Published online Dec 15, 1996. doi: 10.3748/wjg.v2.i4.190
Revised: August 15, 1996
Accepted: October 10, 1996
Published online: December 15, 1996
AIM: To investigate the protective action of various doses of compound zinc (Zn) preparation on liver damages induced by cadmium (Cd) in pregnant rats.
METHODS: The changes in the microstructure and ultrastructure of hepatic cells were observed by microscopy and electron microscopy.
RESULTS: In Cd-toxic group, severe damage of hepatic cells was observed. Electron microscopic analysis showed extensive irreversible pathological changes, such as nearly dissolved hepatic cells with a lot of vacuoles, swelling endoplasmic reticulum and partial degeneration of mitochondrial cristae. However, in 300 μg/mL Zn group, the microstructure and ultrastructure were normal.
CONCLUSION: Zn has a remarkable protective effect on the Cd-induced hepatic cell damage in pregnant rats, and the effect is dose-dependent. Zn at a dose of 300 μg/mL has a significant protective action.
- Citation: Han GA, Jiang HM, Zhen XM, Zhou HJ, Xu CZ, Tian JX. Protective action of various doses of compound zinc preparation on cadmium-induced liver damage. World J Gastroenterol 1996; 2(4): 190-191
- URL: https://www.wjgnet.com/1007-9327/full/v2/i4/190.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i4.190
Cadmium (Cd) is a heavy metal that can cause carcinomas, malformation and mutation. It usually accumulates in the liver and kidney and causes serious damage to the liver and kidney. Zinc (Zn) has an obvious protective effect on Cd-induced liver damage in mice[1]. The present study investigated the protective action of Zn on Cd-induced hepatic cell damage in pregnant rats by observing the microstructure and ultrastructure of hepatic cells.
Sixty Wistar rats weighing 200-240 g were provided by the Experimental Animal Center of Shandong Medical University with a sex ratio of 2 females to 1 male. Self-made Zn preparation containing 450 μg/mL, 400 μg/mL and 300 μg/mL Zn, respectively, CdCl2·2.5H2O (AR grade), optical microscope, JEM-120EX transmission electron microscope and AOE ultramicrotome were used.
Twenty-eight pregnant rats were divided randomly into five groups: A, B, C, D and E. There were 5 rats in each group except that group A had 8 rats. In group A, rats with Cd toxication were given tap water; In groups B, C and D, rats with Cd toxication were given water containing 450 μg/mL, 400 μg/mL and 300 μg/mL Zn, respectively, on the day of gestation, and each was given 29.30 mL per day; In group E (control group), normal rats were given tap water. The pregnant rats in groups A-D were injected with 2 mg/kg Cd into the abdominal cavity on the 7th, 9th, and 11th d of gestation, while the pregnant rats in group E were injected with equal volume of saline solution. All rats were killed on the 20th d of gestation and their livers and kidneys were taken out. Routine sections were observed and photographed by microscopy and electron microscopy.
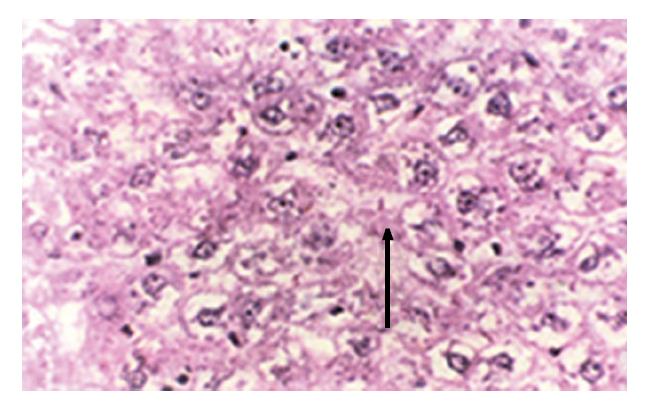
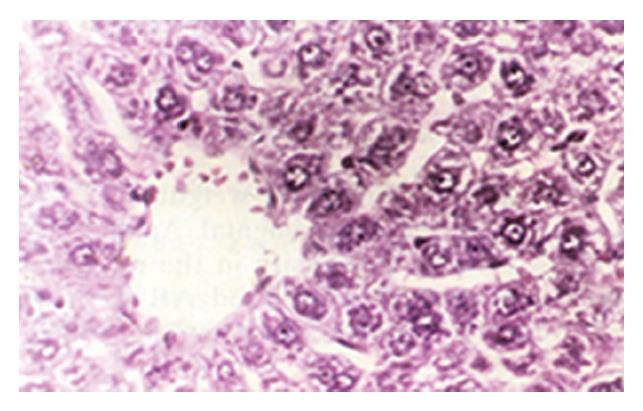
In group E, light microscopic examination revealed that the structure of hepatic cells was normal. Group A showed severe hydropic degeneration and sporadic lytic necrosis of hepatic cells (Figure 1). Group B exhibited diffuse hydropic degeneration of hepatic cells and focal inflammatory cellular infiltration. Group C revealed slight expansion in the central vein of the hepatic lobule, edema and necrosis of hepatic cells, and infiltration of lymphocytes. Group D demonstrated swelling of the hepatic cells and slight hydropic degeneration, which indicated that Zn at a dose of 300 μg/mL had obvious antagonism against Cd-induced damage (Figure 2).
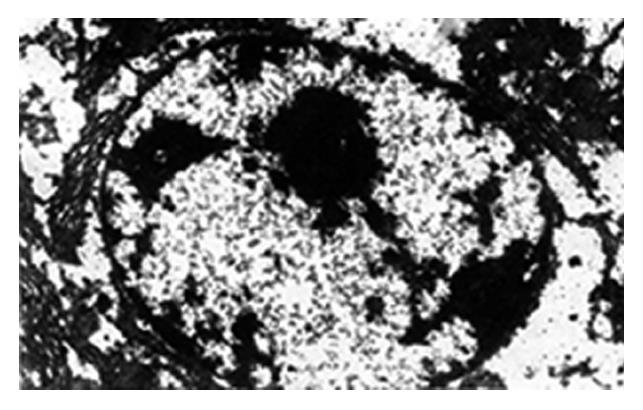
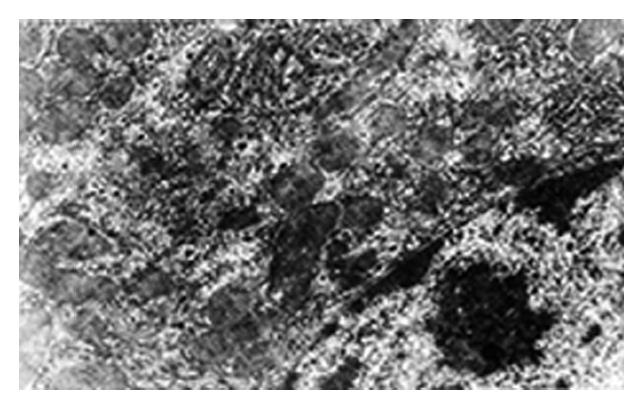
In group E, the ultrastructure of the hepatic cells was normal. Group A revealed the nearly dissolved hepatic cells with a lot of vacuoles, flocculent chromatin in the cytoplasm, unclear karyotheca, swelling endoplasmic reticulum and degenerated mitochondria (Figure 3). In groups B and C, the ultrastructure showed no significant differences compared with that of group A. In group D, the karyotheca of the hepatic cells was clear and regular, the endoplasmic reticulum was almost normal, the bilaminar membrane and cristae of mitochondria were clearly seen but vacuoles were still visible in the cytoplasm (Figure 4). Electron microscopic observations showed that group D had the obvious protective action on Cd induced damage to the ultrastructure of hepatic cells of pregnant rats, while groups B and C had no obvious antagonism, which is consistent with light microscopic examination.
In the present study, various doses of zinc preparation were given orally on the day of gestation before Cd toxication to antagonize Cd-induced damage to the liver of pregnant rats. Groups with 450 μg/mL and 400 μg/mL zinc preparation showed an unremarkable protective effect, but in group with 300 μg/mL zinc preparation, the microstructure and ultrastructure of the hepatic cells remained nearly normal, which suggests that Zn antagonism against Cd-induced damage was closely related to the dose of zinc preparation. The dose of 300 μg/mL Zn is consistent with the dose used in the animal experiment to antagonize Cd-induced malformation[2]. Therefore, it is very necessary to select the proper dose of zinc preparation to get best effect but low toxicity.
The antagonistic mechanism of Zn against Cd-induced liver damage is that one of the important biological functions of Zn in the organism is to induce the formation of metallothionein (MT). Taking zinc before Cd toxication greatly increases the synthesis of MT in the organism. Since Cd has greater power to synthesize Cd-MT, it will replace Zn in Zn-MT, form stable Cd-MT and reduce the concentration of free Cd[1]. Furthermore, taking zinc ahead of time can decrease the quantity of the toxic Cd-high molecular weight protein[3], so the ultrastructure of hepatic cells can be nearly normal. Another biological function of zinc is to protect cells so that they may divide and reproduce in accordance with physiological process[4].
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-).
S- Editor: Cao LB L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Cheng S, Wei ZM, Lu Z, Lei DN. Observation of quantitative electronmicroscopy on zinc protective action on liver from cadmium damage. Zhonghua Yufang Yixue. 1988;22:161-163. |
| 2. | Jiang HM, Han GA, Gao HF, Shi W, Dong YL. The correlation studies of maternal zinc level and fetal malformation. I. Study on the antagonism of compound zinc preparation to cadmium-induced fetal malformation. Shandong Yixueyuan Xuebao. 1995;33:115-118. |
| 3. | Kudo N, Yamashina S, Waku K. Protection against cadmium toxicity by zinc: decrease in the Cd-high molecular weight protein fraction in rat liver and kidney on Zn pretreatment. Toxicology. 1986;40:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Jiang HM, Han GA, Li BQ, Gao HF, Yu YN, Sun SA. Study on the antagonism of zinc and selenium to cadmium-induced lipid peroxidation in rats. Shandong Yixueyuan Xuebao. 1994;32:25-129. |