INTRODUCTION
Excessive accumulation of triglycerides in hepatocytes in the absence of significant alcohol consumption occurs in about 20%-30% of adults[1-5]. Excessive fat in the liver, called nonalcoholic fatty liver disease (NAFLD), predisposes to the development of nonalcoholic steatohepatitis (NASH). NASH constitutes the subset of NAFLD that is most worrisome because it is a significant risk factor for developing cirrhosis and its complications, including hepatocellular carcinoma (HCC)[6-9]. Because the accumulation of excess fat in the liver is a prerequisite for the development of NASH, understanding the underlying causes of NAFLD forms the basis for rational preventive and treatment strategies of this major form of chronic liver disease.
Obesity is a low-grade chronic inflammatory condition and obesity-related cytokines such as interleukin-6 (IL-6), adiponectin, leptin, and tumor necrosis factor (TNF) α may play important roles in the development of NAFLD. The prevalence of NASH is 3% and 20% in nonobese and obese subjects, respectively. Additionally, the prevalence of NASH associated with both cirrhosis and HCC was reported to be high among patients with type-2 diabetes with or without obesity.
OBESITY EPIDEMIC
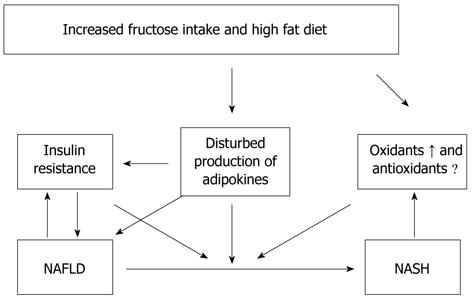
A balance exists between energy demand and intake in the human body. Obesity is one of the major abnormalities of this well preserved equilibrium. Obesity, and its consequences such as insulin resistance and the metabolic syndrome, is a growing threat to the health of people in developed nations[10]. A diet based on high cholesterol, high saturated fat, and high fructose (cafeteria or fast food type) recapitulates features of the metabolic syndrome and NASH with progressive fibrosis (Figure 1).
Figure 1 Diet based on high cholesterol, high saturated fat, and high fructose (cafeteria or fast food type) recapitulates features of the metabolic syndrome and nonalcoholic fatty liver disease and nonalcoholic steatohepatitis with progressive fibrosis in human and mice.
NAFLD: Nonalcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis.
“FAST FOOD” OR “CAFETERIA” TYPE DIET COMPOSED OF HIGH SATURATED FATS, CHOLESTEROL, AND FRUCTOSE
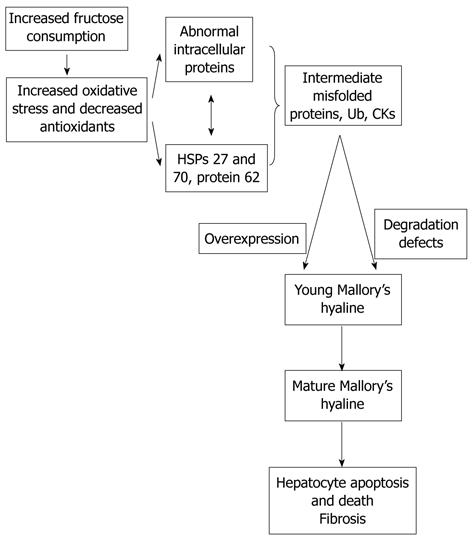
The basis of the composition of “fast food” or “cafeteria” style food is high saturated fats, cholesterol, and fructose[11]. As the high fat diet produces obesity, insulin resistance, and some hepatic steatosis with minimal inflammation and no fibrosis, the fast food diet produces a gene expression signature of increased hepatic fibrosis, inflammation, endoplasmic reticulum stress and lipoapoptosis (Figure 2). Our research group previously showed that consumption of fructose is associated with adverse alterations of plasma lipid profiles and metabolic changes in mice, the American Lifestyle-Induced Obesity Syndrome (ALIOS) model, which included consumption of a high-fructose corn syrup (HFCS) in amounts relevant to that consumed by some Americans[11]. The observation that the ALIOS mice indeed consumed a greater quantity of food beyond the additional calories consumed from the HFCS when fed HFCS compared with control water supports this observation and reinforces the concerns about the role of fructose in the obesity epidemic[12-15]. In adolescents, higher fructose consumption is associated with multiple markers of cardiometabolic risk, but it appears that these relationships are mediated by visceral obesity.
Figure 2 As the high fat diet produces obesity, insulin resistance, and some hepatic steatosis with minimal inflammation with no fibrosis, the fast food diet produces a gene expression signature of increased hepatic fibrosis, inflammation, and endoplasmic reticulum stress and lipoapoptosis.
HSP: Heat shock proteins.
The most commonly used HFCS in soft drinks and other carbohydrate-sweetened beverages is a blend composed of 55% fructose, 41% glucose, and 4% complex polysaccharides. Fructose has increasingly been used as a sweetener since the introduction of high-fructose corn syrups in the 1960s[10-13,16] and is now an abundant source of dietary carbohydrate in the United States. The annual per capita consumption of extrinsic or added fructose was approximately 0.2 kg in 1970 to approximately 28 kg in 1997. This increased consumption has been linked to the increased prevalence of obesity, type 2 diabetes and fatty liver in the United States.
The liver is exquisitely sensitive to changes in nutrient delivery and is uniquely suited to metabolize ingested simple sugars, such as fructose and glucose[13,14]. Stress-activated protein kinases, principally the c-Jun N-terminal kinases (JNK), are activated by cell stress-inducing stimuli. Increased fructose supply provokes a hepatic stress response involving activation of JNK and subsequent reduced hepatic insulin signaling.
UNIQUE METABOLISM OF FRUCTOSE
Fructose, glucose, and galactose are the 3 major dietary monosaccharides. Sucrose (glucose-fructose), lactose (glucose-galactose), and maltose (glucose-glucose) are the major disaccharides. Dietary fructose occurs in 2 forms: mono- or disaccharide. The rate of fructose absorption appears to be between that of mannose and glucose[12-15]. Fructose is absorbed by carrier-mediated facilitated diffusion, an energy-dependent process. The fructose carrier is a member of the glucose transport family and is referred to as glucose transporter 5. Sucrose is cleaved to glucose and fructose by sucrase, an enzyme located in the brush border of small intestine enterocytes.
Fructose was previously accepted as a beneficial dietary component because it does not stimulate insulin secretion. However, since insulin signaling plays an important role in the central mechanisms of NAFLD, this property of fructose may be undesirable[13-15]. Additionally, fructose may prevent suppression of ghrelin secretion, resulting in impaired satiety mechanisms[14]. In large quantities, fructose can also stress the liver by depleting hepatic energy supplies. Normal subjects and patients with NASH exhibited a similar depletion of hepatic ATP levels after an injection of fructose, but recovery of ATP levels after depletion was slower in NASH patients compared with healthy controls. A mixture of fructose and glucose might induce metabolic abnormalities that differ from sucrose, a disaccharide cleaved to fructose and glucose in the small intestine.
Phosphorylation of glucose by glucokinase is a rate-determining step in hepatic glucose metabolism. In contrast to glucose, phosphorylation of fructose in the liver occurs via the enzyme fructokinase. In addition, the metabolism of fructose 1-phosphate in the liver occurs independently of phosphofructokinase, a second rate-determining step in glucose metabolism[13-15]. As a result, the liver is the primary site of fructose extraction and metabolism, with extraction approaching 50% to 70% of fructose delivery. Therefore, increased availability of fructose (e.g., high-fructose corn syrup) will increase not only abnormal glucose flux but also fructose metabolism in the hepatocyte. Thus, the anatomic position of the liver places it in a strategic buffering position for absorbed carbohydrates and amino acids.
Fructose extraction and metabolism by the liver are exceptionally high compared to glucose due both to the extensive amount of fructokinase that phosphorylates fructose to fructose 1-phosphate in the liver and to the subsequent metabolism of fructose 1-phosphate at the triose phosphate level, which bypasses flux control at phosphofructokinase[13-16]. Previous studies comparing the metabolism of fructose and glucose in postabsorptive humans over short intervals have shown that fructose is used faster than glucose and that more is converted to liver glycogen. Fructose oxidation represented a significant portion of fructose metabolism, accounting for 56% to 59% of the ingested fructose and approximately 33% of the infused fructose. It is likely that extrahepatic lactate oxidation subsequent to hepatic fructolysis contributed significantly to the estimated rate of fructose oxidation. Thus, increments in fructose after infusion produced immediate changes in hepatic and extrahepatic substrate metabolism, but did not induce changes in overall glucose production. An immediate fructose infusion in humans induced both hepatic and extrahepatic insulin resistance. These data are consistent with the notion that high concentrations of fructose elicit adaptations in the liver that include metabolic intermediates, gene expression, and insulin action.
SYSTEMIC AND HEPATIC INSULIN RESISTANCE IN NAFLD
While insulin receptor defects cause severe insulin resistance, most patients with insulin resistance have impaired post-receptor intracellular insulin signaling. Insulin binds α-subunits of its receptor, which is a cell surface receptor on the major insulin sensitive cells such as skeletal muscle, adipocytes, and hepatocytes, leading to autophosphorylation of the cytoplasmic domains (β-subunits) of the receptor[2-5,17]. The insulin receptor has intrinsic tyrosine kinase activity activated by insulin binding and the autophosphorylated receptor activates its substrates that include insulin receptor substrate (IRS)-1, IRS-2, Src homology collagen, and adaptor protein with a pleckstrin homology and Src homology 2 domain by tyrosine phosphorylation. These phosphorylated docking proteins bind and activate several downstream components of the insulin signaling pathways. Activated IRS-1 associates with phosphatidyl inositol 3-kinase, which then activates Akt. These events and insulin-dependent inhibition of hepatic glucose output maintain glucose homeostasis. Insulin also affects glucose homeostasis indirectly by its regulatory effect on lipid metabolism. Any interference in this insulin signaling pathway causes glucotoxicity, insulin resistance and, when islet beta cells are capable of responding, compensatory hyperinsulinemia.
Hepatic expression of insulin receptor protein in humans and the levels of both IRS-1 and IRS-2 in animals were decreased in chronic hyperinsulinemic states[11]. IRS-1 was more closely linked to glucose homeostasis with the regulation of glucokinase expression while IRS-2 was more closely linked to lipogenesis with the regulation of lipogenic enzymes sterol regulatory element-binding protein-1c (SREBP-1c) and fatty acid synthase[18,19]. Additional physiological roles of insulin include regulating the metabolism of macronutrients and stimulating cellular growth. Insulin activates synthesis and inhibits catabolism of lipids while shutting off the synthesis of glucose in the liver.
Adipose tissue is one of the major insulin sensitive organs in the human body and the process of differentiation of preadipocytes to adipocytes is induced by insulin[17,18]. Within the adipose tissue, insulin stimulates triglyceride synthesis and inhibits lipolysis by upregulating lipoprotein lipase activity which is the most sensitive pathway in insulin action, facilitating free fatty acid uptake and glucose transport, inhibiting hormone sensitive lipase, and increasing gene expression of lipogenic enzymes.
PROINFLAMMATORY SIGNALING IN INSULIN RESISTANCE
Protein kinase C theta (PKCtheta;) and inhibitor κB kinase β (IKK-β) are two proinflammatory kinases involved in insulin downstream signaling[17,18]. They are activated by lipid metabolites such as high plasma free fatty acid concentrations and there is a positive relationship between the activation of PKCtheta; and the concentration of intermediate fatty acid products. PKCtheta; activates both IKK-β and JNK, leading to increased Ser 307 phosphorylation of IRS-1 and insulin resistance. Activation or overexpression of IKK-β diminishes insulin signaling and causes insulin resistance whereas inhibition of IKK-β improves insulin sensitivity. Inhibition of IKK-β activity prevented insulin resistance due to TNF-α in cultured cells. IKK-β phosphorylates the inhibitor of nuclear factor kappa B (NF-κB), leading to the activation of NF-κB by the translocation of NF-κB to the nucleus. NF-κB is an inducible transcription factor and promotes specific gene expression in the nucleus. For example, NF-κB regulates the production of multiple inflammatory mediators, such as TNF-α and IL-6. TNF-α and reactive oxygen species could also activate NF-κB[19-22]. In contrast, antioxidants inhibit this activation. NF-κB has both apoptotic and anti-apoptotic effects. The finding that NF-κB deficient mice were protected from high-fat diet-induced insulin resistance suggests that NF-κB directly participates in processes that impair insulin signaling. High-dose salicylates also inhibit NF-κB and subsequently improve insulin sensitivity. These subsequently promote hepatic and systemic insulin resistance. The study group also showed that these results were reversed by curcumin which inhibits NF-κB activity. Curcumin also has the ability to induce antioxidant enzymes and scavenge ROS.
Suppressors of cytokine signaling (SOCS) and inducible nitric oxide synthase are two inflammatory mediators recently recognized to play a role in insulin signaling[23-25]. Induction of SOCS proteins (SOCS 1-7 and cytokine-inducible src homology 2 domain-containing protein) by proinflammatory cytokines might contribute to the cytokine-mediated insulin resistance in obese subjects[26-30]. In fact, the isoforms of SOCS are the members of a negative feedback loop of cytokine signaling, regulated by both phosphorylation and transcription events. SOCS-1, and particularly SOCS-3, are involved in the inhibition of insulin signaling either by interfering with IRS-1 and IRS-2 tyrosine phosphorylation or by the degradation of their substrates. SOCS-3 might also regulate central leptin action and play a role in the leptin resistance of obese human subjects. SOCS might be a link between leptin and insulin resistance because insulin levels are increased in leptin resistant conditions due to the diminished insulin suppression effect of leptin because of insufficient leptin levels. Moreover, SOCS proteins might involve insulin/insulin like growth factor-1 signaling. SOCS-1 knockout mice showed low glucose concentrations and increased insulin sensitivity. SREBP-1c is one of the key mediators of lipid synthesis from glucose and other precursors (de novo lipogenesis) in the liver. Indeed, SOCS proteins markedly induce de novo fatty acid synthesis in the liver by both the up-regulation of SREBP-1c and persistent insulin resistance with hyperinsulinemia which stimulates SREBP-1c-mediated gene expression. Liver is the insulin clearance organ. Thus, decreased insulin clearance in patients with NAFLD further elevates insulin levels in the circulation and de novo lipogenesis in the liver. SOCS-1 and SOCS-3 may exert these effects by inhibiting signal transduction and activator of transcription proteins (STAT), particularly STAT-3, via binding Janus tyrosine Kinase (JAK) tyrosine kinase because this binding diminishes the phosphorylation ability of JAK kinase to STAT-3. STAT-3 inhibits the activation of SREBP-1c. Specific STAT-3 knockout mice showed markedly increased expression of SREBP-1c and subsequently increased fat content in the liver. Conversely, inhibition of SOCS proteins, particularly SOCS-3, improved both insulin sensitivity and the activation of SREBP-1c which eventually reduced liver steatosis and hypertriglyceridemia in db/db mice.
Nitric oxide synthase-2 (NOS2) or inducible nitric oxide synthase (iNOS) production are also induced by proinflammatory cytokines[31]. A high-fat diet in rats causes up-regulation of iNOS mRNA expression and increases iNOS protein activity. Increased production of NOS2 might reduce insulin action in both muscle and pancreas and decreased iNOS activity protects muscles from the high-fat diet induced insulin resistance. It was also shown that leptin deficient ob/ob mice without iNOS were more insulin sensitive than ob wild-type mice. Thus, the production of nitric oxide may be one link between inflammation and insulin resistance.
SOURCES OF LIVER FAT
Accumulation of triglycerides as fat droplets within the cytoplasm of hepatocytes is a prerequisite for subsequent events of NASH. Accumulation of excess triglyceride in hepatocytes is generally the result of increased delivery of non-esterified fatty acids (NEFAs), increased synthesis of NEFAs, impaired intracellular catabolism of NEFAs, impaired secretion as triglyceride, or a combination of these abnormalities[32]. Recent techniques, such as isotope methodologies, multiple-stable-isotope approach and gas chromatography/mass spectrometry, provided valuable information regarding the fate of fatty acids during both fasting and fed states[33] such as the relative contribution of three fatty acid sources to the accumulated fat in NAFLD: adipose tissue, de novo lipogenesis, and dietary fat. Additionally, these studies reported that the plasma NEFA pool is the main contributor of both hepatic triglycerides in the fasting state and very low-density lipoproteins (VLDL)-triglycerides in both fasting and fed states.
DYSREGULATED PERIPHERAL LIPOLYSIS
A study showed that adipose tissue makes a major contribution to the plasma NEFA pool, contributing 81.7% in the fasted state and 61.7% in the fed state[33]. Additionally, the contribution of dietary lipids to the plasma NEFA pool was found to be only 26.2% and 10.4% in fed and fasted states, respectively, in the same study. Finally, the contribution of newly made fatty acids (originating from the adipose tissue and liver) to the plasma NEFA pool was 7.0% and 9.4% for the fasted and fed states, respectively.
The liver takes up free fatty acids from the circulating NEFA pool and the rate of uptake depends only on the plasma free fatty acid concentrations. Hepatic NEFA uptake continues despite increased hepatic content of fatty acids and triglycerides[34]. The concentration of free fatty acids is increased in the portal circulation rapidly when lipolysis occurs in visceral adipose tissue. These products directly flux to the liver via the splanchnic circulation and contribute to hepatic triglyceride synthesis, NAFLD, and hepatic insulin resistance.
HEPATIC DE NOVO LIPOGENESIS
Hepatic de novo lipogenesis (fatty acid and triglyceride synthesis) is increased in patients with NAFLD[35-39]. Stable-isotope studies showed that increased de novo lipogenesis (DNL) in patients with NAFLD contributed to fat accumulation in the liver and the development of NAFLD[33]. Specifically, DNL was responsible for 26% of accumulated hepatic triglycerides and 15%-23% of secreted VLDL triglycerides in patients with NAFLD compared to an estimated less than 5% DNL in healthy subjects and 10% DNL in obese people with hyperinsulinemia. Interestingly, Donnelly and colleagues demonstrated the similarity between VLDL-triglycerides and hepatic-triglycerides regarding contributions of fatty acid sources (62% vs 59% for NEFA contribution, respectively; 23% vs 26% for DNL, respectively; and 15% vs 15% for dietary fatty acids, respectively) in NAFLD patients. Substrates used for the synthesis of newly made fatty acids by DNL are primarily glucose, fructose, and amino acids; oleic acid (18:1, a ω-6 monounsaturated fatty acid, which is relatively resistant to peroxidation) is the major end product of de novo fatty acid synthesis[40-42]. Moreover, simple sugars have the ability to stimulate lipogenesis[33]. Ingested carbohydrates are a major stimulus for hepatic delayed neuronal loss and are thus more likely to directly contribute to NAFLD than dietary fat intake[43-46].
In conclusion, fructose has increasingly been used as a sweetener since the introduction of high-fructose corn syrups in the 1960s and is now an abundant source of dietary carbohydrate in the United States[47-50]. The most commonly used HFCS in soft drinks and other carbohydrate-sweetened beverages is a blend composed of 55% fructose, 41% glucose, and 4% complex polysaccharides[51-55]. This increased consumption has been linked to the increased prevalence of obesity and type 2 diabetes and fatty liver in the United States by increased fructose supply, which provokes a hepatic stress response involving activation of JNK and subsequent reduced hepatic insulin signaling[56-59]. Understanding the underlying causes of NAFLD forms the basis for rational preventive and treatment strategies of this major form of chronic liver disease.
P- Reviewers Koutsilieris M, Lee SY S- Editor Gou SX L- Editor O’Neill M E- Editor Zhang DN