Published online Feb 28, 2013. doi: 10.3748/wjg.v19.i8.1314
Revised: December 19, 2012
Accepted: January 17, 2013
Published online: February 28, 2013
Processing time: 119 Days and 0.2 Hours
Gastritis cystica profunda (GCP) is a rare condition caused by ectopic entrapment of gastric glands, probably secondary to the disruption of muscularis mucosae. GCP is often associated with gastric adenocarcinoma, and loss of the KCNE2 subunit from potassium channel complexes is considered a common primary target molecule leads to both GCP and malignancy. In this study, we, for the first time, analyzed the expression of KCNE2 in surgically excised tissue from human gastric cancer associated with GCP and confirmed that reduced KCNE2 expression correlates with disease formation.
- Citation: Kuwahara N, Kitazawa R, Fujiishi K, Nagai Y, Haraguchi R, Kitazawa S. Gastric adenocarcinoma arising in gastritis cystica profunda presenting with selective loss of KCNE2 expression. World J Gastroenterol 2013; 19(8): 1314-1317
- URL: https://www.wjgnet.com/1007-9327/full/v19/i8/1314.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i8.1314
Gastritis cystica profunda (GCP) is a rare condition with nonspecific symptoms and radiographic images, making its diagnosis difficult without definitive surgical resection[1]. Clinically, GCP can be misdiagnosed as gastric lymphoma, stromal tumors, gastric cancer, or Menetrier disease. Histopathologically, GCP shows disruption of the integrity of muscularis mucosa that leads to cystically dilated submucosal glands with superficial inflammation in the lamina propria[1,2]. Since the majority of GCP cases are seen secondary to prolonged chronic inflammation, ischemia, gastric surgery and suturing material, an injury of the muscularis mucosae is assumed to trigger the ectopic entrapment of gastric glands in the submucosa, the muscularis mucosae or serosa and to lead to GCP[1,2]. Moreover, GCP is often associated with gastric adenocarcinoma indicates that it can lead to a secondary malignancy[2]. Indeed, experiments have shown that animals predisposed to Helicobacter infection develop not only secondary GCP but also subsequent gastric carcinoma[3]. This close association between GCP and malignancy has been interpreted as concurrent sharing of causative factors common to both disease conditions[1,3]. Recently, with the use of the KCNE2 (also known as MiRP1) deficient mouse model, loss of the KCNE2 subunit from potassium channel complexes is considered a common primary target molecule that gives rise to both GCP and malignancy[4].
Here, the expression of KCNE2 in surgically excised tissue from human gastric cancer associated with GCP is, for the first time, analyzed to confirm that its reduced level correlates with disease formation.
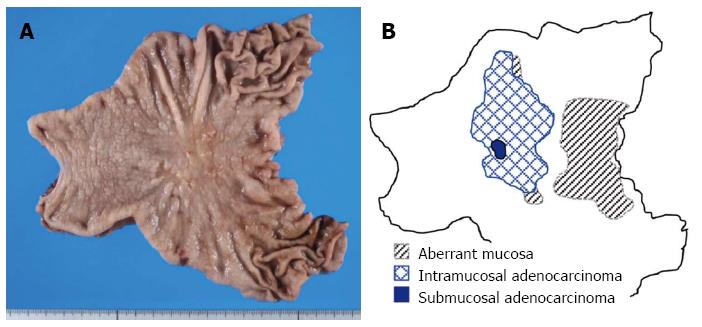
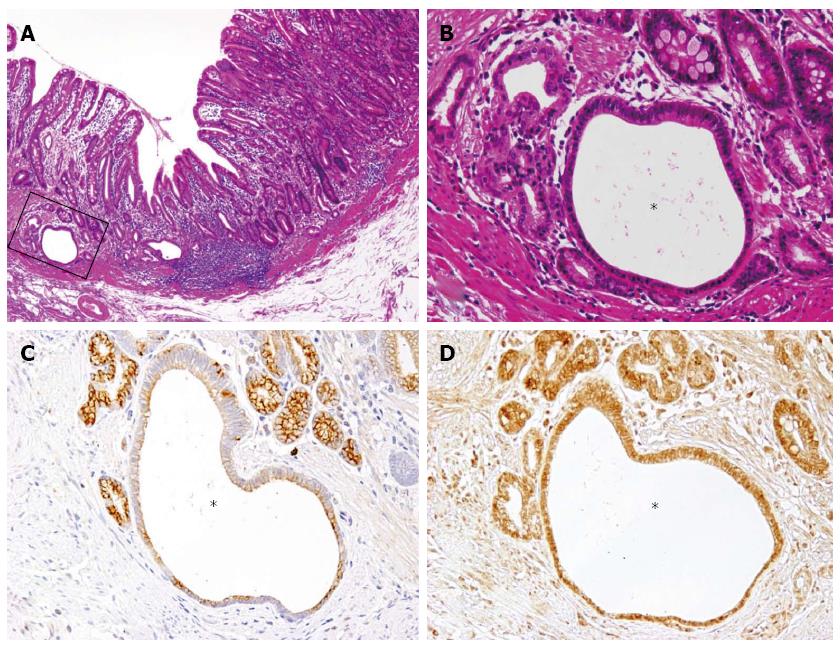
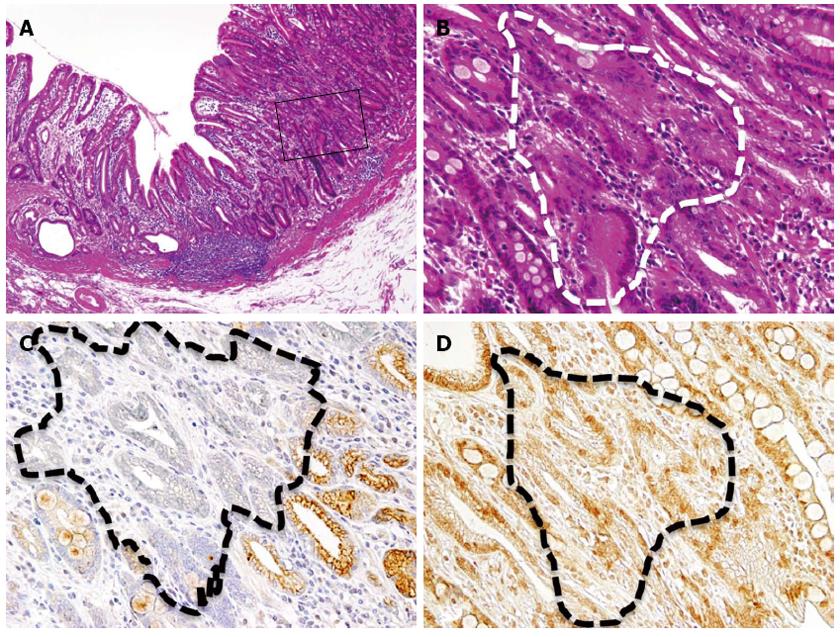
A 63-year-old man was admitted to the hospital with the chief complain of abdominal pain. The patient’s past medical and family history was unremarkable. An endoscopic and other examinations revealed the presence of multiple erosive lesions, and a biopsy demonstrated only severe superficial gastritis with erosion. Under the diagnosis of benign erosive gastritis, the patient was treated with medication and followed up monthly. After one year, however, a follow-up endoscopic examination revealed an irregularly-shaped ulcerating lesion, and the pathological diagnosis of adenocarcinoma was made through biopsy analysis. The patient underwent distal partial gastrectomy, and pathological examination of the excised stomach (Figure 1A) revealed the presence of ectopic cystic mucosa with intestinal metaplasia, especially in the oral side (Figure 1B, dotted area, Figure 2B: HE, × 200). Overlaying the ectopic cystic mucosa, a well- to moderately-differentiated tubular adenocarcinoma was observed in the superficial proper mucosal layer (M), with part of a poorly-differentiated component infiltrating the submucosal layer (SM) in the body of the stomach (Figure 1B, hatched area, Figure 3B: HE, × 200). The pathological diagnosis of gastric adenocarcinoma arising in gastritis cystica profunda was made.
Since targeted deletion of KCNE2 in mice causes gastric lesions resembling gastritis cystica profunda and gastric neoplasia[4], we examined the expression of KCNE2 in the current case immunohistochemically. Also, since signaling through the estrogen receptor (ER) modulates KCNE2 expression[5], we additionally examined the expression of ER. To determine the localization of KCNE2 and ER, rabbit polyclonal anti-KCNE2 antibody (ABCAM, Cambridge, United Kingdom) and rabbit monoclonal anti-ER (EST) antibody (EPIOTOMICS, United States) were used as primary antibodies. Tissue sections were deparaffinized in xylene for 20 min (solvent refreshed at 10 and 5 min) and immersed in absolute ethanol for 10 min (solvent refreshed at 5 min), then rehydrated in 90% and 70% ethanol (5 min each), and finally placed in distilled water for 15 min (solvent refreshed every 5 min). The samples were inactivated in 1 mmol/L EDTA (pH 8.0) plus distilled water in a microwave for 15 min (high heat for 5 min and low heat for 10 min), and then cooled to approximately 25 °C over 1 h. After being undergoing liquid block treatment, the samples were immersed in PBS for 15 min (solvent refreshed every 5 min). After adding blocking buffer to the moisture chamber and incubating the samples with primary antibodies at room temperature for 1 h, they were washed 3 times for 5 min each in PBS, incubated in the moisture chamber at room temperature for 30 min with the secondary antibody diluted at 1:200 with PBS, immersed in PBS for 15 min (solvent refreshed every 5 min), then incubated with DAB for 15 min and washed in PBS.
Immunohistochemical analyses revealed that KCNE2 was universally and strongly expressed on the surface of the cells at the bottom of the cryptic glands, while its expression was diminished in the cystic or dilated lesions (Figure 2C). ER expression was observed in both non-cystic and cystic glands (Figure 2D). In adenocarcinoma, KCNE2 expression was significantly reduced compared with the surrounding non-cancerous gastric mucosa with intestinal metaplasia (Figure 3C), while ER expression was observed in both cancerous and non-cancerous glands (Figure 3D).
In this study the expression status of KCNE2 in surgically excised gastric adenocarcinoma coexisting with GCP was examined in light of that the KCNE2-deficient mouse model develops both GCP and gastric cancer. Since KCNE2 expression is modulated by ER, and since ER expression status per se is related to carcinogenesis and progression stages of gastric cancer[6], we additionally examined the immunohistochemical expression of ER in in the excised tissue.
KCNE2 has originally been identified as a potassium channel protein, and in the stomach, it is expressed mainly in the cytoplasm of parietal cells[5,7,8]. Reduction of KCNE2 in experimental animal models results in profoundly reduced proton secretion, abnormal parietal cell morphology, achlorhydria, hypergastrinemia[5,8], and striking gastric glandular hyperplasia arising from an increase in the number of non-acid-secretory cells[4]. Functionally, KCNE2 also exerts anti-proliferative effects on gastric cancer cells by down-regulating Cyclin D1 and restricting cell growth[5,8]. Indeed, reflecting anti-proliferative or tumor-suppressor functions of KCNE2, long-term observation of KCNE2 -/- mice has revealed that reduced KCNE2 expression causes diffuse hyperplasia in gastric mucosa, resulting in a pathologic condition similar to gastric cancer associated with GCP[4].
We examined here, for the first time, the expression of KCNE2 in surgically excised stomach tissue demonstrating both GCP and adenocarcinoma. While KCNE2 expression in both GCP and adenocarcinoma areas was diminished, that in surrounding non-neoplastic and non-cystic cells was clearly maintained, as determined by immunohistochemical analysis. These data, albeit from a single case study, suggest that selective loss of KCNE2 expression is related to the development and clinical manifestation of GCP with subsequent occurrence of cancer. Furthermore, because selective loss of KCNE2 expression is seen at the level of a single cystic gland and cancer cell nest unit, silencing KCNE2 expression may occur at the level of a single tissue progenitor cell. Although the precise molecular mechanism of such selective loss of KCNE2 expression is largely unknown, it is at least not by the loss of estrogen receptor signaling. We believe that these new data are important because they confirm previous experimental information, and should be assessed in other similar cases. Further genetical and epigenetics studies based on a cumulative case study are needed to elucidate the role of KCNE2 expression in the development of GCP.
We appreciate Ms. Yuki Takaoka for technical assistance.
P- Reviewers Bordas JM, Leitman M S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Fonde EC, Rodning CB. Gastritis cystica profunda. Am J Gastroenterol. 1986;81:459-464. [PubMed] |
| 2. | Mitomi H, Iwabuchi K, Amemiya A, Kaneda G, Adachi K, Asao T. Immunohistochemical analysis of a case of gastritis cystica profunda associated with carcinoma development. Scand J Gastroenterol. 1998;33:1226-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 445] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 4. | Roepke TK, Purtell K, King EC, La Perle KM, Lerner DJ, Abbott GW. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PLoS One. 2010;5:e11451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (105)] |
| 5. | Kundu P, Ciobotaru A, Foroughi S, Toro L, Stefani E, Eghbali M. Hormonal regulation of cardiac KCNE2 gene expression. Mol Cell Endocrinol. 2008;292:50-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Hogan AM, Collins D, Baird AW, Winter DC. Estrogen and gastrointestinal malignancy. Mol Cell Endocrinol. 2009;307:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Yanglin P, Lina Z, Zhiguo L, Na L, Haifeng J, Guoyun Z, Jie L, Jun W, Tao L, Li S. KCNE2, a down-regulated gene identified by in silico analysis, suppressed proliferation of gastric cancer cells. Cancer Lett. 2007;246:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, Brandenburg SA, Litzi TJ, Bunton TE, Limb C. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest. 2000;106:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 219] [Article Influence: 8.8] [Reference Citation Analysis (0)] |