variable | 1 or 2 positive lymph nodes (n = 20) | 3 or more positive lymph nodes (n = 12) | Univariate | Multivariate | ||
| P value | OR | 95%CI | P value | |||
| Age | 0.033a | 3.41 | 1.17-9.92 | 0.025a | ||
| < 60 yr (n = 28) | 9 | 10 | ||||
| ≥ 60 yr (n = 35) | 11 | 2 | ||||
| Gender | 0.043a | 2.36 | 0.78-7.20 | 0.129 | ||
| Male (n = 23) | 14 | 4 | ||||
| Female (n = 40) | 6 | 8 | ||||
| Jaundice | 0.361 | |||||
| Present (n = 12) | 16 | 7 | ||||
| Absent (n = 51) | 4 | 5 | ||||
| CA 19-9 elevation | 0.198 | |||||
| Present (n = 26) | 13 | 5 | ||||
| Absent (n = 37) | 7 | 7 | ||||
| CA 125 elevation | 0.320 | |||||
| Present (n = 9) | 6 | 1 | ||||
| Absent (n = 54) | 14 | 11 | ||||
| CEA elevation | 0.740 | |||||
| Present (n = 13) | 16 | 9 | ||||
| Absent (n = 50) | 4 | 3 | ||||
| Adjacent organ invasion at CT imaging | 0.642 | |||||
| Present (n = 21) | 13 | 7 | ||||
| Absent (n = 42) | 7 | 5 | ||||
| Dilatation of common bile duct at CT imaging | 0.483 | |||||
| Present (n = 11) | 15 | 11 | ||||
| Absent (n = 52) | 5 | 1 | ||||
Only 31.3% (10/32) of patients with LM were detected by CT alone. The sensitivity, specificity and accuracy of radiological diagnosis were 31.3% (10/32), 100.0% (31/31) and 65.1% (41/63), respectively. Among those with pathological LM but failed to be preoperatively detected by CT, 81.8% (18/22) of patients had at least one risk factor, with 31.3% (10/32) who had the risk factor of age < 60 years, and 37.5% (12/32) who had the risk factor of CA 19-9 elevation. This analysis suggested that these risk factors could be used to complement the unsatisfactory results of radiological detection.
In this study, we discussed that preoperative lymphatic staging based on CT imaging was unsatisfying. Though having high accuracy to diagnose invasions of adjacent organs[17] and blood vessels[18], CT has its Achilles’ heel in detection of LM. Only 31.3% of patients with LM were successfully detected by CT. Heterogeneous enhancement and diameter > 1.0 cm were two major radiological criteria to diagnose LM at CT imaging. These diagnostic criteria were based on two characteristics of metastatic lymph nodes: cancerous necrosis for heterogeneous enhancement, and nodal enlargement for increase in diameter. However, based on a study by Ohtani et al[9], more than half (62%) of positive lymph nodes failed to be presented at CT imaging, suggesting that a large percentage of positive lymph nodes share great similarity to normal nodes, such as having small size. A study by Morimoto et al[19] verified this idea. In their research, the optimum cut-off size for positive lymph nodes was calculated as 7.5 mm, and using this standard to judge metastasis only have an unsatisfying sensitivity of 60.8%. Even worse, 23.5% of positive nodes had a diameter less than 5 mm.
Since no currently used radiological modalities could provide satisfying detection of LM, risk factors of LM other than radiological findings should be explored. In this study, we discovered two factors of age < 60 years and CA 19-9 elevation correlating with LM.
As for the factor of age < 60 years, while the mechanism behind the correlation between younger age and LM is unclear yet, except for gallbladder cancer, this correlation was already discovered in breast cancer[20], gastrointestinal stromal tumors (GISTs)[21] and rectal cancer[22]. A possible explanation is that younger patients tend to have lower differentiated types, thus more easily to have LM even when the tumor is within the gallbladder wall. However, due to the relatively small size of this study, there was no statistical significance found in the relation between differentiation type and age or LM (P > 0.05, Table 5). As for the factor of CA 19-9, it is one of the most frequently used tumor biomarkers in biliary cancer, and is also a type of selectin[23] with the major function of facilitating interaction of cancer cells with the endothelium of normal tissue[24,25].
| Differentiation type1 | Age < 60 yr (n = 28) | Age≥60 yr (n = 35) | P value (G1 vs G2-4) | No LM | LM | P value (G1 vs G2-4) |
| G1 | 9 | 13 | 0.679 | 13 | 9 | 0.250 |
| G2-4 | 19 | 22 | 18 | 23 | ||
| G2 | 10 | 12 | 10 | 12 | ||
| G3 | 7 | 10 | 7 | 10 | ||
| G4 | 2 | 0 | 1 | 1 |
In this study, of patients with LM but failed to be detected by CT, 81.8% of patients had at least one of the risk factors discussed above, which proves that these risk factors could complement the unsatisfactory radiological detection in resectable gallbladder cancer.
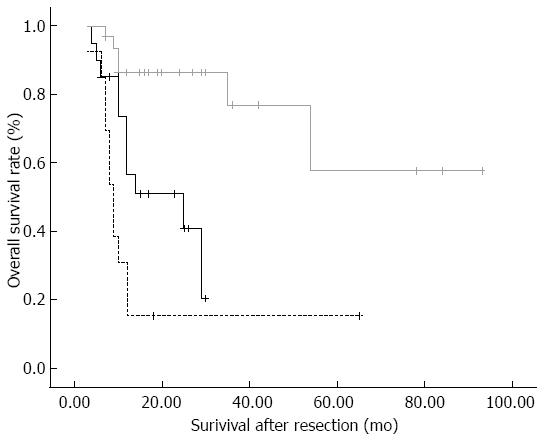
Furthermore, among patients with LM, we discovered age < 60 years as the only risk factor (OR = 3.41) related to the symptom of having 3 or more positive lymph nodes. The pattern of multiple positive lymph nodes was reported to be correlated with worse prognosis. In a study by Sakata et al[14], the cumulative 5-year postoperative survival rates were 62% for patients with one positive node, but only 15% for patients with 4 or more positive nodes. In a study by Shirai et al[26], the number of positive nodes predicts prognostic value better than anatomical location of positive nodes and lymph node ratio. Among patients in that study, the 1- and 3-year survival rates of patients having 3 or more positive nodes were 31% and 15%, respectively, which were significantly worse than those of patients having 1 to 2 positive nodes (74% vs 25%, P < 0.05, Figure 1). Therefore, for the aim of accurately predicting prognosis and avoiding residual of positive nodes, we believe that high quality lymphadenectomy should be ensured in patients aged < 60 years.
The relatively small size (n = 63) is the limitation of this study. This is mainly because of the relatively strict criterion we used for the selection of patients, which is, according to AJCC staging system[15], that an effective lymphadectomy should contain 3 or more dissected lymph nodes.
In conclusion, patients with factors of age < 60 years and CA 19-9 elevation are at a highly increased risk of LM. These two factors could be used as complements to preoperative lymphatic staging, especially for patients in whom CT failed to detect LM. High quality lymphadenectomy should be ensured for patients aged < 60 years since they are more likely to have 3 or more positive lymph nodes, especially when LM is present.
We thank Dr. Peng Hu from Radiological Department for his knowledge in the mechanism of radiological imaging of lymph nodes and soft tissue.
Gallbladder cancer is the most aggressive biliary cancer, and is frequently accompanied with lymphatic metastasis (LM). Precise preoperative evaluation of the extent of invasion, such as LM, is important for clinical surgical decision-making, and helps surgeons to be well-prepared for specific procedure, such as high-quality lymphadectomy.
Unfortunately, by presently used radiological modalities, including computed tomography (CT), magnetic resonance and positron emission tomography/CT, more than half of patients with LM could not be detected preoperatively. For CT, heterogeneous enhancement and diameter larger than 1.0 cm were two major radiological criteria to diagnose LM. However, a large percentage of positive lymph nodes do not have such two characteristic features and resemble normal lymph nodes in appearance. Many studies have reported the unsatisfactory results of radiological detection of LM, but no methods were discovered to solve this problem.
Since even the most advanced radiological methods could not provide effective detection of LM, we seek alternative clues (risk factors) of LM in preoperative findings other than radiological presentations. Age < 60 years (OR = 6.24) and carbohydrate antigen 19-9 elevation (OR = 5.70) were discovered as risk factors to complement radiological detection of LM. Besides, among patients with LM, those whose age were younger than 60 years (OR = 3.41) were more likely to have 3 or more positive lymph nodes.
By combining these two risk factors to direct radiological detection of LM on CT, the total percentage of LM detected preoperatively could have a drastic increase from 31.3% to 81.8%.
This study finds an innovative strategy to complement radiological staging of LM in gallbladder cancer, which is also convenient to use.
P- Reviewers: Aoyagi K, Shao R S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Ma S
| 1. | Fong Y, Wagman L, Gonen M, Crawford J, Reed W, Swanson R, Pan C, Ritchey J, Stewart A, Choti M. Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the National Cancer Database. Ann Surg. 2006;243:767-771; discussion 771-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | You DD, Lee HG, Paik KY, Heo JS, Choi SH, Choi DW. What is an adequate extent of resection for T1 gallbladder cancers? Ann Surg. 2008;247:835-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Agarwal AK, Kalayarasan R, Javed A, Sakhuja P. Role of routine 16b1 lymph node biopsy in the management of gallbladder cancer: an analysis. HPB (Oxford). 2013;Jul 22; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Ito H, Ito K, D’Angelica M, Gonen M, Klimstra D, Allen P, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 2011;254:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Liu GJ, Li XH, Chen YX, Sun HD, Zhao GM, Hu SY. Radical lymph node dissection and assessment: Impact on gallbladder cancer prognosis. World J Gastroenterol. 2013;19:5150-5158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Shirai Y, Sakata J, Wakai T, Ohashi T, Hatakeyama K. “Extended” radical cholecystectomy for gallbladder cancer: long-term outcomes, indications and limitations. World J Gastroenterol. 2012;18:4736-4743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Shirai Y, Wakai T, Hatakeyama K. Radical lymph node dissection for gallbladder cancer: indications and limitations. Surg Oncol Clin N Am. 2007;16:221-232. [PubMed] |
| 8. | Coburn NG, Cleary SP, Tan JC, Law CH. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surg. 2008;207:371-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Ohtani T, Shirai Y, Tsukada K, Hatakeyama K, Muto T. Carcinoma of the gallbladder: CT evaluation of lymphatic spread. Radiology. 1993;189:875-880. [PubMed] |
| 10. | Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, Clavien PA. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43-50. [PubMed] |
| 11. | Corvera CU, Blumgart LH, Akhurst T, DeMatteo RP, D’Angelica M, Fong Y, Jarnagin WR. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206:57-65. [PubMed] |
| 12. | Kim JH, Kim TK, Eun HW, Kim BS, Lee MG, Kim PN, Ha HK. Preoperative evaluation of gallbladder carcinoma: efficacy of combined use of MR imaging, MR cholangiography, and contrast-enhanced dual-phase three-dimensional MR angiography. J Magn Reson Imaging. 2002;16:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Endo I, Shimada H, Tanabe M, Fujii Y, Takeda K, Morioka D, Tanaka K, Sekido H, Togo S. Prognostic significance of the number of positive lymph nodes in gallbladder cancer. J Gastrointest Surg. 2006;10:999-1007. [PubMed] |
| 14. | Sakata J, Shirai Y, Wakai T, Ajioka Y, Hatakeyama K. Number of positive lymph nodes independently determines the prognosis after resection in patients with gallbladder carcinoma. Ann Surg Oncol. 2010;17:1831-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Greene FL, Cancer AJCo, Society AC. AJCC Cancer Staging Manual. London: Springer Limited 2002; . |
| 16. | Fetzner UK, Hölscher AH, Stippel DL. Regional lymphadenectomy strongly recommended in T1b gallbladder cancer. World J Gastroenterol. 2011;17:4347-4348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Kim SJ, Lee JM, Lee JY, Choi JY, Kim SH, Han JK, Choi BI. Accuracy of preoperative T-staging of gallbladder carcinoma using MDCT. AJR Am J Roentgenol. 2008;190:74-80. [PubMed] |
| 18. | Kalra N, Suri S, Gupta R, Natarajan SK, Khandelwal N, Wig JD, Joshi K. MDCT in the staging of gallbladder carcinoma. AJR Am J Roentgenol. 2006;186:758-762. [PubMed] |
| 19. | Morimoto H, Ajiki T, Ueda T, Sawa H, Fujita T, Matsumoto I, Yasuda T, Fujino Y, Kuroda Y, Ku Y. Histological features of lymph node metastasis in patients with biliary tract cancer. J Surg Oncol. 2008;97:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Gann PH, Colilla SA, Gapstur SM, Winchester DJ, Winchester DP. Factors associated with axillary lymph node metastasis from breast carcinoma: descriptive and predictive analyses. Cancer. 1999;86:1511-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Agaimy A, Wünsch PH. Lymph node metastasis in gastrointestinal stromal tumours (GIST) occurs preferentially in young patients & lt; or = 40 years: an overview based on our case material and the literature. Langenbecks Arch Surg. 2009;394:375-381. [PubMed] |
| 22. | Sitzler PJ, Seow-Choen F, Ho YH, Leong AP. Lymph node involvement and tumor depth in rectal cancers: an analysis of 805 patients. Dis Colon Rectum. 1997;40:1472-1476. [PubMed] |
| 23. | Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53:354-361. [PubMed] |
| 24. | Le Pendu J, Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Clément M. ABH and Lewis histo-blood group antigens in cancer. APMIS. 2001;109:9-31. [PubMed] |
| 25. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Shirai Y, Sakata J, Wakai T, Ohashi T, Ajioka Y, Hatakeyama K. Assessment of lymph node status in gallbladder cancer: location, number, or ratio of positive nodes. World J Surg Oncol. 2012;10:87. [PubMed] |