Published online Feb 28, 2013. doi: 10.3748/wjg.v19.i8.1239
Revised: January 3, 2013
Accepted: January 11, 2013
Published online: February 28, 2013
Processing time: 178 Days and 16.1 Hours
AIM: To investigate the effect of high mobility group A2 (HMGA2) gene silencing on gastric cancer MKN-45 cells in vitro.
METHODS: HMGA2 short hairpin RNA (shRNA) expression plasmids were constructed, including a pair of random scrambled sequences. Human gastric cancer cell line MKN-45 cells were divided into three groups: blank control group (non-transfected cells), transfected group (cells transfected with HMGA2 shRNA recombinant plasmid) and scrambled sequence group (transfected with random scrambled plasmid). Cells were transfected with HMGA2 shRNA recombinant plasmids and scrambled plasmid in vitro, and the cells transfection efficiency was assayed by fluorescence microscopy. The HMGA2 messenger RNA (mRNA) expression was detected by reverse transcription polymerase chain reaction, gastric cancer cells apoptosis was detected by flow cytometry, cell proliferation was detected by methyl thiazol tetrazolium, and the protein expression of phosphatidylinositol 3-kinase (PI3K), protein kinase B (Akt), P27, caspase-9 and B-cell leukemia/lymphoma-2 (Bcl-2) were analyzed by Western blotting.
RESULTS: Compared with the blank control group and the scrambled sequence group, the levels of HMGA2 mRNA and protein expression in the transfected group were significantly reduced (P < 0.05). The relative HMGA2 mRNA expression levels of the blank control group, transfected group and scrambled sequence group were 0.674 ± 0.129, 0.374 ± 0.048 and 0.689 ± 0.124, respectively. The relative HMGA2 protein expression levels of the blank control group, transfected group and scrambled sequence group were 0.554 ± 0.082, 0.113 ± 0.032 and 0.484 ± 0.123, respectively. Moreover, transfection with the scrambled sequence had no effect on the expression of HMGA2. After being transfected with shRNA for 24, 48 and 72 h, the cell apoptotic rates of the transfected group were 21.65% ± 0.28%, 39.98% ± 1.82% and 24.51% ± 0.93%, respectively, which significantly higher than those of blank control group (4.72% ± 1.34%, 5.83% ± 0.13% and 5.22% ± 1.07%) and scrambled sequence group (4.28% ± 1.33%, 7.87% ± 1.43% and 6.71% ± 0.92%). After 24, 48 and 72 h, the cell proliferation inhibition rates in the transfected group were 31.57% ± 1.17%, 39.45% ± 2.07% and 37.56% ± 2.32%, respectively; the most obvious cell proliferation inhibition appeared at 48 h after transfection. Compared with the blank control group and scrambled sequence group, after transfection of shRNA for 72 h, the protein expression levels of PI3K (0.042 ± 0.005 vs 0.069 ± 0.003, 0.067 ± 0.05), Akt (0.248 ± 0.004 vs 0.489 ± 0.006, 0.496 ± 0.104) and Bcl-2 (0.295 ± 0.084 vs 0.592 ± 0.072, 0.594 ± 0.109) were significantly reduced. The protein expression levels of P27 (0.151 ± 0.010 vs 0.068 ± 0.014, 0.060 ± 0.013) and caspase-9 (0.136 ± 0.042 vs 0.075 ± 0.010, 0.073 ± 0.072) were significantly upregulated.
CONCLUSION: HMGA2 shRNA gene silencing induces apoptosis and suppresses proliferation of MKN-45 cells.
- Citation: Wei CH, Wei LX, Lai MY, Chen JZ, Mo XJ. Effect of silencing of high mobility group A2 gene on gastric cancer MKN-45 cells. World J Gastroenterol 2013; 19(8): 1239-1246
- URL: https://www.wjgnet.com/1007-9327/full/v19/i8/1239.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i8.1239
Gastric cancer is one of the most common malignancies, being the fourth most common cancer and the second leading cause of cancer related deaths (740 000 deaths in 2008), with nearly one million newly diagnosed cases each year[1]. Up to now, the exact molecular mechanisms involved in carcinogenesis and progression of gastric cancer have remained unclear. Therefore, investigating the cell proliferation and apoptosis involved in these processes is essential for gastric cancer research.
The high mobility group A2 (HMGA2) gene is a member of the high mobility group AT-hook (HMGA) gene family. HMGA2 plays a critical role in several cellular biological processes, including cell transformation, growth, differentiation, senescence and cycle control[2-4]. Normally, HMGA2 is abundantly expressed during embryogenesis, whereas its expression is low or absent in normal adult differentiated tissues[5,6]. Evidence shows that HMGA2 is overexpressed in breast cancer[7], pancreatic cancer[8,9], esophageal squamous cell carcinoma[10], non-small cell lung cancer[11], oral squamous cell cancer[12], bladder cancer[13], ovarian cancer[14,15] and colorectal cancer[16,17]. Its expression is correlated with malignant degree, pathological type and metastasis[18]. Thus, HMGA2 might play a central role in the disease progression of many types of tumors. Overexpression of HMGA2 has also been described in gastric cancer[19]. The study showed that the overexpression of HMGA2 in gastric cancer patients was significantly correlated with poor prognosis and low overall survival. However, the exact mechanism of HMGA2 in gastric cancer is not clear.
The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway plays a central role in many human cancers. Akt regulates cell proliferation, differentiation and apoptosis via activation or inhibition of downstream target proteins, such as P27, caspase-9 and B-cell leukemia/lymphoma-2 (Bcl-2)[20,21].
The aims of the present study were to investigate the effect of HMGA2 expression on gastric cancer cell proliferation and apoptosis. Moreover, the expressions of PI3K, Akt, P27, caspase-9 and Bcl-2 were detected to exploit the possible mechanism.
The human HMGA2 was treated as the target gene (Accession Number: NM-003484) and four pairs of short hairpin RNA (shRNA) oligonucleotides were designed and synthesized (Cyagen Biosciences Inc., United States). A pair of random scrambled sequences, which have no homologous relation with any human gene sequences and whose shRNAs would not interfere with human mRNA were also designed (Table 1). The complementary form was obtained by annealing, which was then cloned and inserted into vector pLLU2 to construct a recombinant plasmid. The plasmid was transformed into stb13 strain. Finally, the plasmid was identified by restriction enzyme digestion and sequenced (Cyagen Biosciences Inc., United States). According to the preliminary experimental results, the first pair shRNA oligonucleotides (Table 1) was the best interfering sequence, and the recombinant plasmid harboring it was used for subsequent experiments.
| Oligo name | Oligo sequence 5’ to 3’ |
| shHMGA2-1-F | TAGTCCCTCTAAAGCAGCTCAACTCGAGTTGAGCTGCTTTAGAGGGACTTTTTTC |
| shHMGA2-1-R | TCGAGAAAAAAGTCCCTCTAAAGCAGCTCAACTCGAGTTGAGCTGCTTTAGAGGGACTA |
| shHMGA2-2-F | TAGGAGGAAACTGAAGAGACATCTCGAGATGTCTCTTCAGTTTCCTCCTTTTTTC |
| shHMGA2-2-R | TCGAGAAAAAAGGAGGAAACTGAAGAGACATCTCGAGATGTCTCTTCAGTTTCCTCCTA |
| shHMGA2-3-F | TCTCACAAGAGTCTGCCGAAGACTCGAG TCTTCGGCAGACTCTTGTGAGTTTTTC |
| shHMGA2-3-R | TCGAGAAAAACTCACAAGAGTCTGCCGAAGACTCGAGTCTTCGGCAGACTCTTGTGAGA |
| Scrambled-F | TGCGCGCTTTGTAGGATTCGCTCGAGCGAATCCTACAAAGCGCGCTTTTTC |
| Scrambled-R | TCGAGAAAAAGCGCGCTTTGTAGGATTCGCTCGAGCGAATCCTACAAAGCGCGCA |
Gastric cancer MKN-45 cells (ATCC, United States) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Solarbio Science and Technology Co., Ltd., Beijing, China) containing 10% placental bovine serum. After 24 h incubation at 37 °C with 5% CO2, logarithmic growth phase cells in good condition were digest by 0.25% trypsin + ethylenediaminetetraacetic acid. After calculating the cell number, cells were seeded into 6-well plates at 1 × 105 cells per well. When the cells reached more than 80% confluence (usually the next day), plasmid and lipofectamine were added dropwise to form a mixture of 1:1 with mixing. After culturing in serum-free and antibiotic-free DMEM for 6 h, the culture medium was replaced by DMEM and the cultured was continued for 24 h. The cells were then transfected using a LipofectamineTM2000 Transfection Kit (Invitrogen, United States) according to the manufacturer’s instructions. Cells were randomly counted in 10 microscope fields at 24, 48 and 72 h after transfection. The ratio of the number of cells with green fluorescent emission and the total cells number indicated the transfection efficiency. Non-transfected cells from the same batch were collected as the blank control group. The cells transfected with the random scrambled sequence plasmid served as the scrambled sequence group.
Total RNA was isolated using TRIzol (Sangon Biotech Co., Ltd., Shanghai, China). A Reverse Transcriptase Kit (Fermentas China Co., Ltd.) was used to create cDNA, according to the manufacturer’s instructions: 1 μg RNA, Oligo dT18 as the primer, and 42 °C for 60 min followed by 70 °C for 5 min. Reverse transcriptase polymerase chain reaction (RT-PCR) assays were carried out using on an ABI 2720 (Applied Biosystems, Foster City, CA, United States). The PCR primers (synthesized by Sangon Biotech Co., Ltd., Shanghai, China) used to detect HMGA2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were as follows: HMGA2, upstream primer 5’-TACTCTGTCTCTGCCTGTGC-3’, downstream primer 5’-GGAGTGAATTGTGTCCCTTGA-3’ (product length 228 bp); GAPDH, upstream primer 5’-GAAGGTGAAGGTCGGAGTC-3’, downstream primer 5’-TCACACCCATGACGAACAT -3’ (product length 401 bp). The 25 μL PCR amplification reaction mixture contained: cDNA, 1.5 μL; Taq Mix, 12.5 μL; 10 μmoL/L; each primer, 1 μL; RNase-free Water, 9 μL. Amplification reaction program: initial denaturation at 95 °C for 5 min; 35 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 35 s, extension at 72 °C for 30 s; and final extension at 72 °C for 7 min. The PCR products were analyzed on agarose gels; gel analysis software was used to analyze the bands. The gray value ratio of the objective band and the β-actin band indicated the relative expression levels of HMGA2 mRNA.
Cells were lysed in RIPA cell lysis buffer (Sangon Biotech Co., Ltd., Shanghai, China). The ratio of lysate and loading buffer was 4:1, degeneration and proteins were extracted. About 80 μg of proteins were fractionated by electrophoresis on sodium dodecyl sulfate-polyacrylamide gels and electroblotted onto a polyvinylidene fluoride membrane. The membrane was blocked with 5% nonfat milk for 2 h at room temperature and incubated overnight with the primary antibody (Santa Cruz Biotechnology, inc., United States). The membrane was washed with Tris-Buffered-Saline with Tween (TBST) three times for 10 min each, and then incubated for 2 h at room temperature with the secondary antibody (Zhongshanjinqiao Biotechnology Co., Ltd., Beijing, China). The membranes were washed with TBST 3 times for 10 min each, before being developed and fixed. β-actin (Zhongshanjinqiao Biotechnology Co., Ltd., Beijing, China) was used as an internal control. Gel analysis software was used to scan and analyze the bands. The gray value ratio of objective band and internal loading control band was used to determine the objective proteins expression levels.
Each group of cells was digested into a single cell suspension using 0.25% trypsin. One hundred μL of buffer from the Annexin v-FITC and PE Apoptosis Detection Kit (BD Co., United States), 5 μL ADD and 5 μL PE were added to the cells, which were incubated without shaking at room temperature for 15 min in the dark. About 400 μL of buffer was then added, then the apoptosis rate of each group of cells was assayed by flow cytometry.
A suspension of each group of cells was obtained by 0.25% trypsin digestion. The cells were centrifuged (1000 g, 5 min), resuspended in 1 mL culture medium and counted. A cell suspension containing 2 × 104/200 μL cells was distributed into 96-well plates: six sets of parallel holes were used for each group. The cells were then incubated at 37 °C in 5% CO2. One 96-well plate was removed daily and 20 μL per well of a fresh new methyl thiazol tetrazolium (MTT) solution (5 mg/mL) was added, and the plate incubated at 37 °C for 4 h. The supernatants were removed, and 150 μL of dimethyl sulfoxide was added to each well and the plates were oscillated for 10 min. The optical density value (A value) of the cells in each well was measured by an enzyme-labeling instrument at a wavelength of 570 nm, and a growth curve for the cells was drawn. The cell proliferation inhibition rate (CPIR) was calculated according to the formula: CPIR (%) = (1 - Aexperimental group /Acontrol group) × 100%.
SPSS 13.0 software (Chicago, United States) was used to perform the statistical analysis. Data were expressed as mean ± SD. One-way analysis of variance was used to analyze the correlation of data among three or more groups, and an SNK test was used for pairwise comparison between groups. P < 0.05 was considered significant.
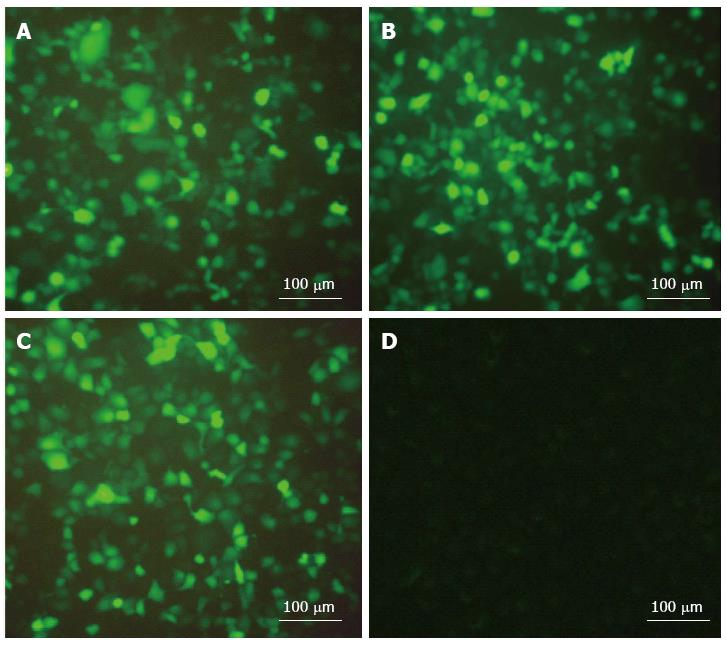
The result showed that the transfection efficiency of the transfected group cells was 60%-70% after transfection for 48 h and was significantly higher than that at 24 h. Compared with 48 h, there was no significant change at 72 h. The scrambled sequence group cells were not transfected at any time (Figure 1).
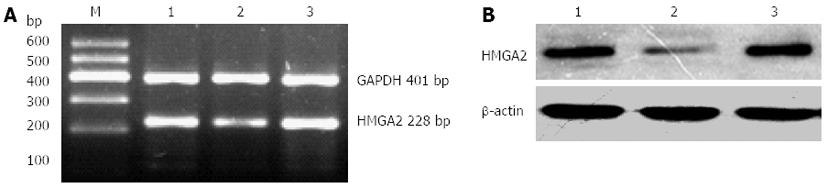
Compared with the blank control group and the scrambled sequence group, the expression of HMGA2 mRNA in the transfected group was significantly reduced (0.374 ± 0.048 vs 0.674 ± 0.129 and 0.689 ± 0.124, P < 0.05); however, the difference between the blank control group and the scrambled sequence group was not statistically significant (P > 0.05) (Figure 2). The results showed that the RNA interference had a targeted inhibition affect on the HMGA2 gene.
Compared with the blank control group and scrambled sequence group, the HMGA2 the protein expression was significantly decreased, the difference was statistically significant (0.113 ± 0.032 vs 0.554 ± 0.082 and 0.484 ± 0.123, P < 0.05); however, the difference between the blank control group and the scrambled sequence group was not statistically significant (P > 0.05) (Figure 2). The results showed that transfection of HMGA2 shRNA expression vector had significantly inhibited the protein expression from the target gene.
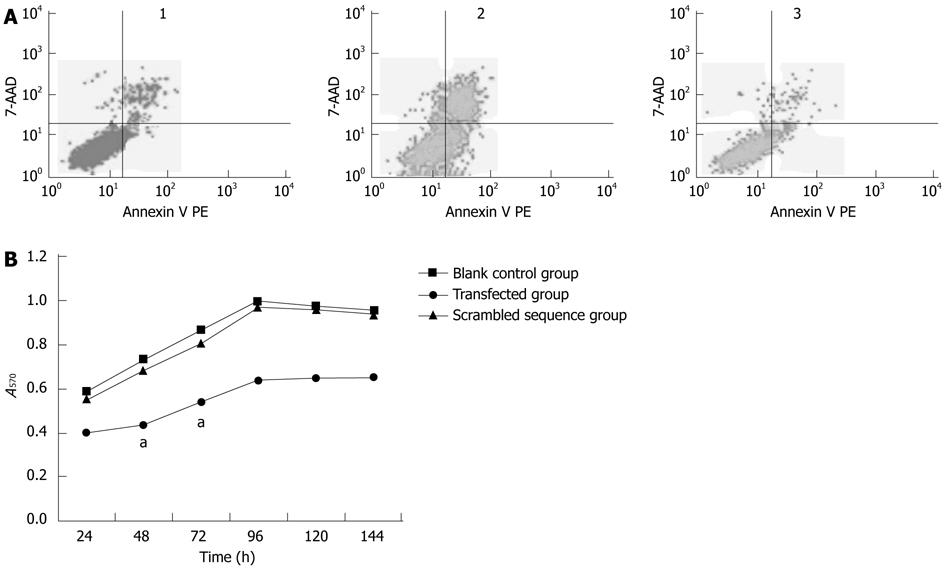
The cell apoptotic rate of the transfected group was significantly higher than that of the blank control group and scrambled sequence group; the difference was statistically significant (P < 0.05). The highest cell apoptotic rate appeared at 48 h after transfection. The difference between the blank control group and the scrambled sequence group was not statistically significant (P > 0.05) (Figure 3).
The MTT assay revealed that there were remarkable differences in cell proliferation at 24, 48 and 72 h after transfection with shRNA. The most obvious cell proliferation inhibition appeared at 48 h after transfection in the transfected group (P < 0.05). There was no significant difference in the inhibition rate of the blank control group and the scrambled sequence group (P > 0.05) (Figure 3).
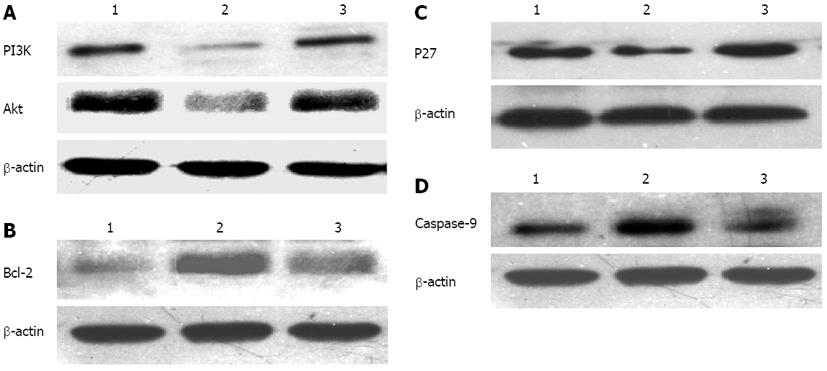
Compared with the blank control group and scrambled sequence group, after transfection of shRNA for 72 h, the protein expression levels of PI3K (0.042 ± 0.005 vs 0.069 ± 0.003 and 0.067 ± 0.05, P < 0.05), Akt (0.248 ± 0.004 vs 0.489 ± 0.006 and 0.496 ± 0.104, P < 0.05) and Bcl-2 (0.295 ± 0.084 vs 0.592 ± 0.072 and 0.594 ± 0.109, P < 0.05) were significantly reduced. Difference between the blank control group and the scrambled sequence group were statistically significant (P > 0.05) (Figure 4A and B). The protein expression levels of P27 (0.151 ± 0.010 vs 0.068 ± 0.014 and 0.060 ± 0.013, P < 0.05) and caspase-9 (0.136 ± 0.042 vs 0.075 ± 0.010 and 0.073 ± 0.072, P < 0.05) were significantly upregulated. The difference between the blank control group and the scrambled sequence group was not statistically significant (P > 0.05) (Figure 4C and D).
Activation of oncogenes, inactivation of suppressor genes and abnormal expression of apoptosis-related genes are closely related to carcinogenesis and progression of gastric cancer. HMGA2 is a transcription factor that is involved in transcriptional regulation of various tumor-related genes[22]. Several studies[14,15,23] have showed that HMGA2 plays a critical role in the processes of many tumors, including enhancing cell growth and invasion. Moreover, the prognosis of patients with high HMGA2 expression is worse than those with low HMGA2 expression[24,25]. The clinical association of HMGA2 with gastric cancer has been reported[19]. The study showed that overexpression of HMGA2 appeared in human gastric cancer, and might be involved in cancer invasion and metastasis. However, the exact mechanism of HMGA2’s involvement in gastric cancer is not clear, especially in relation to signaling pathways and the molecular mechanism.
In the present study, fluorescence microscopy revealed that the transfection efficiency of the transfected group cells can reach about 60%-70%, and that recombinant plasmid was effectively expressed in cells. HMGA2 shRNA transfection could effectively inhibit the expression of HMGA2 gene. The inhibition was achieved without off-target effects, which was confirmed at the mRNA and protein levels by RT-PCR and Western blotting. According to the results of MTT and flow cytometry, cell proliferation was inhibited and apoptosis was increased after transfection. Therefore, we conclude that HMGA2 shRNA gene silencing can effectively induce proliferation inhibition and apoptosis of MKN-45 cells in vitro without off-target effects.
The PI3K/Akt signaling pathway plays important roles in cell survival and resistance to apoptosis[26]; it has been shown to be activated in many cancers and activated Akt acts to phosphorylate P27, caspase-9 and Bcl-2 to promote the resistance of cancer cells to apoptosis[20,21]. A study showed that activation of the PI3K/Akt signaling pathway is induced by overexpression of HMGA2[27]. In this study, western blotting showed that the expressions of PI3K and Akt protein were reduced in the transfected group. Thus, decreased expression of HMGA2 inhibits the activity of thePI3K/Akt signaling pathway.
P27 is a cyclin-dependent kinase (CDK) inhibitor that functions as a fail-safe mechanism in DNA repair and apoptosis[28]. A report showed that the P27 gene reduced cell mitosis division and inhibited cell generation[29]. Overexpression of P27 inhibits CDK activation and entry into the S phase of the cell cycle[30]. The expression level of P27 was positively correlated with cell differentiation, and its loss of function may subsequently contribute to tumorigenesis[31]. Caspase-9, a member of the protease family, is intimately associated with the initiation of apoptosis, and is thought to be activated, while Akt is inhibited[32]. Activated caspase-9 is able to cleave caspase-3, leading to apoptosis[33]. Bcl-2 is a key anti-apoptotic protein involved in the regulation of apoptosis and is overexpressed in many tumors[34-36]. A previous study showed that Bcl-2 delays cell cycle entry by inhibiting Myc activity through the elevation of p27. Bcl-2 can be induced to express via the PI3K/Akt signaling pathway and inhibit cell apoptosis[21,37].
In our study, western blotting revealed that the protein expression levels of Bcl-2 were reduced, whereas the protein expression level of P27 and caspase-9 were upregulated. Thus, decreased expression of HMGA2 inhibits the activity of the PI3K/Akt signaling pathway, and than inhibits cells proliferation and increases apoptosis via upregulation of the protein expression of P27 and caspase-9, and decreased protein expression of Bcl-2.
In summary, our results demonstrated that HMGA2 shRNA gene silencing could induce apoptosis and suppress proliferation of MKN-45 cells in vitro. The PI3K/Akt signaling pathway, P27, caspase-9 and Bcl-2 may play important roles in this HMGA2-mediated effect. We suggest that HMGA2 shRNA gene silencing may be suitable for gastric cancer therapy by suppressing the proliferation and inducing apoptosis of gastric cancer cells.
The newly discovered oncogene, high mobility group A2 (HMGA2), is an endonuclear chromatin protein, which is highly expressed in many malignant tumors. The mechanism by which HMGA2 causes tumors remains unclear, particularly in gastric cancer.
RNA interference can specifically mediate degradation of HMGA2 messenger RNA, block the expression of the gene, inhibit cell proliferation and increase apoptosis, which could be exploited for gastric cancer gene therapy.
This study demonstrated that RNA interference (RNAi) specifically downregulated the expression of HMGA2, inhibited cell proliferation and increased apoptosis. The mechanism appears to be that the decreased expression of HMGA2 inhibits the activity of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway. Inhibition of cell proliferation and increased apoptosis is associated with upregulated protein expression of P27 and caspase-9, and decreasing expression of B-cell leukemia/lymphoma-2 (Bcl-2).
The aims of this study were to investigate the effect of HMGA2 expression on gastric cancer cell proliferation and apoptosis. RNAi was used regulate the expression of HMGA2. Moreover, the expression of PI3K, Akt, P27, caspase-9 and Bcl-2 were detected to investigate the possible mechanism. This will help enrich the theoretical research into the pathogenesis of gastric cancer, and provide an experimental basis for clinical diagnosis and gene therapy of gastric cancer.
This is a well-written manuscript and the results are interesting. It has potential application in gastric cancer gene therapy.
P- Reviewer Mukherjee SK S- Editor Gou SX L- Editor Stewart GJ E- Editor Li JY
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25538] [Article Influence: 1824.1] [Reference Citation Analysis (7)] |
| 2. | Di Cello F, Hillion J, Hristov A, Wood LJ, Mukherjee M, Schuldenfrei A, Kowalski J, Bhattacharya R, Ashfaq R, Resar LM. HMGA2 participates in transformation in human lung cancer. Mol Cancer Res. 2008;6:743-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Lanahan A, Williams JB, Sanders LK, Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992;12:3919-3929. [PubMed] |
| 4. | Narita M, Narita M, Krizhanovsky V, Nuñez S, Chicas A, Hearn SA, Myers MP, Lowe SW. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126:503-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 466] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 5. | Rogalla P, Drechsler K, Frey G, Hennig Y, Helmke B, Bonk U, Bullerdiek J. HMGI-C expression patterns in human tissues. Implications for the genesis of frequent mesenchymal tumors. Am J Pathol. 1996;149:775-779. [PubMed] |
| 6. | Gattas GJ, Quade BJ, Nowak RA, Morton CC. HMGIC expression in human adult and fetal tissues and in uterine leiomyomata. Genes Chromosomes Cancer. 1999;25:316-322. [PubMed] |
| 7. | Fabjani G, Tong D, Wolf A, Roka S, Leodolter S, Hoecker P, Fischer MB, Jakesz R, Zeillinger R. HMGA2 is associated with invasiveness but not a suitable marker for the detection of circulating tumor cells in breast cancer. Oncol Rep. 2005;14:737-741. [PubMed] |
| 8. | Hristov AC, Cope L, Reyes MD, Singh M, Iacobuzio-Donahue C, Maitra A, Resar LM. HMGA2 protein expression correlates with lymph node metastasis and increased tumor grade in pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Piscuoglio S, Zlobec I, Pallante P, Sepe R, Esposito F, Zimmermann A, Diamantis I, Terracciano L, Fusco A, Karamitopoulou E. HMGA1 and HMGA2 protein expression correlates with advanced tumour grade and lymph node metastasis in pancreatic adenocarcinoma. Histopathology. 2012;60:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Liu Q, Lv GD, Qin X, Gen YH, Zheng ST, Liu T, Lu XM. Role of microRNA let-7 and effect to HMGA2 in esophageal squamous cell carcinoma. Mol Biol Rep. 2012;39:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Wu Y, Song Y, Liu H. Expression and its clinical significance of HMGA2 in the patients with non-small cell lung cancer. Zhongguo Feiai Zazhi. 2008;11:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Miyazawa J, Mitoro A, Kawashiri S, Chada KK, Imai K. Expression of mesenchyme-specific gene HMGA2 in squamous cell carcinomas of the oral cavity. Cancer Res. 2004;64:2024-2029. [PubMed] |
| 13. | Langelotz C, Schmid P, Jakob C, Heider U, Wernecke KD, Possinger K, Sezer O. Expression of high-mobility-group-protein HMGI-C mRNA in the peripheral blood is an independent poor prognostic indicator for survival in metastatic breast cancer. Br J Cancer. 2003;88:1406-1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Park SM, Shell S, Radjabi AR, Schickel R, Feig C, Boyerinas B, Dinulescu DM, Lengyel E, Peter ME. Let-7 prevents early cancer progression by suppressing expression of the embryonic gene HMGA2. Cell Cycle. 2007;6:2585-2590. [PubMed] |
| 15. | Malek A, Bakhidze E, Noske A, Sers C, Aigner A, Schäfer R, Tchernitsa O. HMGA2 gene is a promising target for ovarian cancer silencing therapy. Int J Cancer. 2008;123:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Huang ML, Chen CC, Chang LC. Gene expressions of HMGI-C and HMGI(Y) are associated with stage and metastasis in colorectal cancer. Int J Colorectal Dis. 2009;24:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Wang X, Liu X, Li AY, Chen L, Lai L, Lin HH, Hu S, Yao L, Peng J, Loera S. Overexpression of HMGA2 promotes metastasis and impacts survival of colorectal cancers. Clin Cancer Res. 2011;17:2570-2580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Chau K, Arlotta P, Patel UA, Crane-Robinson C, Manfioletti G, Ono SJ. A novel downstream positive regulatory element mediating transcription of the human high mobility group (HMG) I-C gene. FEBS Lett. 1999;457:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Motoyama K, Inoue H, Nakamura Y, Uetake H, Sugihara K, Mori M. Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microRNA family. Clin Cancer Res. 2008;14:2334-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339-345. [PubMed] |
| 21. | Wang XT, Pei DS, Xu J, Guan QH, Sun YF, Liu XM, Zhang GY. Opposing effects of Bad phosphorylation at two distinct sites by Akt1 and JNK1/2 on ischemic brain injury. Cell Signal. 2007;19:1844-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Zhang Q, Wang Y. Homeodomain-interacting protein kinase-2 (HIPK2) phosphorylates HMGA1a at Ser-35, Thr-52, and Thr-77 and modulates its DNA binding affinity. J Proteome Res. 2007;6:4711-4719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Watanabe S, Ueda Y, Akaboshi S, Hino Y, Sekita Y, Nakao M. HMGA2 maintains oncogenic RAS-induced epithelial-mesenchymal transition in human pancreatic cancer cells. Am J Pathol. 2009;174:854-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Venkatesan N, Kandalam M, Pasricha G, Sumantran V, Manfioletti G, Ono SJ, Reddy MA, Krishnakumar S. Expression of high mobility group A2 protein in retinoblastoma and its association with clinicopathologic features. J Pediatr Hematol Oncol. 2009;31:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Liu X, Lai L, Wang X, Xue L, Leora S, Wu J, Hu S, Zhang K, Kuo ML, Zhou L. Ribonucleotide reductase small subunit M2B prognoses better survival in colorectal cancer. Cancer Res. 2011;71:3202-3213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Sos ML, Fischer S, Ullrich R, Peifer M, Heuckmann JM, Koker M, Heynck S, Stückrath I, Weiss J, Fischer F. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci USA. 2009;106:18351-18356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 27. | Ikeda K, Mason PJ, Bessler M. 3’UTR-truncated Hmga2 cDNA causes MPN-like hematopoiesis by conferring a clonal growth advantage at the level of HSC in mice. Blood. 2011;117:5860-5869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501-1512. [PubMed] |
| 29. | Zhang WG, Yu JP, Wu QM, Tong Q, Li SB, Wang XH, Xie GJ. Inhibitory effect of ubiquitin-proteasome pathway on proliferation of esophageal carcinoma cells. World J Gastroenterol. 2004;10:2779-2784. [PubMed] |
| 30. | Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9-22. [PubMed] |
| 31. | Choi HR, Tucker SA, Huang Z, Gillenwater AM, Luna MA, Batsakis JG, El-Naggar AK. Differential expressions of cyclin-dependent kinase inhibitors (p27 and p21) and their relation to p53 and Ki-67 in oral squamous tumorigenesis. Int J Oncol. 2003;22:409-414. [PubMed] |
| 32. | Shultz JC, Goehe RW, Wijesinghe DS, Murudkar C, Hawkins AJ, Shay JW, Minna JD, Chalfant CE. Alternative splicing of caspase 9 is modulated by the phosphoinositide 3-kinase/Akt pathway via phosphorylation of SRp30a. Cancer Res. 2010;70:9185-9196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Yamakawa N, Takahashi A, Mori E, Imai Y, Furusawa Y, Ohnishi K, Kirita T, Ohnishi T. High LET radiation enhances apoptosis in mutated p53 cancer cells through Caspase-9 activation. Cancer Sci. 2008;99:1455-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Azad N, Vallyathan V, Wang L, Tantishaiyakul V, Stehlik C, Leonard SS, Rojanasakul Y. S-nitrosylation of Bcl-2 inhibits its ubiquitin-proteasomal degradation. A novel antiapoptotic mechanism that suppresses apoptosis. J Biol Chem. 2006;281:34124-34134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci. 2006;43:1-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Greider C, Chattopadhyay A, Parkhurst C, Yang E. BCL-x(L) and BCL2 delay Myc-induced cell cycle entry through elevation of p27 and inhibition of G1 cyclin-dependent kinases. Oncogene. 2002;21:7765-7775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |