Published online Feb 28, 2013. doi: 10.3748/wjg.v19.i8.1278
Revised: November 28, 2012
Accepted: December 22, 2012
Published online: February 28, 2013
Processing time: 167 Days and 21.4 Hours
AIM: To determine whether fluid injection during radiofrequency ablation (RFA) can increase the coagulation area.
METHODS: Bovine liver (1-2 kg) was placed on an aluminum tray with a return electrode affixed to the base, and the liver was punctured by an expandable electrode. During RFA, 5% glucose; 50% glucose; or saline fluid was infused continuously at a rate of 1.0 mL/min through the infusion line connected to the infusion port. The area and volume of the thermocoagulated region of bovine liver were determined after RFA. The Joule heat generated was determined from the temporal change in output during the RFA experiment.
RESULTS: No liquid infusion was 17.3 ± 1.6 mL, similar to the volume of a 3-cm diameter sphere (14.1 mL). Mean thermocoagulated volume was significantly larger with continuous infusion of saline (29.3 ± 3.3 mL) than with 5% glucose (21.4 ± 2.2 mL), 50% glucose (16.5 ± 0.9 mL) or no liquid infusion (17.3 ± 1.6 mL). The ablated volume for RFA with saline was approximately 1.7-times greater than for RFA with no liquid infusion, representing a significant difference between these two conditions. Total Joule heat generated during RFA was highest with saline, and lowest with 50% glucose.
CONCLUSION: RFA with continuous saline infusion achieves a large ablation zone, and may help inhibit local recurrence by obtaining sufficient ablation margins. RFA during continuous saline infusion can extend ablation margins, and may be prevent local recurrence.
- Citation: Ishikawa T, Kubota T, Horigome R, Kimura N, Honda H, Iwanaga A, Seki K, Honma T, Yoshida T. Radiofrequency ablation during continuous saline infusion can extend ablation margins. World J Gastroenterol 2013; 19(8): 1278-1282
- URL: https://www.wjgnet.com/1007-9327/full/v19/i8/1278.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i8.1278
Radiofrequency ablation (RFA) is a technique by which deposition of electromagnetic energy is used to thermally ablate hepatic tumor tissue[1] and is now the standard method for local treatment of hepatic malignancy[2-9].
Several types of device are currently used for such therapy, such as the Cool-tip system (single electrode) made by Covidien, and the RFA systems made by Boston Scientific and AngioDynamics (the Starburst XL electrode)[10]. The former uses a single-electrode puncture needle, whereas the latter both employ a needle electrode with an expandable array of fine needles extending from the tip. The expandable array of the StarBurst XL electrode is also fitted with an infusion port. To date, various techniques have been devised to secure adequate ablation margins[11]. We investigated whether ablation margins could be extended by fluid infusion from the infusion port of an expandable electrode.
We conducted an ex vivo experiment on bovine liver to investigate whether ablation margins could be extended by fluid infusion from a StarBurst XL electrode in order to avoid the influence of the cooling effect in vivo experiment.
A StarBurst XL electrode (AngioDynamics; Queensbury, NY) and RITA1500X generator (AngioDynamics) were used to provide RFA. One to two kilograms of bovine liver form an abattoir was used which had been exposed to room temperature for several hours until the baseline mean liver temperature ranged between 15 °C and 21 °C, placed on an aluminum tray with a return electrode affixed to the base and the liver was punctured by the StarBurst XL electrode, with a maximum array expansion of 5 cm in diameter, under ultrasonographic guidance by convex probe using a Logiq 7 ultrasound device (GE Healthcare, Tokyo, Japan). Stepped ablation was performed, with the electrode first expanded to 2 cm after reaching the set temperature of 105 °C, then expanded by 3 cm after reaching the set temperature of 105 °C, with single ablation maintained at each diameter for 7 min. An infusion line was connected to the infusion port on the electrode, and the other end of the infusion line was connected to a syringe pump. Continuous liquid infusion was initiated at the same time as radiofrequency output. Three infusion liquids were used with the continuous infusion rate was set at 1.0 mL/min: 5% glucose; 50% glucose; and saline[12].
