Published online Feb 14, 2013. doi: 10.3748/wjg.v19.i6.882
Revised: November 5, 2012
Accepted: November 24, 2012
Published online: February 14, 2013
Processing time: 207 Days and 15.9 Hours
AIM: To determine the splanchnic blood flow and oxygen uptake in healthy-subjects and patients and to relate the findings to body-composition.
METHODS: The total splanchnic blood flow (SBF) and oxygen uptake (SO2U) were measured in 20 healthy volunteers (10 women) and 29 patients with suspected chronic intestinal ischemia (15 women), age 40-85 years, prior to and after a standard meal. The method is based on the Fick principle using the continuous infusion of an indicator (99mTechnetium-labelled mebrofenin) and catheterization of an artery and the hepatic vein. An angiography of the intestinal arteries was performed during the same investigation. A whole-body dual-energy x-ray absorptiometry scan was performed in healthy volunteers to determine body composition.
RESULTS: Angiography revealed no atherosclerotic lesions in the intestinal arteries. The mean baseline SBF was 1087 mL/min (731-1390), and this value increased significantly to 1787 mL/min after the meal in healthy volunteers (P < 0.001). The baseline SBF in patients was 1080 mL/min, which increased to 1718 mL/min postprandially (P < 0.001). The baseline SBF was independent of age, sex, lean body mass and percentage of body fat. The mean meal-induced increase in SBF was equal to 282 mL/min + 5.4 mL/min × bodyweight, (P = 0.025). The SO2U in healthy volunteers and patients was 50.7 mL/min and 48.0 mL/min, respectively, and these values increased to 77.5 mL/min and 75 mL/min postprandially, respectively. Both baseline and postprandial SO2U were directly related to lean body mass. Age and sex exerted no impact on SO2U.
CONCLUSION: A direct correlation between body weight and the postprandial increase in SBF was observed. The effect of body weight should be considered in the diagnosis of chronic intestinal ischemia.
- Citation: Zacho HD, Henriksen JH, Abrahamsen J. Chronic intestinal ischemia and splanchnic blood-flow: Reference values and correlation with body-composition. World J Gastroenterol 2013; 19(6): 882-888
- URL: https://www.wjgnet.com/1007-9327/full/v19/i6/882.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i6.882
Hepatic and total splanchnic blood flow (SBF) can be quantified using the Fick principle with the continuous infusion of an indicator, the catheterization of the liver vein and subsequent blood sampling. This method has been thoroughly described in the literature[1,2], and it has been validated[3]. SBF is used for the investigation of splanchnic oxygen uptake (SO2U) or liver metabolism[4] and the examination of the liver clearance of endogen substances and pharmaceuticals[5]. This method is used in Scandinavian countries as a diagnostic tool for chronic intestinal ischemia (CII)[6,7]. SBF is measured at baseline and after a meal to quantify the meal-induced SBF response.
The diagnosis of CII is based on the inability to enhance SBF after a standard meal, and an increase less than 250 mL/min is abnormal[6-8]. Reference values for SBF are based on few and often young individuals without consideration of their body composition or the morphology of their intestinal arteries[6,8,9]. The lack of this information is deplorable because atherosclerotic lesions in the intestinal arteries are commonly observed in a population of otherwise healthy middle-aged individuals[10,11]. Furthermore, patients suffering from CII are frequently severely underweight[7,12], and the large variation in the anthropometrics of individuals may influence SBF values. Therefore, investigations are required to explore the association between SBF, SO2U, age, and body composition.
The present study determined the SBF and SO2U prior to and after a standard meal in a group of middle-aged healthy volunteers with angiography-proven normal intestinal arteries. This study also related SBF and SO2U values to anthropometric measures of the body in healthy volunteers and in a cohort of patients with suspected chronic intestinal ischemia due to weight loss and abdominal pain but with angiography-proven normal intestinal arteries.
