INTRODUCTION
Acute-on-chronic liver failure (ACLF) has a poor prognosis and a high mortality. It may result in multiple syndromes, including jaundice, coagulopathy, ascites, and even multiple organ dysfunction syndrome induced by precipitating factors (e.g., hepatotropic virus reactivation, infection/sepsis, and hepatotoxic drugs), and secondary compensated liver diseases (e.g., chronic hepatitis and stable liver cirrhosis). ACLF is characterized by acute deterioration of hepatic function, which develops aggressively[1,2]. Given the long-term survival without liver transplantation (LTx) or self-recovery in ACLF subjects is very low, emergency LTx has been introduced as a first-line therapy to significantly improve the short- and long-term survival of the patients[3], in spite of the recently emerging debate on the benefit of artificial liver support to improve the short-term prognosis[4-7].
ABO blood group is considered as a barrier to LTx. Immune rejection, especially antibody-mediated rejection (AMR), is the main cause of graft loss in ABO-incompatible (ABO-I) LTx. Due to the presence of A/B antigen on the surface of vascular endothelium, bile duct epithelium and liver sinusoidal endothelial cells, an ABO-I graft might be attacked by the existing hemagglutinin, causing damage to the blood vessel and bile duct of the graft, characterized by liver parenchymal necrosis and biliary strictures. Other causes of graft loss include vascular or biliary complications, and recurrence of viral hepatitis.
Therapeutic advancement has improved the outcomes of ABO-I LTx and made a long-term survival possible[8-10]. In this report, we present a case of severe hepatic necrosis of unknown reasons after ABO-I LTx. However, the patient was cured and discharged.
CASE REPORT
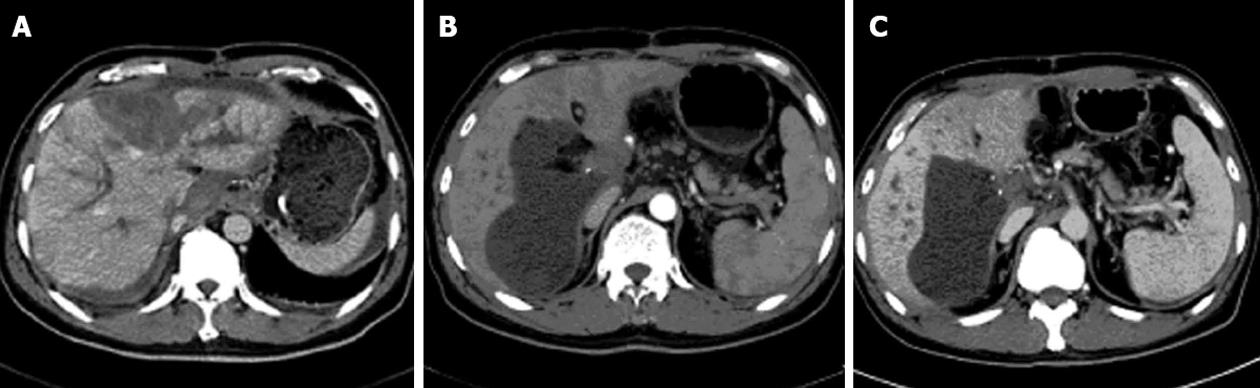
A 53-year-old male patient was transferred from the internal medical ward to the liver transplant unit after he was diagnosed as having severe hepatitis B and ACLF, and he then underwent an emergency ABO-I LTx. The liver graft was obtained from a cardiac-death donor with blood group B, which mismatched the recipient’s blood group O. Cold ischemia time of the graft was 5 h. The modified piggy orthotopic liver transplantation was performed with a 60-min anhepatic phase and without splenectomy. Massive ascites (about 4000 mL), omentum and gallbladder edema, enlarged tough spleen and severe atrophic and cirrhotic liver were visualized after a right upper incision, confirming the diagnosis of ACLF. The operation was successful. Basiliximab 20 mg and methylprednisolone 1000 mg were administered for immune-induction and hepatitis B immunoglobulin (HB-Ig) 1000 U for prevention of hepatitis B virus (HBV) recurrence before anhepatic phase. A quadruple therapy was given to prevent rejection: (1) Rituximab 100 mg on post-operative day (POD) 1 and Basiliximab 20 mg on POD 4 for induction of immune tolerance; (2) Tacrolimus (Tac) at a dose adjusted according to a blood level of 10-15 ng/mL; (3) Methylprednisolone (MP) at a dose gradually reduced from 1000 mg qd to a maintenance of 10 mg bid; and (4) Mycophenolatemofetil, 0.5 g, bid. Other treatments included administration of antibiotics, antiviral therapy with lamivudine, proton pump inhibitor and liver-protective medication. Primary monitoring examinations included blood routine, liver function, coagulation function, HBV serum virology, serum drug level of Tac, B-ultrasound (B-US) and computed tomography (CT) scan. Pathological examination, lymphocyte subsets analysis and ImmuKnow Assay (T cell function test) results showed no rejection. On POD 16, the patient had fever and erythra. B-US displayed a sub-hepatic, pelvic and thoracic fluid sonolucent area, while CT scan showed abnormal left liver lobe infusion and slight intrahepatic bile duct dilatation (Figure 1). Both alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were elevated (ALT 54.3 U/L and AST 26.5 U/L vs ALT 1204.2 U/L and AST 1555.3 U/L at POD 12 and 16, respectively). All these results indicated a severe hepatic necrosis. After a 10-d MP pulse therapy, the patient’s symptoms were relieved and aminotransferase was reduced. The patient was discharged on POD 35, and was followed up once a month. The patient is doing well without any recurrent disease.
Figure 1 Computed tomography scan and computed tomography angiography images.
A: Intrahepatic bile duct expansion and left lobe liver necrosis on post-operative day (POD) 19; B: A low-density cyst (6.1 cm × 13 cm) and a filling defect (2.1 cm × 1.9 cm) at portal area on POD 48; C: Computed tomography (CT) scan and CT angiography on POD 118 showed a smaller low-density cyst (5 cm × 10 cm) and a same-sized filling defect (2.1 cm × 1.9 cm) at portal area on POD 118 compared with earlier findings.
DISCUSSION
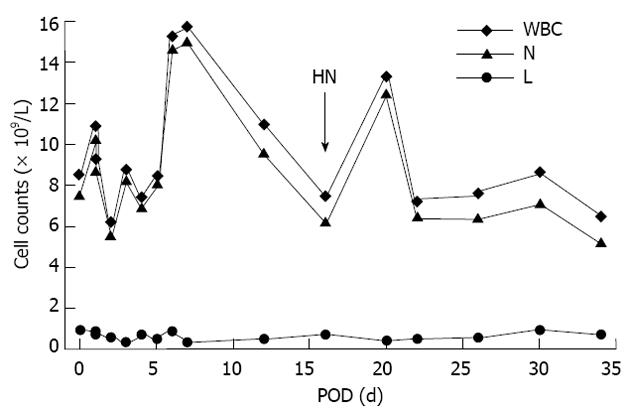
The initial clinical impression of severe hepatic necrosis on POD 16 was rejection. AMR is triggered by alloantibody binding and compliment activation. In the case of ABO-I solid organ transplantation, A, B or AB blood group antigens expressed on graft could be detected by specific natural antibodies. Incompatible blood group antigens activated B cells directly with the help of T cells and resulted in transcription of cytokines and antibodies[11]. AMR can be caused by performed antibodies, anamnestic B-cell responses, early de novo and late de novo B-cell responses. The strength of isohemagglutinins and natural antibodies in ABO-I recipients can be detected and quantified by multiple methods. Antibody titer is reported to correlate with the survival of recipients. ABO-I living donor liver transplantation with high preoperative and postoperative immunoglobulin (Ig) G and IgM levels sustained bile duct complication and hepatic necrosis, respectively[12], while the safe value of isohemagglutinin titer is ≤ 1:16 for kidney transplantation[13,14] and ≤ 1:4 for pediatric heart transplantation[15,16]. In this case, on POD 16, the recipient run a fever, and had poor liver function, and CT scan showed intrahepatic bile duct dilatation and liver necrosis in the left lobe (Figure 1A). But lymphocyte subset analysis indicated a slight increase of T cells and a drop of B cells (CD3+ 77.9%, CD3+CD4+ 49.7%, CD3+CD8+ 23.9%, CD3-CD16+56 14.4% and CD19+ 1%), which was contradictory to AMR with an enhanced B cell function. Meanwhile, ImmuKnow Assay (T cell function tests) before LTx and on POD 34 and 90 were 156.7 ng/mL, 101.7 ng/mL and 70.3 ng/mL, respectively, suggesting a depression of T cell function after LTx resulting from immunosuppressive therapy[17,18]. The total number of white blood cell was mainly affected by neutrophils (Figure 2). Levels of IgG, IgA, IgM, C3 and C4 were normal or almost normal before LTx and on POD 118 (18.1 g/L, 3.45 g/L, 0.94 g/L, 0.20 g/L and 0.0159 g/L vs 7.1 g/L, 1.38 g/L, 0.51 g/L, 1.07 g/L and 0.305 g/L, respectively). Therefore, rejection was not considered as the cause of hepatic necrosis initially.
Figure 2 Blood routine test results after ABO-incompatibleliver transplantation.
Due to treatment with tacrolimus and methylprednisolone, the number of lymphocyte stayed stable before and after the occurrence of severe hepatic necrosis, while the total number of white blood cell counts was mainly affected by neutrophil changes, which might be induced by non-specific reasons, e.g., phagocytose and absorption of necrotic tissues. LTx: Liver transplantation; POD: Post-operative day; HN: Hepatic necrosis; WBC: White blood cell; L: Lymphocyte; N: Neutrophil.
Another factor leading to severe hepatic necrosis after LTx was recurrent diseases. To prevent recurrent hepatitis B, HB-Ig and/or lamivudine was administered. A 10-year follow-up study in 24 individuals with chronic hepatitis B demonstrated the effectiveness of HB-Ig and/or lamivudine[19]. Nineteen patients were alive after the follow-up duration with well-functioning graft. None of them developed recurrent hepatitis B. Among them, 12 patients received HB-Ig and lamivudine, 3 received lamivudine only, 1 received adefovir, and 3 received no antivirals. HbsAg and HbcAb were both positive in the serum virology test in this patient before LTx. Antiviral treatments included HB-Ig 1000 U injection during LTx and lamivudine p.o. after operation. Post-LTx HBV examination results were all negative. Pathological examination of the explanted liver showed classic necrotic changes, without hepatocellular carcinoma. Taken together, there was no recurrent hepatitis or carcinoma till the last follow-up in this case, although CT scan showed dilation of bile duct and inflammation of left liver lobe (Figure 1A), which could result from recurrent viral hepatitis[20].
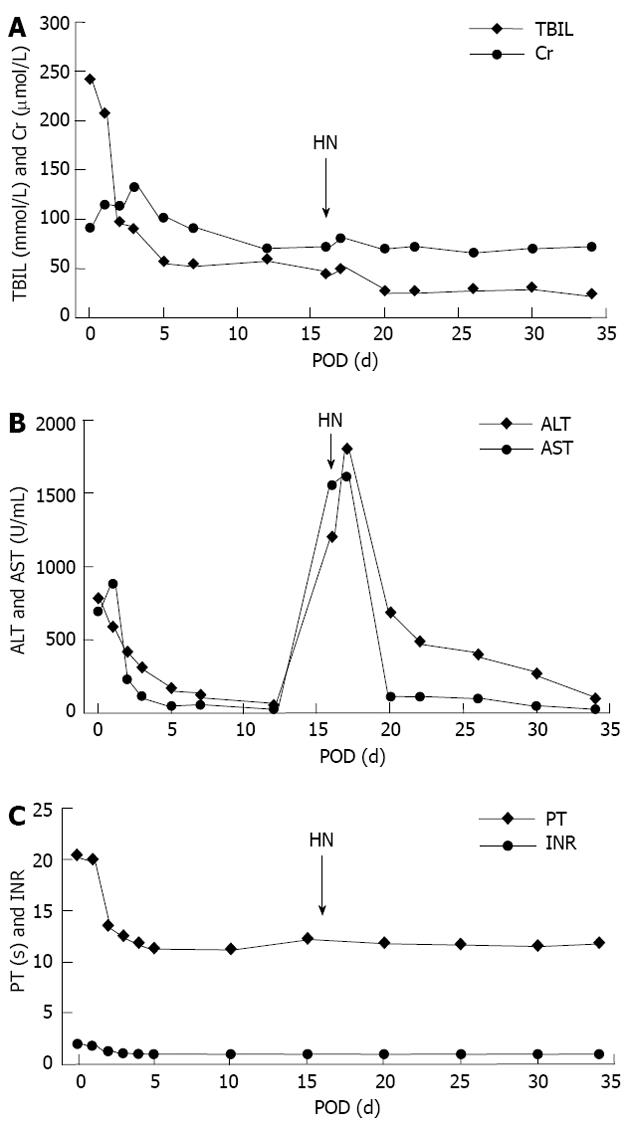
Blood supply disorders and biliary complications could also lead to hepatic necrosis after LTx. CT scan and CT angiography (CTA) also suggested normal hemodynamics on POD 3, but a low-density cystic echo (6.1 cm × 13 cm) and a filling defect (2.1 cm × 1.9 cm) at the portal area (Figure 1B). However, as shown in Figure 3A, total bilirubin and creatinine levels turned normal on POD 7 according to Li et al[21], and remained stable during hepatic necrosis after LTx. Besides, ALT and AST levels started to fall on POD 20, i.e., 4 d after an MP pulse therapy for severe hepatic necrosis, indicating that liver function had been improved (Figure 3B). As demonstrated in Figure 3C, PT and INR became normal on POD 5[21], and B-US on POD 16 showed no sign of intrahepatic thrombosis and stricture. To prevent postoperative bleeding, hemocoagulase was injected which would not result in prothrombin increase and thrombosis. So we excluded vascular or biliary complications. Low molecular weight heparin calcium injection was also given till POD 30 for 14 d. However, CT scan and CTA on POD 118 showed a smaller low-density cyst (5 cm × 10 cm) and a same-sized filling defect (2.1 cm × 1.9 cm) at portal area compared with the earlier findings (Figure 1C), which indicated no deterioration of blood supply.
Figure 3 Total bilirubin and creatine, liver function, prothrombin time and international normalized ratio changes after liver transplantation.
A: Total bilirubin (TBIL) and creatine (Cr) levels elevated after graft implantation and then decreased in 2 wk. Severe hepatic necrosis resulted in a slight rise, which was reversed by methylprednisolone(MP) pulse therapy; B:Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) dropped significantly within 5 d after liver transplantation (LTx), and then remained in a low level till post-operative day (POD) 16. Severe hepatic necrosis led to a sharp increase of both ALT and AST which were under control after 4-d intravenous drip of 500 mg MP. Dosage of MP was reduced gradually to 40 mg on POD 25; C: Prothrombin time (PT) and international normalized ratio (INR) changes after LTx. Coagulation function became normal within 5 d. HN: Hepatic necrosis.
In conclusion, coagulatory, immunologic and hepatic functions after LTx were complicated, and we have not encountered such case before at our center. This patient had severe hepatic necrosis on POD 16, which might be attributed to the rejection with atypical manifestation due to immune suppressive therapy. However, the patient recovered in liver function after treatment with MP, and discharged on POD 34. Till the last follow-up 10 mo after LTx, there were no symptoms or signs indicating bad prognosis. Although the mechanism for hepatic necrosis and recovery of similar cases needs further studies, our experience has provided evidence that emergency ABO-I LTx is an effective treatment option for ACLF, and secondary liver necrosis of unknown causes might be treated by MP.