Published online Sep 15, 1996. doi: 10.3748/wjg.v2.iSuppl1.22
Revised: January 27, 1996
Accepted: May 1, 1996
Published online: September 15, 1996
- Citation: Wood J. Enteric nervous system in pathogenesis of gastrointestinal motility disorders. World J Gastroenterol 1996; 2(Suppl1): 22-25
- URL: https://www.wjgnet.com/1007-9327/full/v2/iSuppl1/22.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.iSuppl1.22
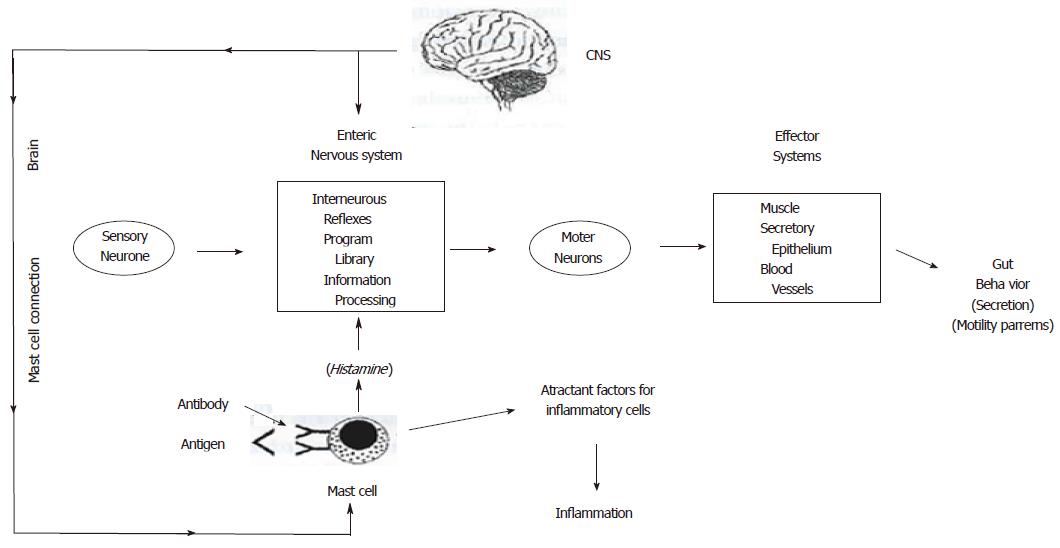
The concept of the little brain in the gut is basic to neurogastroenterology and understanding of gastrointestinal motility disorders. It refers to the presence in the gastrointestinal tract of a nervous system with functional properties similar to those of the brain and spinal cord, as well as the independent integrative systems responsible for behavior in simpler animals such as arthropods and mollusks[1]. The conceptual model (Figure 1) for the enteric nervous systems is the same as for other integrative nervous systems[2]. It is comprised of sensory neurons, interneurons and motor neurons with the directionality of information flow in this respective order.
Sensory neurons function to provide informatin in the state of the intestine. This consists of parameters such as contractile tension, mechanical brushing of the mucosal surface, osmolarity, pH, and glucose concentration. The information generated by sensory neurons is processed in microcircuits comprised of interneurons. Synaptic connections among the interneurons form the interneurons processing circuits.
Motor neurons innervate the gastrointestinal effector systems. The primary effector systems are the musculature, secretory epithelium and the blood vascular system. Motor neurons are the final common pathways from the interneuronal microcircuits. They transmit the commands to the effector systems that initiate or suppress activity of the effector systems. Outflow in motor neurons to the different effectors is coordinated by the interneuronal microcircuits to determine the overall behavior of a particular region of the gut consistent with the digestive state at any moment in time.
This overview will start with the neurophysiology of enteric motor neurons and relations of normal function to pathophysiology. This will be followed by review of the synaptic properties of the interneuronal microcircuits. References to current concepts of enteric neuroimmune communication and the implications of this for understanding gastrointestinal disorders will conclude the overview.
Basic knowledge of enteric neurophysiology has been obtained from standard methods of recording the electrical and synaptic behavior of enteric neurons in the various specialized regions of the digestive tract[3]. These are the same electrophysiological methods used productively for investigation of neuronal behavior in the brain and spinal cord. The methodology involves impalement of individual neurons with fine tipped glass micropipette electrodes. With appropriate amplifiers and accessory electronic devices, the electrodes record resting membrane potentials, action potential discharge and synaptic potentials in single neurons representative of the transmission and processing of signals within the microcircuits.
Synaptic events are recorded by the microelectrodes after impalement of the neuron. Synaptic potentials may be recorded as spontaneously occurring events or be evoked experimentally by electrical stimulation of the presynaptic axon that forms the synapse with the postsynaptic neuron. Synaptic potentials are evoked experimentally by electrical shocks from fine stimulating electrodes manipulated onto either the fiber tracts connect enteric ganglia or onto the surface of a ganglion.
Segments of the enteric nervous system are obtained for study by microdissection of the gastrointestinal wall and placement of the preparations into recording chambers for microscopic observation. The preparations are bathed by physiologic al solutions circulated by pumps providing continuous superfusion. The neurons are exposed to neurotransmitters, modulators and pharmacological agents of experimental interest by addition of the agent to the superfusion solution or by micropressure ejection from fine-tipped pipettes brought into close proximity to the neuron under study.
Enteric motor neurons are the final common pathways from the enteric microcircuits to the effector systems consisting of the musculature, the mucosa and the intramural blood vascular system. There are two populations of motor neurons, one of which is excitatory and the other inhibitory. One population of motor neurons to the musculature releases inhibitory transmitters that suppress contraction and relax tension in the muscle, whereas a second population releases excitatory neurotransmitters to evoke contraction. The intestinal crypts are innervated by a population of excitatory secretomotor neurons that, when active, evoke secretion of water, electrolytes and mucin from the crypts. Enteric vasomotor neurons release neurotransmitters that act to dilate the submucosal vasculature and thereby increase mucosal blood flow in support of secretory behavior.
Inhibitory motor neurons to the musculature have Dogiel Type I morphology as revealed by intraneuronal dye injection from recording electrodes and neuronal tracing methods. These are neurons with many short dendrites around the perimeter of relatively flat cell bodies with one long axon projecting from the cell bod y to the effector system. Most evidence suggests that the neurotransmitters released from inhibitory motor neurons are vasoactive intestinal peptide and nitric oxide.
The functional significance of inhibitory motor neurons is appropriately timed relaxation of tension in the musculature. During peristaltic propulsion, timed activation of inhibitory motor neurons relaxes the intestinal circular muscle ahead of the advancing bolus. In sphincteric regions, such as the lower esophageal sphincter or internal anal sphincter, the inhibitory motor neurons are silent and turned-on by the microcircuits with appropriate timing to relax the sphincter and allow passage of the intraluminal contents. In both the sphincteric smooth muscle and the circular musculature of the body of the small and large intestine, activation of the motor neurons and release of inhibitory neurotransmitter results in hyperpolarization of the membrane potential of the muscle cells to produce electrical potentials called inhibitory junction potentials.
Some of the inhibitory motor neurons in the body of the intestine are spontaneously active. Ongoing action potential discharge releases inhibitory neurotransmitters to maintain the autogenic musculature in a continuous state of inhibition. Evidence of the ongoing inhibition is found in experimental situations in vitro. For example, segments of cat small intestine, after equlibration in the organ bath, show electrical slow waves, but no action potentials or circular muscle contractions. Blockade of the ongoing activity of the inhibitory motor neurons by tetrodotoxin releases the inhibition resulting in the appearance of action potentials with each slow wave and the occurrence of large amplitude contractions at the frequency of the slow waves.
The behavior of enteric inhibitory motor neurons differs between sphincters and non-sphincteric regions of the gut. In the microcircuits of the sphincters, the inhibitory motor neurons are normally silent and turned-on transiently with appropriate timing for relaxation of the myogenic tone in the sphincter. On the other hand, some of the inhibitory motor neurons in the non-sphincteric intestinal regions are continuously active to maintain the autogenic musculature in a state of ongoing suppression. This accounts in part for the state of physiological ileus seen, for example, during the interdigestive state between the arrival of migrating motor complexes. Contractile responses of the muscle during normal behavior of the intestine require swithing-off the discharge of the inhibitory neurons. When the inhibitory neurons are off triggering of contractions by the electrical slow waves can occur.
The neurophysiology of inhibitory motor neurons suggests that any condition with ablation of the neurons will result in achalasia and discoordinated motor behavior. Hirschsprung’s disease is a classic example where congenital absence of inhibitory motor neurons, as well as other circuit elements, results in uncoordinated autogenic contractions of the musculature and failure of relaxation of the internal anal sphincter. Achalasia of the lower esophageal sphincter is associated with degenerative loss of the neurons in this region of the gut. Likewise, some forms of biliary dyskinesia are undoubtedly related to loss of the inhibitory innervation and achalasia in the sphincter of Oddi. In paraneoplastic syndrome, similarity of antigenic components of the small cell carcinomas in the lung and enteric neurons results in inflammatory ablation of the neurons. This eliminates inhibitory neural control of the musculature accounting for spasticity and non-propulsive contractile behavior with symptoms of intestinal pseudo-obstruction. A similar situation occurs in Chagas disease where similarity of surface antigens between the blood parasite Trypanosoma cruzi and the enteric neurons leads to inflammatory ablation of the integrative circuits of the intestinal minibrain[4]. Loss of enteric neurons in cases of intestinal pseudo-obstruction of unknown etiology suggest idiopathic degenerative disease of the enteric nervous system as a poorly understood factor in gastrointestinal motility disorders.
Like the motor neurons to the musculature, the secretomotor neurons to the intestinal crypts have Dogiel type I morphology. The neurotransmitters released from the neurons to evoke secretion include vasoactive intestinal peptide and acetyl choline. Acetylcholine acts at muscarinic receptors on the epithelial cells to initiate the cholinergic component of the secretory response.
Axons of the secretomotor neurons in guinea-pig small intestine give off collaterals that innervate blood vessels in the submucosa. When the secretomotor neurons fire, they evoke both secretion from the crypts and vasodilation which supports the secretory response with increased blood flow. The axon collaterals to the arterioles release acetylcholine that in turn activates release of nitric oxide from the vasculature endothelium to relax the vascular smooth muscle.
One kind of synaptic input to the secretomotor neurons consists of inhibitory inputs. When these inputs are activated, they result in hyperpolarization and suppression of action potential discharge by the neuron. The overall effect is to shut-off the secretomotor neurons and thereby prevent neurally evoked secretion from the crypts. Inhibitory synaptic inputs to the secretomotor neurons are provided by both the sympathetic innervation of the intestine and the intrinsic microcircuits. When active, the input from the sympathetic nervous system suppresses secretion by releasing norepinephrine to act at alpha-2 adrenoceptors on the secretomotor neurons. The inhibitory neurotransmitter released from intrinsic neural elements to suppress the secretomotor neurons appears to be somatostatin.
Disruption of normal function of intestinal secretomotor neurons leads to predictable states of secretory disease, particularly in the colon. Hyperactivity of the secretomotor neurons is associated with diarrheal states. This includes immune responses in which foreign antigens (e.g., parasites and food antigens) degran-ulate mast cells to release mediators that activate the secretomotor neurons. Cholera toxin which is taken up by submucosal neurons converts the neuronal behavior to an hyperexcitable state.
Hypoactivity of the secretomotor neurons is associated with constipation. Opiate abuse is a classical situation of constipation related to suppression of activity of intestinal secretomotor neurons. Opiates and opioid peptides when aplied to enteric neurons in vitro studies hyperpolarize the neurons and thereby suppress spike discharge. In animals addicted to morphine, application of naloxone dramatically increases the firing rate of the neurons.
The action of effective antidiarrheal drugs are explained by their inhibitory actions on secretomotor neurons. Clonidine, octreotide and loperamide all act to hyperpolarize and suppress spike discharge in secretomotor neurons. Clonidine acts at alpha-2 adrenoceptors to produce this effect, whereas octreotide is a somatostatin analog and loperamide an opioid.
Apart from the brain-in-the-gut, the digestive tract is recognized as the largest lymphoid organ in the body together with a unique compliment of mast cells. In its position at one of the dirtiest of interfaces between the body and outside world, the intestinal mucosal immune system continuously encounters dietary antigens, bacteria, viruses and toxins. Physical and chemical barriers at the epithelial interface are insufficient to exclude fully the large antigen load thereby allowing chronic challenges to the mucosal immune system.
Observations in antigen sensitized animal models suggest direct communication between the mucosal immune system and the minibrain in the intestine[4]. The communication is meaningful and results in adaptive behavior of the bowel in response to circumstances within the lumen that are threatening to the functionalintegrity of the whole animal. Communication is chemical in nature (paracrine) and incorporates specialized sensing functions of the immune cells for specific antigens together with the capacity of the enteric nervous system for intelligent interpretation of the signals. Immuno-neural integration progresses sequentially beginning with immune detection followed by signal transfer to enteric microcircuits followed by neural interpretation and then selection of a specific neural program of coordinated mucosal secretion and motor propulsion that effectively clears the antigenic threat from the intestinal lumen. Experimental approaches to immuno-neural interaction brings together the disciplines of mucosal immunology and enteric neurophysiology[5-7].
The intestinal tract is colonized from birth by a complement of immune cells that fluctuate with luminal conditions and pathophysiological states[8]. A variety of cell types including polymorphonuclear leukocytes, lymphocytes, macrophages, dendrocytes and mast cells are present in varying numbers. These are often found in close association with the neuronal elements of the enteric nervous system. The histoanatomy as well as immunophysiological observations indicate that elements of the enteric immune system are strategically positioned to establish a first line of defense against foreign invasion at a vulnerable interface between the body and the outside environment[5].
Foreign antigens in the form of foodstuffs, toxins or invading organisms sensitizes the enteric immune system. After sensitization, a second exposure to the same antigen triggers predictable integrated behavior of the intestinal effector systems. Coordinated activity of the muscle, mucosa and blood vasculature results in organized behavior of the whole intestine that rapidly expels the antigenic threat. Recognition of an antigen by the sensitized immuno-neuro apparatus calls forth a specialized propulsive motor program that is coordinated with copious secretion of water, electrolytes and mucus into the intestinal lumen. Detection by the enteric immune system and signaling to the enteric minibrain initiates the adaptive behavior[4].
The neurally organized pattern of muscle behavior in response to an offending antigen in the sensitized intestine is called power propulsion. The specialized form of intestinal motility forcefully and rapidly propels any material in the lumen over long distances and effectively strips the lumen clean. Its occurrence is accompanied by abdominal distress and diarrhea.
Power propulsion is one of the neural programs contained in the library of programs of the enteric minibrain. Output of the program reproduces the same stereotype d motor behavior in response to radiation exposure, mucosal contact with noxious stimulants or antigenic detection by the sensitized enteric immune system. The neural program for power propulsion incorporates connections for coordination of mucosal secretion with the motor behavior. The program is organized to stimulate copious secretion that flushes and lubricates the mucosa in the receiving segment ahead of the powerful propulsive contractions, which in turn, empty the lumen. The overall benefit for the well being of the animal is undoubtedly rapid elimination of material recognized by the immune system as threatening. The side-effects for the individual are lower gastrointestinal distress and diarrhea.
Several kinds of immune cells are potential sources of paracrine signals to the enteric minibrain[4]. Among these are lymphocytes, macrophages, polymorphonuclear leukocytes and mast cells. Most is known about signaling between mast cells and the elements of the local neural networks.
Intestinal mast cells proliferate during exposure to dangerous invaders such as Trichinella spiralis. Following an initial exposure to the nematode, immunoglobulin bound to receptors on the mast cells recognized the sensitizing antigens. Cross-linking of a specific antigen with the bound antibody triggers degranulation of the mast cells. Degranulation releases a variety of paracrine messengers which may include serotonin, histamine, prostaglandins, leukotrienes, platelet-activating factor and cytokines (Figure 1). Among these, histamine is implicated as a significant messenger in communication between the enteric immune system and the enteric nervous system[5,9].
Twentieth century progress in understanding the neurophysiological basis of integrative functions of the enteric nervous system supports the conclusion that the system functions like a minibrain. Most of the nervous functions associated with the central nervous system operate in the enteric nervous system. The implications of this are significant for understanding functional disorders of the gastrointestinal tract. Functional disorders of the central nervous system, such as Parkinson’s disease and Huntington’s chorea, were impossible to understand prior to the advancement of knowledge of the neurophysiology of the midbrain microcircuits responsible for programming of somatomotor behavior. This analogy holds true for functional bowel disorders where the underlying nervous malfunctions are not yet well understood. Understanding of many gastrointestinal disorders now categorized as functional disorders pivots on continued momentum in the development of knowledge of the neurophysiology of the enteric nervous system. Scientific investigation in this area has spawned the new subdiscipline of neurogastroenterology which is currently one of the exciting frontiers of gastrointestinal research.
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-).
E- Editor: Liu WX
| 1. | Wood JD. Congenital megacolon. Spontaneous Animal Models of Human Disease. New York: New York, Academic Press 1979; 29-34. |
| 2. | Wood JD, Cooke HJ. Murine models for congenital megacolon: hirschsprung’s Disease. Animal Models of Intestinal Disease. Boca Raton: C. R. C. Press 1985; 181-195. |
| 3. | Wood JD. Electrical and synaptic behavior of enteric neurons. Handbook of Physiology, The Gastrointestinal System, Motility and Circulation. Bethesda, Maryland: American Physiological Society 1989; 465-517. |
| 4. | Wood JD. Communication between minibrain in gut and enteric immune system. News Physiol Sci. Bethesda, Maryland: American Physiological Society 1991; 64-69. |
| 5. | Wood JD. Histamine signals in enteric neuroimmune interactions. Neuro Immuno Physiology of the Gastrointestinal Mucosa. New York: Ann New York Acad Sci 1991; 275-283. |
| 6. | Wood JD. Gastrointestinal neuroimmune interactions. Advances in the innervation of the Gastrointestinal Tract. Amsterdam: Elsevier Scientific Press 1992; 607-615. |
| 7. | Wood JD. Enteric neuroimmune interactions. Immunophysiology of the Gut. New York: Academic Press 1993; 207-227. |
| 8. | Wood JD. Physiology of the enteric nervous system. Physiology of the Gastrointestinal Tract. New York: Raven Press 1994; 423-482. |
| 9. | Wood JD, Progress in neurogastroenterology. In: Kirsner JB, editor. The Growth of Gastroenterologic Knowledge in the Twentieth Century. Malvern, Pennsylvania: Lea and Febiger 1994; 159-180. |