The protocol was performed under a license from the Ethical Committee, Central Denmark Region, and the Danish Data Protection Agency. Individually signed informed consent forms were obtained according to the Helsinki II declaration. No complications or side effects were encountered in the study. Twenty healthy volunteers aged 40-70 year (10 women) participated in the present study. None of the volunteers exhibited any signs of cardiovascular disease, abdominal complaints or weight loss one year prior to the investigation. Apart from appendectomy n = 2, no former abdominal surgery was performed in any of the volunteers. A total of 180 patients with suspected CII were routinely referred for SBF measurements due to weight loss and abdominal pain from June 2002 to October 2011. Patients with a digital subtraction angiography that revealed three normal intestinal arteries were included in the present study for comparison only. Therefore, 32 patients participated in the study; 31 (97%) patients suffered from postprandial abdominal pain, and 25/32 had experienced unintentional weight loss (mean 10.4 kg). Three patients were excluded: two patients due to ischemic colitis as verified on biopsy and one patient due to pulmonary cancer that had metastasized to the ventricle and colon. Table 1 presents the anthropometric data of the healthy volunteers and patients.
| Healthy volunteers | Patients suspected of CII, normal angiography | ||||
| Female | Male | Female | Male | P value | |
| n = 10 | n = 10 | n = 15 | n = 14 | ||
| Age (yr) | 53.8 | 54.5 | 63 | 60.4 | 0.01 |
| (40-64) | (43-69) | (44-85) | (45-76) | ||
| Weight (kg) | 72 | 83 | 57.9 | 74.9 | 0.02 |
| (55.0-92.0) | (65.0-94.8) | (34.0-114) | (52.0-98.0) | ||
| Height (cm) | 165 | 177 | 165 | 176 | 0.88 |
| (160-170) | (170-188) | (153-172) | (170-186) | ||
| BMI (kg/m2) | 26.5 | 26.6 | 21.4 | 24 | 0.02 |
| (21.2-34.5) | (21.7-30.6) | (12.5-41.9) | (15.0-31.6) | ||
| Body fat (%) | 34.4 | 21.9 | NA | NA | |
| (28.0-48.2) | (13.1-29.4) | ||||
| LBM (kg) | 47.4 | 65.5 | NA | NA | |
| (39.6-54.6) | (55.8-70.6) | ||||
| BSA (m2) | 1.81 | 2.01 | 1.61 | 1.89 | 0.02 |
| (1.56-2.07) | (1.77-2.16) | (1.25-2.29) | (1.64-2.19) | ||
All of the healthy volunteers and patients underwent the following protocol.
Subjects underwent catheterization of the femoral artery and vein in the morning following an overnight fast using the Seldinger technique under local analgesia. A 5F sheath was used for the artery, and a 7F sheath was used for the vein. The venous catheter (Schwan-Ganz, Edwards, CA, United States) was placed in a central hepatic vein using fluoroscopy, and the arterial catheter (pigtail catheter, Cordis, NJ, United States) was positioned in the abdominal aorta.
The SBF equals the hepatic blood flow in normal subjects, and it was measured using a standardized protocol of the indirect Fick principle with 99mTechnetium labeled Bridatec (Mebrofenin®, GE Healthcare, Suluggia, Italy) [99mTc-Mebrofenin (MBF)] as the indicator. This method was originally introduced by Bradley[1], and it was used with corrections for unsteady state and small urinary excretion of 99mTc-MBF, as recommend by Henriksen et al[2].
Briefly, a bolus injection of 99mTc-MBF was administered followed by a constant infusion at 2.02 mL/min (range: 1.96-2.17 mL/min), which equaled an infusion rate of 0.323 MBq/min. Less than 80 MBq was used in total. An equilibration period of 20 min was interposed before blood samples were collected to obtain steady state measurements. Splanchnic plasma flow (SPF) was calculated as SPF = E/(Ca-Cv), where E is the hepato-biliary excretion rate of 99mTc-MBF, which equals the corrected infusion rate[2]. Ca and Cv are the concentrations of 99mTc-MBF in the abdominal aorta and the hepatic vein, respectively. The level of 99mTc-MBF in plasma samples was determined using a Cobra II Auto-Gamma counter (Packard Bioscience Company, Frankfurt, Germany). At least 10 000 counts were obtained and corrected for decay, background and dead time.
SBF was calculated as SPF/(1-hematocrit fraction). The splanchnic oxygen uptake (SO2U) was calculated as hemoglobin × (arterial oxygen saturation-hepatic venous oxygen saturation) × SBF × 1.34 mL O2/g of hemoglobin. The blood samples were analyzed using an ABL 700 series (Radiometer Medical A/S, Brønshøj, Denmark), which was operated according to the manufacturer’s instructions.
Blood samples were taken from a central hepatic vein and the abdominal aorta simultaneously every ten minutes during the first hour. A mean baseline value was calculated. The participants ingested a 4000 kJ/400 mL standard liquid meal that consisted of 33% protein, 33% carbohydrates and 33% fat after one hour. Blood samples were collected every ten minutes for an additional hour, and blood glucose levels were measured at each sampling time as a control of gastric emptying and intestinal absorption. The extraction fraction (EF) of 99mTc-MBF was calculated as (Ca-Cv)/Ca.
The wedged and free hepatic vein pressures were measured using a capacitance transducer at the end of the session to exclude the presence of portal hypertension and resulting porto-systemic shunting. The mean gradient wedged-to-free hepatic vein pressure was 2.5 mmHg (range 1-5 mmHg).
An angiography was performed during the same session via a 4F pigtail catheter in the abdominal aorta as a control for the morphology of the intestinal arterial blood supply. The angiography included an antero-posterior and lateral horizontal abdominal projection to visualize the individual origin of the mesenteric artery branches. Iomeprol (Iomeron® 200 mg iodine/mL, Bracco, Milan, Italy; 15 mL for each image) was used as a contrast agent, and a total of 45 mL was administered on average.
Each artery was classified as normal (0%-9% lumen reduction) or exhibiting slight stenosis (10%-49% lumen reduction), moderate stenosis (50%-69% lumen reduction), significant stenosis (70%-99% lumen reduction) or occlusion. The investigator who evaluated the angiographies was blinded to the results of the SBF, and both positive and negative controls were included.
Only healthy volunteers were subjected to a dual-energy x-ray absorptiometry (DEXA) scan. Body composition, which was evaluated as the percentage of body fat and fat-free mass, was measured using whole-body scanning in a Delfi Discovery A S/N 70879 and software version 12.6 (Hologic, MA, United Stastes) for quantification. Each participant was immobilized in supine position during evaluation with the arms and legs away from the body. The equipment was calibrated daily according to the manufacturer’s instructions.
Statistical analysis was performed using STATA®11 (StataCorp LP, Station, Texas, United States). All results are presented as mean values, SD and ranges. Paired and unpaired Student’s t tests were used for intergroup comparisons. Relationships between variables were analyzed using linear or multiple linear regression analysis and repeated measurements when appropriate. A P value < 0.05 was considered statistically significant.
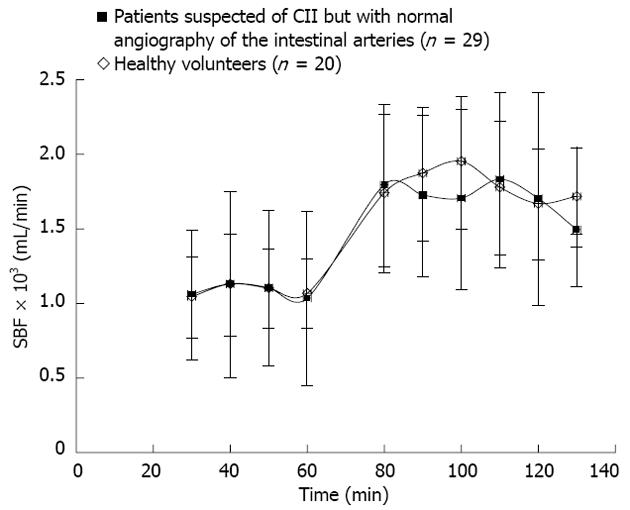
The average values and ranges of SBF and SO2U during fasting and after the meal are presented in Table 2. Significant differences (P < 0.01) between these values were observed at baseline and after the standard meal. The mean body weight among patients (65.4 kg) was significantly (P = 0.016) smaller than the group of healthy volunteers (77.5 kg). Eleven of the 29 patients were underweight [defined as body mass index (BMI) < 20]. None of the healthy volunteers were underweight. However, no differences in the mean baseline SBF were observed between patients (1080 mL/min) and healthy volunteers (1087 mL/min) (P = 0.95) or the mean postprandial SBFs, which were 1718 mL/min in patients and 1787 mL/min in healthy volunteers. Figure 1 presents the mean SBF and standard deviation as a function of time in healthy volunteers and the patient group.
| Healthy volunteers | Patients suspected of CII, normal angiography | ||||
| Female | Male | Female | Male | P value | |
| n = 10 | n = 10 | n = 15 | n = 14 | ||
| Fasting plasma glucose (mmol/L) | 5.7 | 5.8 | 5.7 | 5.6 | 0.59 |
| (5.1-6.9) | (5.2-6.9) | (4.5-11.6) | (4.7-7.5) | ||
| Postprandial plasma glucose (mmol/L) | 6.8b | 7.2b | 7.0b | 7.2b | 0.27 |
| (5.9-7.8) | (6.3-8.8) | (5.4-12.1) | (5.9-9.9) | ||
| Mean fasting SBF (mL/min) | 1044 | 1129 | 1040 | 1123 | 0.95 |
| (731-1319) | (800-1390) | (619-2781) | (429-2740) | ||
| Mean postprandial SBF (mL/min) | 1731b | 1844b | 1581b | 1863b | 0.54 |
| (1386-1987) | (1485-2343) | (906-2340) | (1332-3163) | ||
| Postprandial rise in SBF (mL/min) | 686 | 714 | 542 | 707 | 0.47 |
| (515-915) | (314-1145) | (-441-1014) | (293-1325) | ||
| Mean fasting oxygen consumption (mL/min) | 43.4 | 58.1 | 44.5 | 51.8 | 0.48 |
| (32.1-53.8) | (40.5-84.5) | (34.6-76.1) | (15.2-78.2) | ||
| Mean postprandial oxygen consumption (mL/min) | 65.5b | 89.6b | 68.3b | 82.2b | 0.63 |
| (49.1-84.0) | (60.1-118.9) | (46.2-104.7) | (45.0-118.2) | ||
Visceral arteriography revealed no significant stenotic vessels or occlusions in the group of healthy volunteers. A slight stenosis (10%-49% lumen reduction) of the celiac artery was present in five cases. The presence of these minor stenoses did not impact the SBF at baseline (1001 mL/min, P = 0.31) or alter the postprandial increase (648 mL/min, P = 0.49). Baseline SBF and SO2U values did not change over time (1045 mL/min to 1068 mL/min, P = 0.89 and 48 mL O2/min to 50 mL O2/min, P = 0.66, respectively). Variations in SBF were significantly larger between individuals than within individuals (P = 0.002), and the mean baseline SBF was 1087 mL/min (range 731-1390 mL/min), which increased to 1787 mL/min (range 1387-2343 mL/min) postprandially (P < 0.001). The corresponding SO2U increased from 50.7 mL O2/min (range 32.1-84.5 mL O2/min) to 77.5 mL O2/min (range 49.1-118.9 mL O2/min) (P < 0.01).
The baseline values and the increase in SBF after meals were independent of age, sex, body weight, body surface area, and lean body mass in the group of healthy volunteers. However, SO2U differed significantly between genders (P = 0.001). This difference disappeared when gender was corrected for lean body mass (P = 0.99). Therefore, the gender-related differences in baseline SO2U and the postprandial increase were solely attributed to the gender-related difference in lean body mass (P = 0.001). Lean body mass did not influence SBF. Therefore, the SO2U increase was accommodated by a larger oxygen extraction from the blood, which lowered hepatic venous blood saturation (data not shown).
The percentage of body fat influenced baseline SO2U, but it did not influence the postprandial increase (P = 0.003). The baseline SO2U decreased by 4.4 mL/min for a five percent increase in body fat percentage.
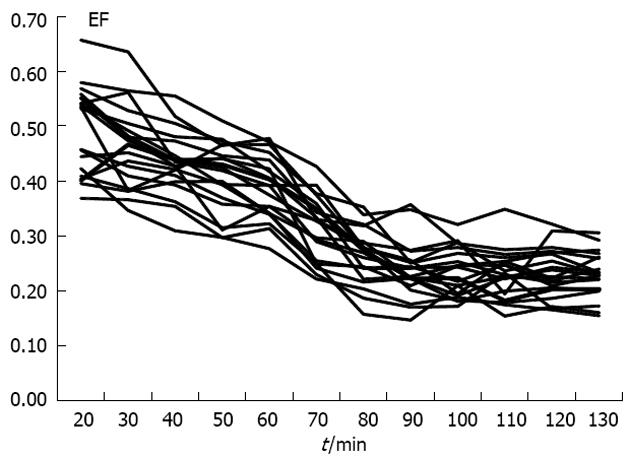
Figure 2 illustrates the EF as a function of time in each healthy volunteer. The arrow indicates the time of the standard meal. The mean EF of 99mTc-MBF decreased significantly (P < 0.01) from 0.46 to 0.39 during the baseline period. A steep drop in EF from 0.39 to 0.24 (P < 0.01) and an increase in SBF were observed 20 min after the meal. The EF did not change thereafter.
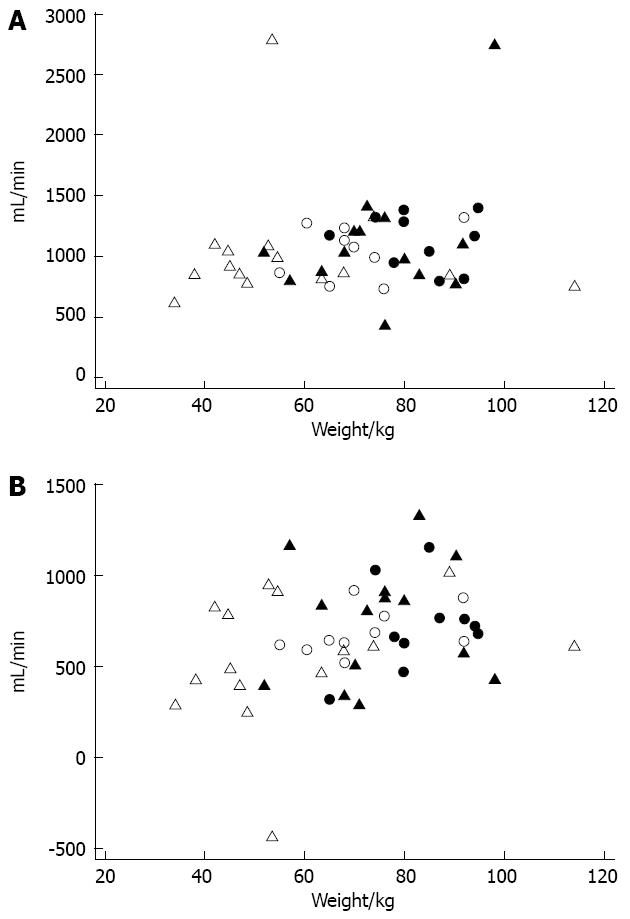
Figure 3A presents the mean baseline SBF for individual patients and healthy volunteers as a function of weight. Two of the patients exhibited high baseline SBF values (2740 and 2781 mL/min). Figure 3B presents the postprandial increase in SBF in individuals as a function of weight. One of the outliers with hyperperfusion at baseline experienced a decreased SBF after the meal; this individual had the negative increase in Figure 3B.
The increase in baseline and postprandial SBF did not depend on weight (P = 0.47 and P = 0.12, respectively). However, weight did exert a significant (P = 0.025) impact on the postprandial increase but not the baseline SBF in all individuals (n = 49) independent of health status. Therefore, an average person weighing 60 kg would demonstrate a 605 mL/min (95%CI: 510-701 mL/min) postprandial increase in SBF. Each additional kg in bodyweight would augment the postprandial increase by 5.4 mL/min. No significant difference between genders or any relationship to age was observed. Baseline and postprandial SO2U values were 48.0 mL O2/min and 75.0 mL O2/min, respectively, and these values in patients did not differ significantly from the healthy volunteers (P = 0.48 and P = 0.68). Patients also exhibited a significant difference between genders in postprandial SO2U (P = 0.03).
Despite the redundancy in the visceral circulation, with its numerous interconnections between the three abdominal arteries, the celiac trunk, and the superior and inferior mesenteric arteries, symptoms of CII ultimately arise when the genuine and collateral arteries can no longer accommodate the postprandial oxygen demand. Therefore, investigations of the morphology of the intestinal arteries cannot stand alone; functional tests that evaluate the physiological consequences are required[12]. An understanding of splanchnic hemodynamics and SO2U in healthy volunteers is crucial. None of the healthy volunteers with stable weight exhibited a BMI < 20. Therefore, a group of patients who underwent SBF measurements due to a suspicion of CII with weights of 34 to 114 kg and angiography-proven normal intestinal arteries served as a control group. The mean baseline SBF of 1087 mL/min and the increase to 1787 mL/min after the ingestion of a standard meal that was observed in this study are consistent with Madsen et al[8], who performed a study in a comparable population of healthy volunteers but without knowledge of arterial morphology.
Previous investigations of SBF have been performed in volunteers who were 20 to 30 years of age. This age group is not representative of the population of interest for CII. These studies reported a total splanchnic plasma flow of 900 mL/min during fasting[4,9,13], which corresponds to an SBF of 1500 mL/min given a normal hematocrit. These flow values are 35% higher than the results in the present study. Although we did not observe an age-related effect in participants aged 40 to 85 years, age may be a factor in comparisons of young adults to middle-aged and elderly individuals.
The frequent presence of atherosclerotic changes in asymptomatic 60- to 70-year-old adults renders the knowledge of arterial morphology in a study population essential. However, this knowledge has been neglected in previous studies. The present study demonstrated no atherosclerosis in any mesenteric arteries in healthy volunteers, and only patients with normal intestinal arteries were included.
Healthy volunteers demonstrated no correlation between SBF and body composition, which was described as body weight, body surface area and lean body mass. This result contrasts a previous study[8] in which both baseline SBF and SO2U were directly related to the body surface area. One reason for this discrepancy may be that the ranges of body surface area or lean body mass in the healthy volunteers in the present study were too small to detect a correlation. This limitation was encountered because of the use of a group of patients with a weight range from 34 to 114 kg. The inclusion of weight in the analysis further augmented the postprandial increase by 5.4 mL/min for each kg bodyweight. Even patients who weighed 34 kg, which represented the lightest patient at our clinic, should have experienced a mean postprandial SBF increase of 465 mL/min (95%CI: 275-656) using this calculation. The exclusion of two patients with hyperperfusion at baseline slightly changed these values (the mean postprandial SBF increased 4.9 mL/min for each kg of bodyweight). These results support previous studies[6,8], which have reported that all individuals should demonstrate a postprandial response larger than 250 mL/min independent of body weight. However, the present study recommends the consideration of body weight when postprandial SBF is used to diagnose CII.
Interestingly, weight did not influence baseline SBF. A minor tendency toward an increase in baseline SBF of 2.2 mL/min (95%CI: -1.6 to 6.1, P = 0.25) for each kg increase in body weight was observed. However, a significant correlation between weight and baseline SBF must be rejected.
A significant difference in SO2U between genders was observed in both groups. These differences between genders were uniquely correlated to the differences in lean body mass in the healthy volunteers who underwent a DEXA scan. This difference may also apply to the patient group, but this group did not undergo DEXA scans. Therefore, the correlation of gender differences in SO2U to differences in body mass remains unknown in this group. The whole-body DEXA scan can reveal the body composition in each region of the body. A large total lean body mass is closely associated with a large truncal lean body mass, which may explain the increased demand for oxygen in the splanchnic territory that was observed in the present study.
The observed decrease in the EF of 99mTc-MBF in healthy volunteers was most pronounced in the 20 min following the meal. This decrease is an inherent consequence of the Fick principle in which the EF decreases with an increase in flow according to the following formula: SPF = E/(Ca-Cv). However, the decrease in EF throughout the baseline period, which was characterized by a constant SBF, remains unexplained. The decrease in EF during continuous infusion has been observed previously in pigs[3] and patients with fatty liver and cirrhosis[2]. This observation may be due to the limited capacity of hepatocytes to process 99mTc-MBF from the blood to the bile.
International guidelines[14,15] on CII suggest the exclusive use of investigations that describe the morphology of the intestinal arteries for the diagnosis of CII. The diagnostic criteria include a combination of relevant symptoms, such as postprandial abdominal pain and weight loss, and at least two significant stenoses/occlusions in the intestinal arteries. This approach ignores the well-documented knowledge of the frequent presence of inconsequential arterial stenosis[11,16,17]. Furthermore, patients with single-vessel disease or non-occlusive mesenteric ischemia are neglected.
The major drawbacks of physiological investigations, such as SBF measurements or tonometry, are that these techniques are often time consuming[18,19] and invasive. Therefore, the use of non-invasive investigations, such as duplex ultrasound (DUS) and MRI, are widely used. The wide availability and non-invasive nature of DUS make it an attractive screening tool. The overall accuracy, sensitivity and specificity for the identification of stenotic or occluded vessels for both the CA and the SMA are above 90% compared to DSA[20]. The meal-induced differences in blood flow velocity responses and the resistivity index in the celiac and superior mesenteric arteries can be assessed using DUS[21] as a surrogate marker for blood flow in healthy young volunteers. However, the calculation of blood flow volume is dependent on an accurate cross-sectional area of the vessel, which is difficult to obtain using DUS, especially in the intestinal arteries, and this area is almost impossible to obtain in patients with disseminated atherosclerosis. Therefore, DUS exhibits a disadvantage because only the morphology of the vessels is examined. Another major disadvantage of DUS is the requirement of an experienced examiner because obesity and overlying bowel gas may compromise the results and the lack of the visualization of the inferior mesenteric artery.
MRI has demonstrated promising results for the quantification of blood flow in the portal and superior mesenteric veins[22,23], and MR angiography has a short acquisition time (17 s)[24]. Another promising study reported the possible quantification of small bowel perfusion for the differentiation of normal individuals from patients with CII[25]. However, the capability of a functional assessment of CII using MRI has not been applied in a clinical setting despite the promise of previous reports. This fact assigns the measurement of SBF as one of a few clinical applicable investigations for the functional evaluation of CII.
In conclusion, the present study recommends the consideration of body weight in the diagnosis of CII using the postprandial increase in SBF.
Chronic intestinal ischemia is characterized by postprandial abdominal pain and weight loss. Atherosclerosis of the intestinal arteries is the most common cause of chronic intestinal ischemia. However, the rich collateral blood supply in the intestines may further develop in the presence of arterial stenosis to provide an adequate blood flow despite the significant stenosis of the intestinal arteries. Therefore, the application of physiological testing is required. The measurement of the splanchnic blood flow prior to and after a meal allows for an assessment of the physiological consequences of arterial stenosis in the intestines.
Reference values for the total splanchnic blood flow and splanchnic oxygen uptake are lacking. The effect of body size and body composition should be considered because the majority of patients are underweight.
Previous investigations of the splanchnic blood flow have not considered the morphology of the intestinal arteries or the possible effect of body composition. An intestinal angiography and a whole-body dual-energy X-ray absorptiometry scan were performed in each of the healthy volunteers in the present study. A group of patients was included as a second group to achieve a weight range that represented individuals with very low bodyweight. All patients exhibited a completely normal angiography of the intestinal arteries. The splanchnic blood flow during fasting was independent of age, sex, body weight and body composition, but the meal-induced increase was directly related to body weight.
The study suggests that the meal-induced increase in splanchnic blood flow is directly related to body weight. This relationship should be considered in the diagnosis of chronic intestinal ischemia based on splanchnic blood flow measurements.
Chronic intestinal ischemia is a clinical entity that is characterized by abdominal pain after eating. The pain causes the patient to eat smaller meals at longer intervals, which often leads to weight loss. The total splanchnic blood flow equals the blood flow through the liver, which can be calculated using the Fick principle.
The paper makes sense to search for and refine new diagnostic tools, especially with regard to the functional aspects of the chronic mesenteric ischemia. The study design is valid and the data is sufficient.
P- Reviewers Mann O, Kopacova M S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Bradley SE, Ingelfinger FJ, Bradley GP, Curry JJ. The estimation of hepatic blood flow in man. J Clin Invest. 1945;24:890-897. [PubMed] |
| 2. | Henriksen JH, Winkler K. Hepatic blood flow determination. A comparison of 99mTc-diethyl-IDA and indocyanine green as hepatic blood flow indicators in man. J Hepatol. 1987;4:66-70. [PubMed] |
| 3. | Zacho HD, Kristensen NB, Henriksen JH, Abrahamsen J. Validation of mTechnetium-labeled mebrofenin hepatic extraction method to quantify meal-induced splanchnic blood flow responses using a porcine model. J Appl Physiol. 2012;112:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Enevoldsen LH, Simonsen L, Macdonald IA, Bülow J. The combined effects of exercise and food intake on adipose tissue and splanchnic metabolism. J Physiol. 2004;561:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Peeters MY, Aarts LP, Boom FA, Bras LJ, Tibboel D, Danhof M, Knibbe CA. Pilot study on the influence of liver blood flow and cardiac output on the clearance of propofol in critically ill patients. Eur J Clin Pharmacol. 2008;64:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Hansen HJ, Engell HC, Ring-Larsen H, Ranek L. Splanchnic blood flow in patients with abdominal angina before and after arterial reconstruction. A proposal for a diagnostic test. Ann Surg. 1977;186:216-220. [PubMed] |
| 7. | Zacho HD, Abrahamsen J. Functional versus radiological assessment of chronic intestinal ischaemia. Clin Physiol Funct Imaging. 2010;30:116-121. [PubMed] |
| 8. | Madsen JL, Søndergaard SB, Møller S. Meal-induced changes in splanchnic blood flow and oxygen uptake in middle-aged healthy humans. Scand J Gastroenterol. 2006;41:87-92. [PubMed] |
| 9. | Simonsen L, Coker R, A L Mulla N, Kjaer M, Bülow J. The effect of insulin and glucagon on splanchnic oxygen consumption. Liver. 2002;22:459-466. [PubMed] |
| 10. | Chandra A, Quinones-Baldrich WJ. Chronic mesenteric ischemia: how to select patients for invasive treatment. Semin Vasc Surg. 2010;23:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Järvinen O, Laurikka J, Sisto T, Salenius JP, Tarkka MR. Atherosclerosis of the visceral arteries. Vasa. 1995;24:9-14. [PubMed] |
| 12. | Mensink PB, Moons LM, Kuipers EJ. Chronic gastrointestinal ischaemia: shifting paradigms. Gut. 2011;60:722-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Jørgensen M, Tønnesen KH, Petersen KR, Jespersen J, Gram J, Vinberg N. Splanchnic extraction and clearance of tissue plasminogen activator (t-PA) after injection of recombinant t-PA (Actilyse) in resting healthy subjects is proportional to the arterial concentration. Blood Coagul Fibrinolysis. 2002;13:331-338. [PubMed] |
| 14. | American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroenterology. 2000;118:951-953. [PubMed] |
| 15. | Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17:1383-1397; quiz 1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 16. | Korotinski S, Katz A, Malnick SD. Chronic ischaemic bowel diseases in the aged--go with the flow. Age Ageing. 2005;34:10-16. [PubMed] |
| 17. | Zeller T, Macharzina R. Management of chronic atherosclerotic mesenteric ischemia. Vasa. 2011;40:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Mensink PB, Geelkerken RH, Huisman AB, Kuipers EJ, Kolkman JJ. Twenty-four hour tonometry in patients suspected of chronic gastrointestinal ischemia. Dig Dis Sci. 2008;53:133-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Zacho HD, Abrahamsen J. Chronic intestinal ischaemia: diagnosis. Clin Physiol Funct Imaging. 2008;28:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Zwolak RM, Fillinger MF, Walsh DB, LaBombard FE, Musson A, Darling CE, Cronenwett JL. Mesenteric and celiac duplex scanning: a validation study. J Vasc Surg. 1998;27:1078-1087; discussion 1088. [PubMed] |
| 21. | Someya N, Endo MY, Fukuba Y, Hayashi N. Blood flow responses in celiac and superior mesenteric arteries in the initial phase of digestion. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1790-R1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Burkart DJ, Johnson CD, Reading CC, Ehman RL. MR measurements of mesenteric venous flow: prospective evaluation in healthy volunteers and patients with suspected chronic mesenteric ischemia. Radiology. 1995;194:801-806. [PubMed] |
| 23. | Laissy JP, Trillaud H, Douek P. MR angiography: noninvasive vascular imaging of the abdomen. Abdom Imaging. 2002;27:488-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Sutter R, Heilmaier C, Lutz AM, Weishaupt D, Seifert B, Willmann JK. MR angiography with parallel acquisition for assessment of the visceral arteries: comparison with conventional MR angiography and 64-detector-row computed tomography. Eur Radiol. 2009;19:2679-2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |