Published online Feb 28, 2013. doi: 10.3748/wjg.v19.i8.1322
Revised: January 9, 2013
Accepted: January 23, 2013
Published online: February 28, 2013
Processing time: 109 Days and 17.4 Hours
Multiple endocrine neoplasia type 1 (MEN1) is a rare hereditary syndrome known to predispose subjects to endocrine neoplasms in a variety of tissues such as the parathyroid glands, pituitary gland, pancreas and gastrointestinal tract. We herein report a patient with a past history of pituitary adenoma, presenting with symptoms of chronic diarrhea for nearly one year and a sudden upper gastrointestinal hemorrhage as well as perforation without signs. Nodules in the duodenum and in the uncinate process and tail of pancreas and enlargement of the parathyroid glands were detected on preoperative imaging. Gastroscopy revealed significant ulceration and esophageal reflux diseases. The patient underwent subtotal parathyroidectomy and autotransplantation, pylorus-preserving pancreaticoduodenectomy and pancreatic tail resection and recovered well. The results observed in our patient suggest that perforation and bleeding of intestine might be symptoms of Zollinger-Ellison Syndrome in patients with MEN1.
- Citation: Lu YY, Zhu F, Jing DD, Wu XN, Lu LG, Zhou GQ, Wang XP. Multiple endocrine neoplasia type 1 with upper gastrointestinal hemorrhage and perforation: A case report and review. World J Gastroenterol 2013; 19(8): 1322-1326
- URL: https://www.wjgnet.com/1007-9327/full/v19/i8/1322.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i8.1322
Multiple endocrine neoplasia type 1 (MEN1) syndrome is transmitted as an autosomal dominant mutation of a suppressor gene located on chromosome 11q13 and it is a rare congenital disease. The classical clinical manifestation of MEN1 is composed of parathyroid hyperplasia, pancreatic endocrine tumor and pituitary adenoma[1]. The definition of MEN1 is the coincidence of at least two of the above mentioned tumors[1].
We herein report a case of MEN1 with sudden upper gastrointestinal hemorrhage and perforation 14 years after surgery for pituitary adenoma.
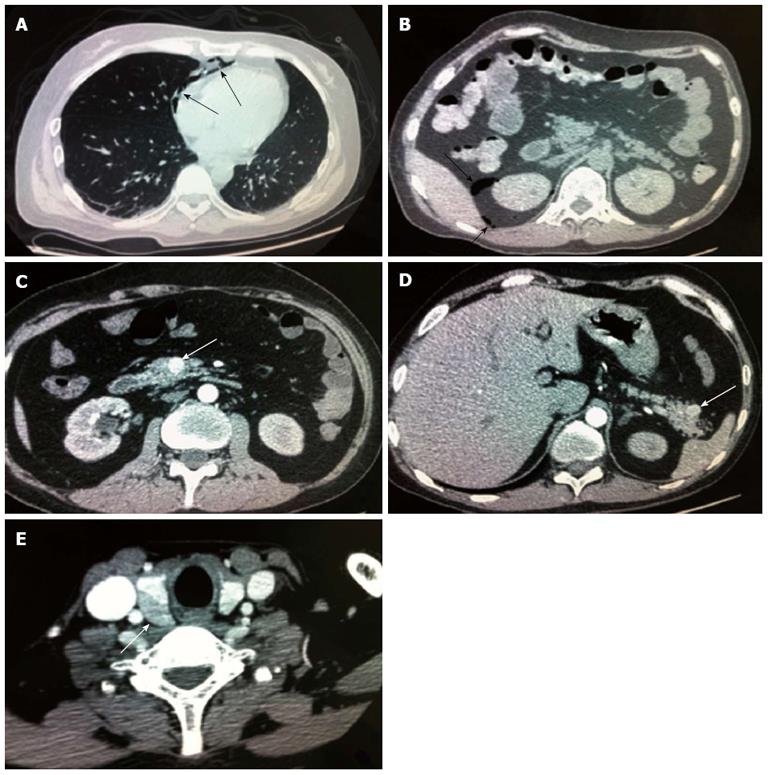
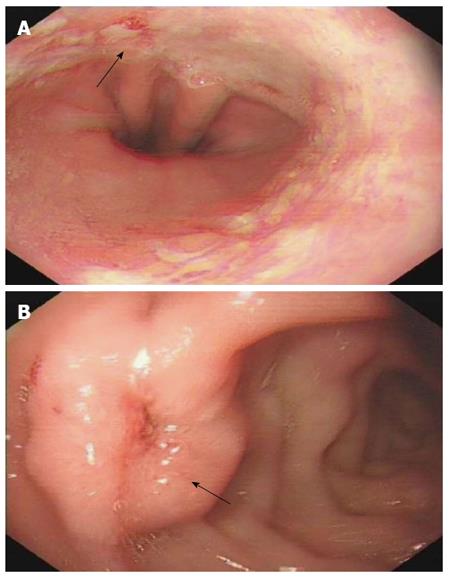
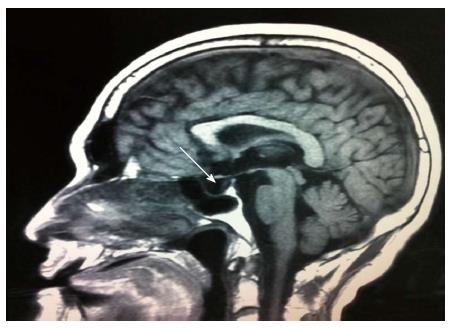
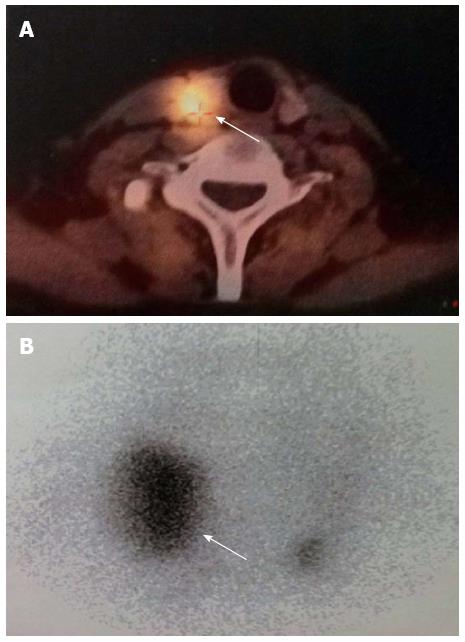
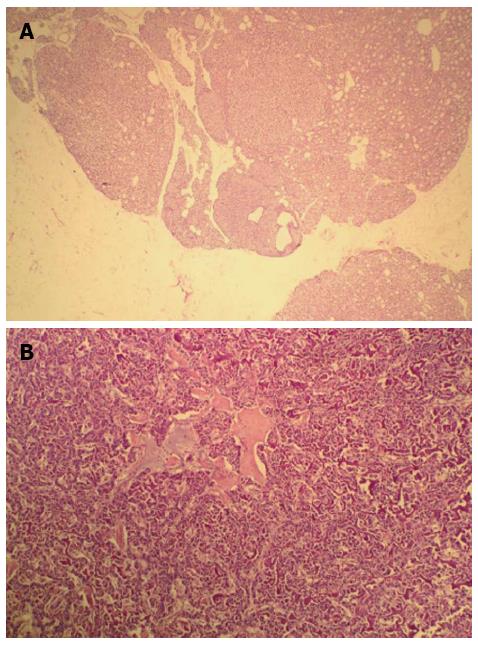
A 42-year-old man was hospitalized with symptoms of nausea, vomiting and diarrhea for more than one year. The patient, presenting with decreased vision, diminished interest in sex, fatigue, weakness and polyuria, was diagnosed with pituitary adenoma in 1998. His blood prolactin level was high (more than 200 ng/mL, reference range, 2-23 ng/mL). The patient underwent removal of the tumor via the trans-sphenoidal approach in February 1998. Histological examination of the surgical specimen showed a chromophore cell adenoma. The patient’s family history was notable for his brother also having a pituitary adenoma. In 2009, the patient underwent surgical procedures to remove a kidney stone. Recently, the patient had diarrhea, nausea and vomiting lasting for more than one year while gastroscopy and colonoscopy were performed prior to admission. Gastroscopy showed reflux esophagitis, esophageal protrusive lesion, gastric polyp and duodenal ulcer. Colonoscopy was normal. The patient was admitted on March 18, 2012. Physical examination revealed hypopigmentation of skin, sparse hair with normal blood pressure, body temperature, breath sounds and heart rhythms. The abdomen was prominently tender with normal bowel sounds and there was no shifting dullness found on the mass. Rectal examination was normal. Laboratory tests showed the following data: leukocytosis (22.40 × 109), mildly low hemoglobin level (115 g/L), elevated serum calcium (3.05 mmol/L, reference range, 2.1-2.55 mmol/L), low phosphorus level (0.57 μmol/L, reference range, 0.81-1.45 μmol/L), extremely high level of parathyroid hormone (PTH) (627 pg/mL, reference range, 15-65 pg/mL). Serum magnesium, calcitonin, electrolytes, glucose, liver and renal function were normal. Computed tomography (CT) scan of the chest revealed mediastinal emphysema with hypostasis in the inferior lobe of the lung (Figure 1A). Epigastric CT scan showed accumulation of gas and fluid in the anterior pararenal space just adjacent to the thickened wall of horizontal duodenum as well as accumulation of gas in the right perirenal space, which implied the possibility of duodenal perforation. CT imaging also found renal and hepatic cysts (Figure 1B). The patient complained of melena two days after admission and the complete blood count showed medium anemia (hemoglobin 86 g/L) and positive stool occult blood (OB). Emergency gastroscopy was performed and revealed chronic superficial gastritis with erosion, reflux esophagitis LA grade C and multiple deep ulcers in the descending part of the duodenum (Figure 2). Pathological analysis showed chronic inflammation of duodenal mucosa. CT scan of the small intestine showed bowel wall thickening and strong enhancement of the horizontal part of the duodenum. A nodular mass with rich blood supply in the uncinate process and tail of pancreas led to a diagnosis highly suspicious for Zollinger-Ellison syndrome (ZES), and an adenoma on the left adrenal gland and multiple liver cysts were also found in this CT image (Figure 1C-D). Magnetic resonance imaging (MRI) of the pituitary showed no space-occupying lesion in the sellar region (Figure 3). PTH was re-examined and the result was 401 pg/mL, which was still significantly higher than the normal value. Laboratory tests also revealed elevated serum gastrin (342.27 pg/mL, reference range, 0-108 pg/mL), elevated prolactin (127.50 ng/mL, reference range, 4.97-23.3 ng/mL) and low testosterone level (0.19 ng/mL, reference range, 2.8-8 ng/mL). Serum progesterone, follicle-stimulating hormone (FSH), luteotropic hormone, estradiol, cortisol, adrenocorticotropic hormone (ACTH), aldosterone and thyroid hormones were normal. CT scan of the thyroid gland showed a mild nodular goiter with nodules posterior and lateral to the thyroid gland, which might originate from an enlargement of the parathyroid glands (Figure 1E). A radioisotope scan revealed soft tissue masses posterior to the thyroid gland and abnormal uptake of 99mTc-MIBI, considered to be parathyroid adenoma or hyperplasia (Figure 4). The patient was diagnosed with MEN1 presenting as hyperparathyroidism, gastrinoma and prolactinoma. He underwent subtotal parathyroidectomy and autotransplantation on April 9, 2012. The pathology of all parathyroid glands was consistent with chief cell hyperplasia with immunohistochemical expression of CK19, CK8, CgA, Syn and NSE and negative expression of TTF-1, TG and Ki67 (Figure 5A). After operation, the levels of serum calcium and PTH fell to normal. The patient had no evidence of metastatic disease on preoperative studies. With the pancreatic endocrine tumors being well-located in the CT image, pylorus-preserving pancreaticoduodenectomy and pancreatic tail resection were performed on April 24, 2012 for removing gastrinoma and cure of ZES. Pathological analysis showed that tumor cells had an acidophilic cytoplasm and round nucleoli which were uniform in size and shape, arranging in tubular, organoid and gyriform patterns (Figure 5B). The pathological diagnosis was a well-differentiated neuroendocrine tumor, infiltrating the muscular layer of the duodenal bowel wall, with no blood vessels and nerves involved and a well-differentiated neuroendocrine tumor also formed in the tail of pancreas, with immunohistochemical expression of NSE, Syn, CK8, CgA and α-AT, weakly positive expression of CK, CD56, Vim, CK19 and Ki67, and negative expression of 5-HT, insulin and ACTH. Pathologic examination of three lymph nodes near the duodenum and the head of pancreas showed chronic lymphadenitis. The post-operative level of serum gastrin was 39.34 pg/mL, and was returning to normal. The patient was discharged two weeks after the successful surgical resection of the tumor and is currently doing well but requires careful follow-up.
MEN1 can cause combinations of more than 20 different endocrine and nonendocrine tumors. Pituitary adenomas affect approximately 30% of patients and usually they are prolactin-secreting micro-adenomas or “nonfunctional”tumors[2]. Fasting prolactin concentration exceeding the upper limit of normal by 20-fold allows the diagnosis of prolactinoma[3]. In our case, the high prolactin level and CT imaging led to a diagnosis of pituitary adenoma which was further confirmed by histological analysis after operation. The patient had no other symptoms and was then considered to be cured. After a period of time, he suffered from chronic diarrhea lasting for nearly one year. There was little information regarding the cause of diarrhea until CT scan showed mediastinal emphysema and accumulation of gas and fluid in the anterior pararenal and right perirenal space which strongly implied the possibility of intestinal perforation. The patient had no positive abdominal physical signs that caused our bewilderment. While we were looking for the possible site of bowel perforation, the patient complained of melena unexpectedly and stool OB was positive, which led to the diagnosis of acute upper gastrointestinal bleeding. Emergency gastroscopy was performed and revealed multiple deep ulcers in the descending part of the duodenum, leading to the suspicion of gastrinoma, which was further confirmed by the elevated serum gastrin level and contrast-enhanced CT; and treatment with high-dose proton pump inhibitors was given. It is now evident that many affected patients have adrenal lesions. A non-functional adrenal cortical tumor was described in up to 40% of MEN1 cases and diagnosed by radiological imaging[4]. Our patient had an adenoma of the left adrenal gland but with normal testosterone, progesterone, FSH, ACTH, LH and aldosterone levels.
The patient had a significantly high serum calcium and PTH level which led to the diagnosis of hyperparathyroidism. Imaging showed parathyroid adenoma or hyperplasia. Hypercalcemia reminded us that the patient underwent a surgical procedure to remove a kidney stone three years ago. The patient stayed asymptomatic then and no further testing was performed, which suggested that clinical manifestation of MEN-1 is mostly mild for a long period of time and a lack of regular screening might result in numerous complications[5].
The fraction of MEN-1 in patients with primary hyperparathyroidism (HPT) is estimated at 1%-5%[6]. As is known, calcium is a secretagogue for gastrin, so HPT may exacerbate gastric acid secretion in concomitant ZES[7]. In practical terms, when serum calcium approaches or exceeds 2.75 mmol/L, surgery is recommended. The most common options for initial surgical management are either total parathyroidectomy and cervical thymectomy with heterotopic parathyroid autotransplantation or subtotal parathyroidectomy and cervical thymectomy. Subtotal parathyroidectomy is preferred because of the advantage of a lower risk of permanent hypoparathyroidism and an acceptable risk of recurrent HPT. In our patient, subtotal parathyroidectomy and autotransplantation were performed and the level of serum calcium decreased immediately. No hypoparathyroidism was detected post-operatively.
In MEN1, pancreatic neuroendocrine tumors occur in 40%-80% of patients and are mostly non-functioning tumors or gastrinomas. The treatment of pancreaticoduodenal disease is quite controversial because of the inability to achieve biochemical cure consistently. In one retrospective analysis, surgical treatment for MEN-1-associated pancreatic tumors < 2 cm showed no advantage over conservative treatment[8]. However, analysis of another cohort of patients revealed that early detection and surgery are beneficial for MEN-1-associated pancreatic tumors[9]. Gastrinomas are the most common functional gastrointestinal neuroendocrine tumors, presenting in up to 54% of MEN1 patients. The diagnosis of ZES depends on proving elevated gastrin levels in the presence of high gastric acidity. Approximately one-third of patients with ZES can be diagnosed by a serum gastrin > 1000 pg/mL with a gastric pH < 3.0. The other two-thirds of patients require provocative tests. Our patient had a moderate increase in serum gastrin but serum gastrin remained < 1000 pg/mL and might require provocative tests to confirm the diagnosis. As the secretin supply was limited, nodules of our patient detected on preoperative imaging that caused significant ulceration and perforation, esophageal reflux symptoms and chronic diarrhea made the diagnosis valid. Prior to surgery, all MEN1 patients should be evaluated for coexisting neuroendocrine and other tumors as well as for the presence of metastases. Preoperative imaging is selected to define the extent of resection, and to identify tumors that would be outside the scope of resection. In our case, contrast-enhanced CT scan of the small intestine showed bowel wall thickening and strong enhancement of the horizontal part of the duodenum, a nodular mass with a rich blood supply in the uncinate process and tail of pancreas, but with no regional lymph nodes and hepatic metastases found. The lesions were well-located in CT imaging, hence no further imaging techniques, such as endoscopic ultrasound or somatostatin receptor scintigraphy, were necessary. The goals of surgical resection of pancreatic endocrine tumors are complete removal of the gross tumor burden, and preservation of pancreatic function. This often results in the subtotal resection of the distal pancreas, and enucleation of tumors in the head of the pancreas and duodenum. In all patients with gastrinoma, the duodenum should be opened and submucosal tumors resected. Total pancreatectomy is rarely indicated. According to all the information collected pre-operatively, our patient received pylorus-preserving pancreaticoduodenectomy and pancreatic tail resection. Postoperative complications were carefully monitored and an optimistic outcome was acquired. The level of serum gastrin returned to normal soon after surgery and the patient recovered well. We hope the patient is completely cured with no requirement for medication, but continued surveillance of all MEN1 patients is necessary and this patient is now under our careful follow-up.
We wish to thank Xiang-Jun Sun from the Pathology department of our hospital for providing pathological results.
P- Reviewer Loffroy R S- Editor Jiang L L- Editor A E- Editor Zhang DN
| 1. | Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658-5671. [PubMed] |
| 2. | Corbetta S, Pizzocaro A, Peracchi M, Beck-Peccoz P, Faglia G, Spada A. Multiple endocrine neoplasia type 1 in patients with recognized pituitary tumours of different types. Clin Endocrinol (Oxf). 1997;47:507-512. [PubMed] |
| 3. | Tortosa F, Chico A, Rodriguez-Espinosa J, Ruscalleda J, de Leiva A. Prevalence of MEN 1 in patients with prolactinoma. MEN1 Study Group of the Hospital de la Santa Creu i Sant Pau of Barcelona. Clin Endocrinol (Oxf). 1999;50:272. [PubMed] |
| 4. | Skogseid B, Larsson C, Lindgren PG, Kvanta E, Rastad J, Theodorsson E, Wide L, Wilander E, Oberg K. Clinical and genetic features of adrenocortical lesions in multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 1992;75:76-81. [PubMed] |
| 5. | Chudek J, Piecha G, Nieszporek T, Marini F, Brandi ML, Wiecek A. Novel 1113delC menin gene mutation in a Polish family with multiple endocrine neoplasia type 1 syndrome. Eur J Intern Med. 2006;17:447-449. [PubMed] |
| 6. | Uchino S, Noguchi S, Sato M, Yamashita H, Yamashita H, Watanabe S, Murakami T, Toda M, Ohshima A, Futata T. Screening of the Men1 gene and discovery of germ-line and somatic mutations in apparently sporadic parathyroid tumors. Cancer Res. 2000;60:5553-5557. [PubMed] |
| 7. | Gogel HK, Buckman MT, Cadieux D, McCarthy DM. Gastric secretion and hormonal interactions in multiple endocrine neoplasia type I. Arch Intern Med. 1985;145:855-859. [PubMed] |
| 8. | Triponez F, Goudet P, Dosseh D, Cougard P, Bauters C, Murat A, Cadiot G, Niccoli-Sire P, Calender A, Proye CA. Is surgery beneficial for MEN1 patients with small (& lt; or = 2 cm), nonfunctioning pancreaticoduodenal endocrine tumor? An analysis of 65 patients from the GTE. World J Surg. 2006;30:654-62; discussion 663-4. [PubMed] |
| 9. | Kouvaraki MA, Shapiro SE, Cote GJ, Lee JE, Yao JC, Waguespack SG, Gagel RF, Evans DB, Perrier ND. Management of pancreatic endocrine tumors in multiple endocrine neoplasia type 1. World J Surg. 2006;30:643-653. [PubMed] |