After stopping radiofrequency output, specimens were sectioned along the longitudinal and transverse axes of each lesion. Measurement of the coagulation diameter perpendicular to the electrode axis was based on consensus of two observers (Ishikawa T, Kubota T). Macroscopic changes in specimens have been demonstrated to correlate well with coagulation necrosis at histological examination[13]. Furthermore, the central white area of the RF-induced ablation zone was found to correspond to the necrotic zone[14].
The liver blocks containing lesions were dissected along the axis along which the electrode was inserted, and the central white area of the RF-induced ablation zone was found to correspond to the necrotic zone[15]. For macroscopic examination, two observers used calipers to measure, in the central white area of coagulation necrosis in each pathologic specimen, the overlapping width, the longest diameter of each ablation sphere along the electrode, and the shortest diameter at the midpoint between the two electrodes[16].
The region of discoloration indicating ablation of the bovine liver was measured, and the volume of ablation was calculated using the formula for calculating the volume of an ellipsoid (V = 4/3abcπ).
An intergroup comparison of calculated thermocoagulated volumes was performed using Student’s t-test with Bonferroni correction.
In addition, the generated Joule heat was calculated from the data for output changes over time during the RFA experiment.
In the intergroup comparison of calculated thermocoagulated volumes, an unpaired Student’s t-test was used and P values were calculated with a correction of the α significance level by Bonferroni’s method based on the number of test repetitions. For all statistical analyses, values of P < 0.05 were considered statistically significant.
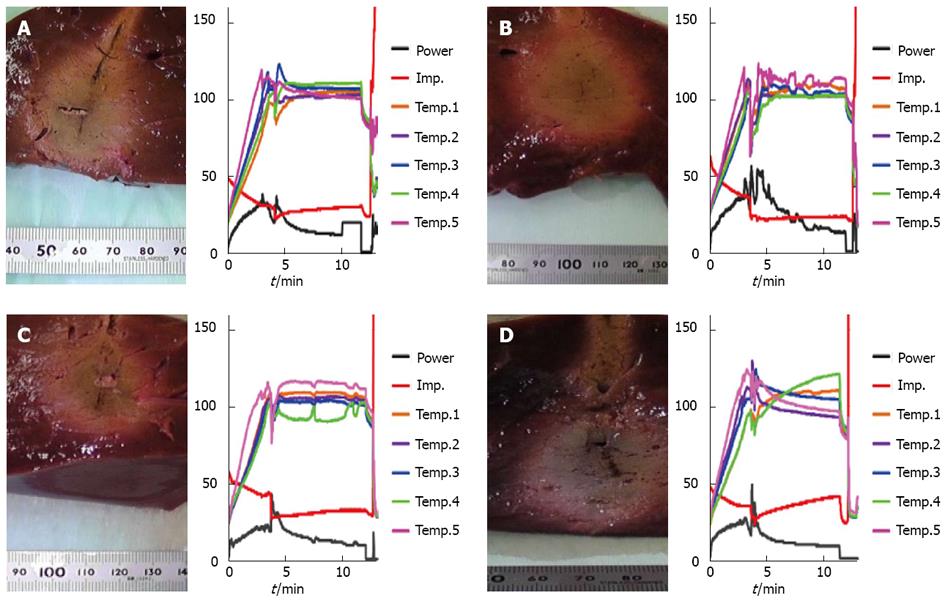
Figure 1 shows cross-sectional photographs of ablated regions of liver and graphs of changes over time in temperature, output and impedance during RFA for each continuous infusion condition. Changes in impedance over time after array expansion to 3 cm tended to gradually increase in RFA without infusion and with glucose, but the steady state of impedance was seen when RFA was used with saline (Figure 1). The ablation zone also widened and presented a circular shape when saline was used.
Experimental result shows mean ± SD values for dimensions of ablation, calculated volume, mean output during RFA, and calculated Joule heat. Mean thermocoagulated volume was significantly larger with continuous infusion of saline (29.3 ± 3.3 mL) than with 5% glucose (21.4 ± 2.2 mL), 50% glucose (16.5 ± 0.9 mL) or no liquid infusion (17.3 ± 1.6 mL) (P < 0.01). The ablated volume for RFA with saline was approximately 1.7-times greater than for RFA with no liquid infusion, representing a significant difference between these two conditions.
An increasing trend in ablation volume was also seen in RFA with 5% glucose, although not to the same extent as with saline. In RFA with 50% glucose, the ablated volume was very similar to RFA with no liquid infusion, and differed significantly from RFA with saline.
Mean radiofrequency output tended to be higher in RFA with saline than with no liquid infusion, but tended to be low in RFA with glucose. The mean total Joule heat generated during RFA was highest with saline, and low with glucose.
RFA is a therapeutic technique that utilizes the principle of conductive heating, in which heat is generated by friction from moving electrons when current, is passed through a conductor[17]. Specifically, by passing electricity through a needle electrode inserted into the human body, coagulation and necrosis of a malignant neoplasm or other lesion tissue is induced[18,19]. The basic principle of RFA as treatment for hepatic malignancy is the generation of Joule heat by high-frequency current in living tissue between the needle electrode and a return electrode. Although the heat generated by this high-frequency current arises in all the conducting tissue pathways, high temperatures only develop in tissues near the electrode, where the current density is high. Barely any heat arises in tissues further from the electrode because of the fall in current density and the cooling (or radiator) effect of blood flow.
Although this principle brings about ablation in tissue near the electrode, the vapor microbubbles generated in the ablation region are removed by blood flow, lowering the electrical conductivity of tissue and consequently sometimes preventing adequate ablation of the target zone[2]. Efficient generation of conductive heating is therefore necessary to achieve adequate ablation margins[20]. Temperature-controlled systems for facilitating adequate ablation of the target zone by preventing excessive heating of tissue have been commercialized, but our experiments aimed to test the effect of RFA used in combination with continuous infusion of a liquid to the target tissue. Specifically, we conducted ex vivo experiments on bovine liver to investigate whether infusion of a liquid from the infusion port of a StarBurst XL electrode could enhance the efficiency of conductive heating.
The mean ablated volume when RFA was performed by StarBurst XL electrode with no liquid infusion was 17.3 ± 1.6 mL, similar to the volume of a 3-cm diameter sphere (14.1 mL). On the other hand, when RFA was used with a continuous infusion of liquid, the mean ablation volume was 29.3 ± 3.3 mL with saline, 21.4 ± 2.2 mL with 5% glucose, and 16.5 ± 0.9 mL with 50% glucose (P < 0.01). This shows that the ablated region expanded significantly when saline was used. In RFA with continuous liquid infusion, the ablated volume was significantly higher than in RFA without liquid infusion, and when saline in particular was used, the mean point estimate volume increased approximately 1.7-fold. In order to maintain the steady state of impedence for RFA during saline continuous injection, current density between electrodes should therefore be decreased to within the range within which current causes an increase in tissue temperatures, without boiling. We speculate that changing the electrical conductance of the tissue by infusing saline could be a good solution. Furthermore, we propose the following three reasons for these results.
First, in RFA without liquid infusion, water in tissues near the electrode forms microbubbles, some of which move to the area around the tissue. The moisture percentage near the electrode thus decreases and electrical conductivity of the tissue falls. This is supported by the finding that impedance gradually increased during RF output. In contrast, when RFA is used with continuous liquid infusion, the moisture percentage in tissue near the electrode remains stable or is increased, maintaining or increasing electrical conductivity and leading to expansion of the ablated region. Furthermore, saline was likely more effective in extending the ablated region than glucose when used with RFA because saline is a conductive solution.
Second, ablation of tissue near the electrode results from the thermal conversion effect of Joule heat due to a high current density. The thermal conversion effect of Joule heat alone is insufficient to induce ablation of the surrounding tissue, and we speculate that such ablation is the result of synergistic effects involving, for example, heat conduction from tissue near the electrode to surrounding tissue, or transfer of heated water or high-temperature microbubbles generated near the electrode to the surrounding tissue. Infusion of liquid would promote this transfer of heated water or high-temperature microbubbles to the surrounding tissue, with the ultimate effect of extending the ablated region.
Third, the type of infusion liquid was relevant, with a significant difference in ablated volume seen between the saline and 50% glucose solutions. This is probably because although a highly concentrated glucose solution would be expected to extend the ablation region through the effects described in the two points above, the non-ionic nature of the solution may lower the electrical conductivity of the target tissue. Considering that ablated volumes were similar in RFA with 50% glucose infusion and without any liquid infusion, the negative effects of this highly concentrated but non-ionic solution cancelled out the effects described in the first two points above, resulting in no marked extension of the ablated region.
Shorter treatment time (RFA time) may be another effect of RFA with continuous liquid infusion. This could be due to an increase in thermocoagulation efficiency at the target tissue arising from the transfer of high-temperature microbubbles or heated water to the surrounding tissue, as suggested in the second point above.
Furthermore, RFA with continuous liquid infusion tended to produce a larger thermocoagulated region with a lower total Joule heat compared to RFA without liquid infusion. Saline infusion in particular demonstrated high ablation efficiency at the target tissue, and could therefore prove highly useful[21].
This high ratio reveals the strong ablative action on the target tissue, and suggests that RFA with continuous saline infusion can achieve efficient ablation of tissue near the electrode. While this ex-vivo procedure cannot be directly adopted for use in clinical settings, RFA during continuous saline infusion can achieve a large thermocoagulation region in a short time and may be able to inhibit local recurrence by securing ablation margins.
There are several limitations to this study. The most important limitation was the absence of blood flow. In vivo, the volume of coagulation will be smaller and the amount of applied energy will be higher due to the removal of heat by the presence of blood flow[22]. This ex-vivo procedure cannot be directly adopted for use in a clinical setting. Using the same RF device, the size of the ablation zone in vivo was smaller than when ex vivo[23]. However, RFA during saline injection has potentially broad clinical merit and can assist in the planning of a methodological strategy. The relationship between parameters was calculated for a particular RF system, therefore, a different multipolar RF system would change the mathematical modeling. Moreover, the physiologic variability of thermal and electrical tissue properties is supposed to lead to a variable efficiency of applied energy under in vivo conditions. Consequently, the accuracy of mathematical modeling derived from ex vivo data will be restricted in the clinical application of multipolar RF ablation. Therefore, mathematical modeling has to be adjusted for in vivo conditions and due to the physiologic variability[24] of parameters the accuracy of predicted volumes is supposed to be reduced.
Finally, the findings of this study reflect certain model-specific characteristics, such as its hypovascularity, outcomes in other more clinically relevant tumor model may differ. Therefore, extrapolation to other types of tumors should be performed with caution.
In conclusion, we confirmed the effect of continuous liquid infusion in expanding the RFA thermocoagulated region in ex vivo experiments using a Starburst XL electrode system. Our results indicate that RFA with continuous saline infusion can significantly expand the volume of thermocoagulation in temperature-controlled RFA systems. RFA with continuous saline infusion can achieve a large thermocoagulation region in a short time and may be able to inhibit local recurrence by securing ablation margins[25,26].
Radiofrequency ablation (RFA) is now the standard method for local treatment of hepatic malignancy. To date, various techniques have been devised to secure adequate ablation margins. This study was investigated whether ablation margins could be extended by fluid infusion from the infusion port of an expandable electrode.
In this study, authors confirmed that ablation margins by RFA could be extended by saline infusion from the infusion port of an expandable electrode.
While this ex-vivo procedure cannot be directly adopted for use in clinical settings, RFA during continuous saline infusion can achieve a large thermocoagulation region in a short time and may be able to inhibit local recurrence by securing ablation margins.
In this study, RFA with continuous saline infusion can achieve a large thermocoagulation region in a short time and may be able to inhibit local recurrence by securing ablation margins.
This is a very novel study that Starburst RFA device and infusion saline and dextrose to study differences in burn volume with RFA. The results are interesting and suggest that RFA during continuous saline infusion can extend ablation margins.
P- Reviewers Ooi LL, Kambadakone A S- Editor Gou SX L- Editor A E- Editor Lu YJ
| 1. | Callstrom MR, Charboneau JW. Technologies for ablation of hepatocellular carcinoma. Gastroenterology. 2008;134:1831-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Rossi S, Di Stasi M, Buscarini E, Cavanna L, Quaretti P, Squassante E, Garbagnati F, Buscarini L. Percutaneous radiofrequency interstitial thermal ablation in the treatment of small hepatocellular carcinoma. Cancer J Sci Am. 1995;1:73-81. [PubMed] |
| 3. | Livraghi T. Treatment of hepatocellular carcinoma by interventional methods. Eur Radiol. 2001;11:2207-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Rhim H, Lim HK, Choi D. Current status of radiofrequency ablation of hepatocellular carcinoma. World J Gastrointest Surg. 2010;2:128-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Rhim H, Lim HK. Radiofrequency ablation of hepatocellular carcinoma: pros and cons. Gut Liver. 2010;4 Suppl 1:S113-S118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Livraghi T, Mäkisalo H, Line PD. Treatment options in hepatocellular carcinoma today. Scand J Surg. 2011;100:22-29. [PubMed] |
| 7. | Künzli BM, Abitabile P, Maurer CA. Radiofrequency ablation of liver tumors: Actual limitations and potential solutions in the future. World J Hepatol. 2011;3:8-14. [PubMed] |
| 8. | Izumi N. Recent advances of radiofrequency ablation for early hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26 Suppl 1:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Himoto T, Kurokohchi K, Watanabe S, Masaki T. Recent advances in radiofrequency ablation for the management of hepatocellular carcinoma. Hepat Mon. 2012;12:e5945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Denys AL, De Baere T, Kuoch V, Dupas B, Chevallier P, Madoff DC, Schnyder P, Doenz F. Radio-frequency tissue ablation of the liver: in vivo and ex vivo experiments with four different systems. Eur Radiol. 2003;13:2346-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Goldberg SN, Stein MC, Gazelle GS, Sheiman RG, Kruskal JB, Clouse ME. Percutaneous radiofrequency tissue ablation: optimization of pulsed-radiofrequency technique to increase coagulation necrosis. J Vasc Interv Radiol. 1999;10:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Livraghi T, Goldberg SN, Monti F, Bizzini A, Lazzaroni S, Meloni F, Pellicanò S, Solbiati L, Gazelle GS. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology. 1997;202:205-210. [PubMed] |
| 13. | McGahan JP, Griffey SM, Budenz RW, Brock JM. Percutaneous ultrasound-guided radiofrequency electrocautery ablation of prostate tissue in dogs. Acad Radiol. 1995;2:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001;2:151-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaginghistopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001;2:151-158. [PubMed] |
| 16. | Lee JM, Han JK, Kim SH, Sohn KL, Lee KH, Ah SK, Choi BI. A comparative experimental study of the in-vitro efficiency of hypertonic saline-enhanced hepatic bipolar and monopolar radiofrequency ablation. Korean J Radiol. 2003;4:163-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Solbiati L, Ierace T, Goldberg SN, Sironi S, Livraghi T, Fiocca R, Servadio G, Rizzatto G, Mueller PR, Del Maschio A. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology. 1997;202:195-203. [PubMed] |
| 18. | Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633-646. [PubMed] |
| 20. | Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, Tsuchihashi T, Saigenji K. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 21. | Miao Y, Ni Y, Yu J, Zhang H, Baert A, Marchal G. An ex vivo study on radiofrequency tissue ablation: increased lesion size by using an “expandable-wet” electrode. Eur Radiol. 2001;11:1841-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002;178:47-51. [PubMed] |
| 23. | de Baere T, Denys A, Wood BJ, Lassau N, Kardache M, Vilgrain V, Menu Y, Roche A. Radiofrequency liver ablation: experimental comparative study of water-cooled versus expandable systems. AJR Am J Roentgenol. 2001;176:187-192. [PubMed] |
| 24. | Montgomery RS, Rahal A, Dodd GD, Leyendecker JR, Hubbard LG. Radiofrequency ablation of hepatic tumors: variability of lesion size using a single ablation device. AJR Am J Roentgenol. 2004;182:657-661. [PubMed] |
| 25. | Gananadha S, Morris DL. Saline infusion markedly reduces impedance and improves efficacy of pulmonary radiofrequency ablation. Cardiovasc Intervent Radiol. 2004;27:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Hänsler J, Frieser M, Schaber S, Kutschall C, Bernatik T, Müller W, Becker D, Hahn EG, Strobel D. Radiofrequency ablation of hepatocellular carcinoma with a saline solution perfusion device: a pilot study. J Vasc Interv Radiol. 2003;14:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |