Published online Feb 14, 2013. doi: 10.3748/wjg.v19.i6.829
Revised: November 12, 2012
Accepted: November 14, 2012
Published online: February 14, 2013
Insulinomas, the most common cause of hypoglycemia related to endogenous hyperinsulinism, occur in 1-4 people per million of the general population. Common autonomic symptoms of insulinoma include diaphroresis, tremor, and palpitations, whereas neuroglycopenenic symptoms include confusion, behavioural changes, personality changes, visual disturbances, seizure, and coma. Diagnosis of suspected cases is based on standard endocrine tests, especially the prolonged fasting test. Non-invasive imaging procedures, such as computed tomography and magnetic resonance imaging, are used when a diagnosis of insulinoma has been made to localize the source of pathological insulin secretion. Invasive modalities, such as endoscopic ultrasonography and arterial stimulation venous sampling, are highly accurate in the preoperative localization of insulinomas and have frequently been shown to be superior to non-invasive localization techniques. The range of techniques available for the localization of insulinomas means that blind resection can be avoided. Intraoperative manual palpation of the pancreas by an experienced surgeon and intraoperative ultrasonography are both sensitive methods with which to finalize the location of insulinomas. A high proportion of patients with insulinomas can be cured with surgery. In patients with malignant insulinomas, an aggressive medical approach, including extended pancreatic resection, liver resection, liver transplantation, chemoembolization, or radiofrequency ablation, is recommended to improve both survival and quality of life. In patients with unresectable or uncontrollable insulinomas, such as malignant insulinoma of the pancreas, several techniques should be considered, including administration of ocreotide and/or continuous glucose monitoring, to prevent hypoglycemic episodes and to improve quality of life.
- Citation: Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K. Diagnosis and management of insulinoma. World J Gastroenterol 2013; 19(6): 829-837
- URL: https://www.wjgnet.com/1007-9327/full/v19/i6/829.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i6.829
Insulinomas are the most common functioning endocrine neoplasm of the pancreas[1-4]. They are insulin-secreting tumors of pancreatic origin that cause hypoglycemia[5-7]. Insulinomas occur in 1-4 people per million in the general population and represent 1%-2% of all pancreatic neoplasms[8-10]. Insulinomas can occur at any age and have an equal gender distribution. As many as 90% of insulinomas have been reported to be benign, 90% are solitary, > 90% occur at intrapancreatic sites, and 90% are < 2 cm in diameter[10-13]. Insulinomas are evenly distributed over the entire pancreas. Most insulinomas are located in the pancreas or are attached directly to the pancreas. Extrapancreatic insulinomas causing hypoglycemia are extremely rare (incidence < 2%); extrapancreatic insulinomas are most commonly found in the duodenal wall[8]. The etiology and pathogenesis of insulinomas are not known.
Following biological and biochemical confirmation of an insulinoma, preoperative localization is sought using computed tomography (CT)[14-16], magnetic resonance imaging (MRI)[16-19], endoscopic ultrasonography (EUS)[20-22], intra-arterial calcium stimulation test with hepatic venous sampling[23], and/or angiography and arterial stimulation venous sampling (ASVS)[24-28]. Surgical resection is the primary treatment modality for insulinomas, and so accurate localization of the tumor before or during surgery is important. Intraoperative manual palpation of the pancreas by an experienced surgeon and intraoperative ultrasonography are both sensitive methods with which to localize insulinomas, supporting the argument by some surgeons that preoperative localization of the tumors is not necessary[29-33]. The present review describes some of the latest findings regarding the clinical diagnosis and medical management of insulinomas in the adult population that are not associated with either multiple endocrine neoplasms or von Hippel-Lindau disease.
Insulinomas are the most common cause of hypoglycemia related to endogenous hyperinsulinism. The episodic nature of the hypoglycemic attack is due to the intermittent secretion of insulin by the tumor[8]. Common autonomic symptoms of an insulinoma include diaphroresis, tremor, and palpitations, whereas neuroglycopenenic symptoms include confusion, behavioral changes, personality changes, visual disturbances, seizures and coma[34,35].
Diagnosis of insulinomas can be challenging. Although it was originally considered that symptoms only became evident in the fasting state or following exercise, it is now known that patients with an insulinoma can also present with postprandial symptoms[36,37]. The classical diagnosis of insulinoma depends on satisfying the criteria of Whipple’s triad, which remains the cornerstone of the screening process: (1) hypoglycemia (plasma glucose < 50 mg/dL); (2) neuroglycopenic symptoms; and (3) prompt relief of symptoms following the administration of glucose (Table 1)[38]. In adults with symptoms of neuroglycopenia or documented low blood glucose levels, the gold standard for biochemical diagnosis remains measurement of plasma glucose, insulin, C-peptide, and proinsulin during a 72-h fast (Table 1). This prolonged fasting test can detect up to 99% of insulinomas[39]. Endogenous hypoglycemia due to insulinomas was previously based on findings of abnormal serum levels of insulin, C-peptide, and, more recently, proinsulin at the time of fasting hypoglycemia. To date, there is some general agreement regarding the diagnostic thresholds that must be reached for insulin, C-peptide, and proinsulin levels to be considered abnormal. Several years ago, ratios calculated from insulin and blood glucose levels were used, with the insulin/C-peptide ratio in patients diagnosed with insulinoma reported to be < 1.0[40,41]. It is of note that a normal insulin level does not exclude the disease, because the absolute insulin level is not elevated in all patients with insulinoma. In addition, because the proportion of proinsulin secreted by insulinoma cells is generally higher than that secreted by normal β-cells, high proinsulin levels have been suggested as being diagnostic of insulinoma, regardless of concomitant blood glucose levels[42]. The availability of proinsulin assays has led to the use of serum proinsulin thresholds as a diagnostic tool: it has been recommended that a cut-off level of 20 pmol/L proinsulin at the time of hypoglycemia < 45 mg/dL is indicative of the presence of an insulinoma (Table 1)[42-44].
| Classical diagnosis |
| Hypoglycemia (plasma glucose < 50 mg/dL) |
| Neuroglycopenic symptoms |
| Prompt relief of symptoms following the administration of glucose |
| Present consensus |
| At the time of hypoglycemia during a 72-h fasting test: |
| 5 mIU/L (36 pmol/L) insulin threshold |
| 0.6 ng/mL (0.2 nmol/L) C-peptide threshold |
| Insulin/C-peptide ratio < 1.0 |
| 20 pmol/L proinsulin cut-off level |
| Absence of sulfonylurea (metabolites) in the plasma or urine |
Delays in the diagnosis of insulinoma are common because the symptoms usually precede detection of a tumor and there may be misattribution of the symptoms to psychiatric, cardiac, or neurological disorders[45]. Once a diagnosis of insulinoma is considered, it is important that patients are managed in a timely and safe manner. As a general rule, patients with insulinoma can be cured by surgical resection of the tumor. The need for preoperative localization of insulinomas and the methods used remain contentious. In the past, confirmation of the presence of the Whipple triad (symptoms known or likely to be caused by hypoglycemia; a low plasma glucose measured at the time of the symptoms; relief of symptoms when the glucose is raised to normal) usually meant that a patient was led directly to surgery[46,47]. However, at present, most agree that knowledge of the site of the tumor before surgery is helpful in that it allows one to determine not only whether enucleation (the surgical removal of a mass) of the neoplasm or pancreatic resection is likely to be required, but also whether the tumor is amenable to removal via a laparoscopic approach. Preoperative localization should also mean that the operation itself can be performed more quickly, thereby reducing associated morbidity and mortality[27]. It is important to remember that most tumors are intrapancreatic, 90% are solitary, 90% are < 2 cm in diameter, and the tumors are distributed equally within the head, body and tail of the pancreas.
A number of non-invasive techniques are available for the localization of a suspected insulinoma, including transabdominal ultrasonography, CT and/or MRI. The sensitivity of transabdominal ultrasonography in the localization of insulinomas is poor (ranging from 9% to 64%)[48]. However, insulinomas demonstrate characteristic features when imaged with both CT and MRI and the sensitivity of these techniques has been reported to be 33%-64% and 40%-90%, respectively[10,49]. The sensitivity and specificity of MRI is generally superior to that of CT, as is the detection of extrapancreatic extensions[16].
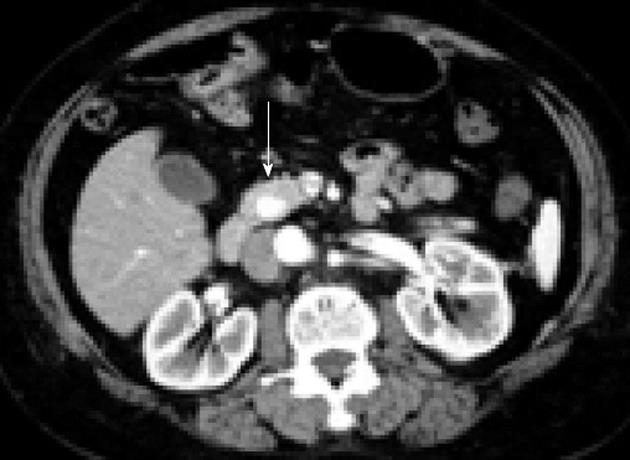
CT is a safe and simple procedure to perform that is operator independent. CT visualizes the exact location of an insulinoma, its relationship to vital structures, and the presence of metastases[49]. Typically, insulinomas are hypervascular and, as a result, demonstrate a greater degree of enhancement than normal pancreatic parenchyma during the arterial and capillary phases of contrast bolus (Figure 1)[16]. An atypical CT appearance of insulinomas is occasionally encountered and can include hypovascular and hypodense lesions post-contrast, hyperdense lesions precontrast, cystic masses, and calcified masses[16]. Calcification, when it occurs, tends to be discrete and nodular, and is more common in malignant than benign tumors[50,51]. Technical advances have improved the quality of CT, with a recent study reporting that using a multidetector CT enabled visualization of 94.4% of insulinomas[52]. CT is currently accepted as the first-line investigation for the visualization of insulinomas.
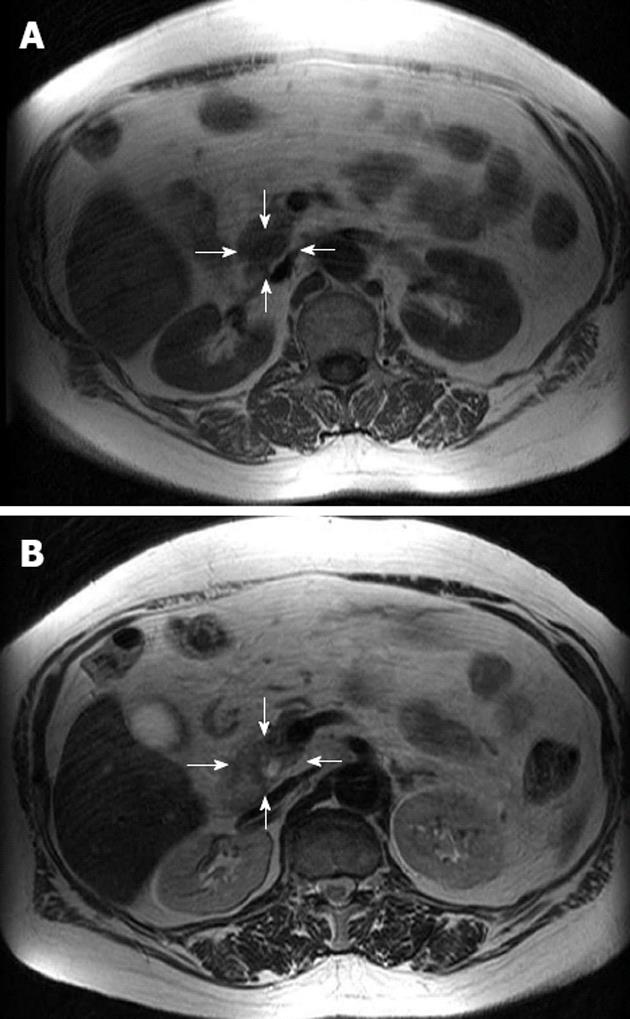
Currently, there is strong evidence emerging for the use of MRI in the imaging of insulinomas, and investigators have shown a high sensitivity for MRI in the detection of insulinomas[16,49]. Like CT, MRI is safe, non-invasive, rapid, and facilitates the detection of metastases. Insulinomas generally demonstrate low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (Figure 2)[50]. However, limitations in the use of MRI in the detection of insulinomas include the standard contraindications for MRI. The modern MRI system allows rapid triphasic, breath-held T1 rapid gadolium-enhanced sequences, and/or diffusion-weighted imaging[19,49]. These sequences significantly reduce motion artifacts and enable accurate assessment of the pancreas in both the arterial and venous phase[49]. MRI has all the advantages of CT and recent evidence suggests that it may be the more sensitive tool. In current practice, MRI is a second-line investigation for the localization of insulinomas, but it could potentially take over from CT in the future as it becomes more widely available and expertise improves.
The diagnostic procedure in cases of suspected insulinoma is based on standard endocrine examinations, especially the prolonged fasting test. Non-invasive imaging procedures are used to localize the source of pathological insulin secretion after a diagnosis of insulinoma has been established[20]. Invasive modalities, such as EUS and ASVS, have been shown to be highly accurate in the preoperative localization of insulinomas, and have frequently been shown to be superior to non-invasive localization techniques.
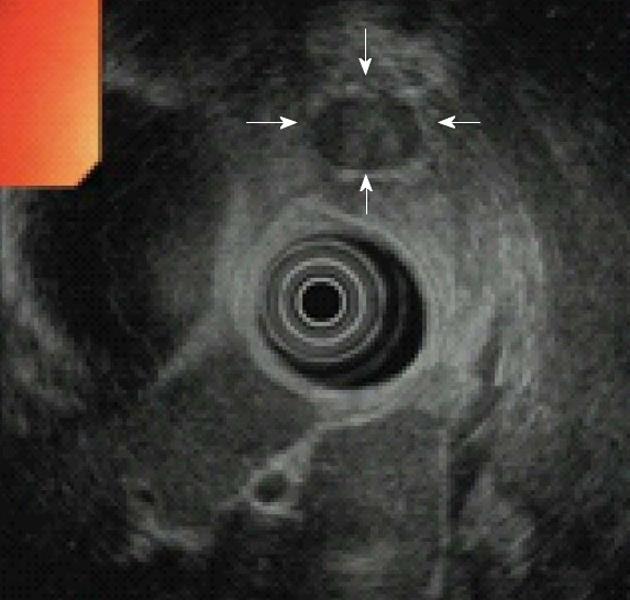
EUS is currently the test of choice in most Western centers, with reported detection rates of 86.6%-92.3%[10,46]. The appearance of insulinomas on EUS is quite characteristic, with most tumors homogeneously hypoechoic, rounded in shape, and with distinct margins (Figure 3). Although EUS is a highly reliable procedure for the preoperative localization of insulinomas, there are several problems associated with the detection of these tumors using EUS. First, EUS may yield both false-positive and false-negative results, with the quality of the EUS findings largely dependent on the examiner’s experience[22]. Second, some insulinomas are missed by preoperative EUS because they are completely isoechoic. A low body mass index, female gender, and young age may be risk factors for negative imaging[20]. Third, the sensitivity of EUS for insulinomas depends on the location and size of the tumor; sensitivity is greatest for tumors in the head of the pancreas and lowest for those in the tail of the pancreas or those that are extrapancreatic[10]. Once the site of the tumor has been determined, fine-needle aspiration (FNA) of the pancreas allows for a preoperative diagnosis of insulinoma. Advances in EUS have made EUS-guided FNA particularly useful in the diagnosis of insulinomas, because most functioning tumors are small. EUS-guided FNA is becoming increasingly popular, and it seems likely that it will eventually become the standard for the diagnosis and staging of pancreatic tumors[9].
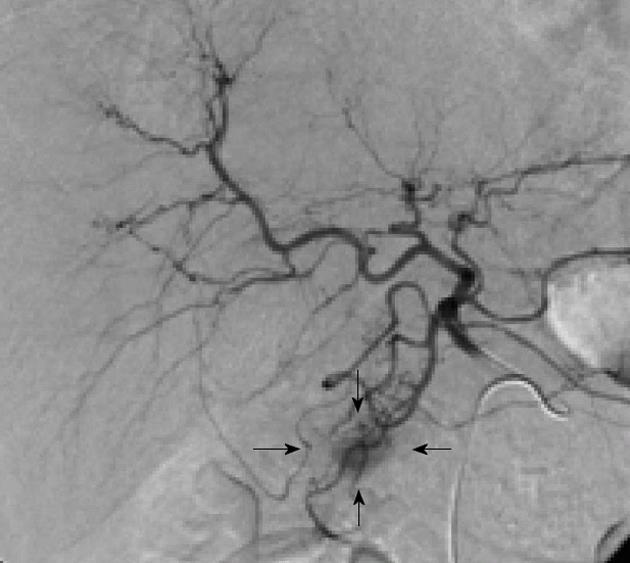
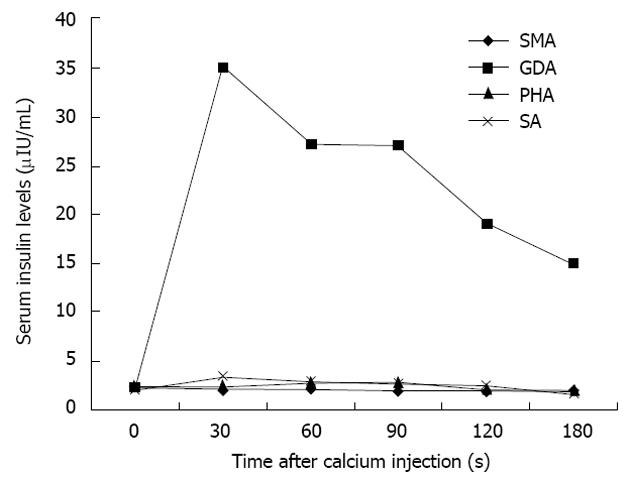
There can be little doubt that angiography combined with ASVS should not precede non-invasive investigations, such as CT and MRI, but it remains a highly sensitive technique for the precise localization of insulinomas and will usually provide more information than EUS[27]. Morphological imaging modalities do not reflect hormonal function; however, the addition of ASVS helps regionalize a tumor by verifying hormonal function[24]. The use of ASVS allows for a more accurate surgical approach and can minimize the likelihood of re-operation[24]. For atypical insulinomas, preoperative localization of insulinomas by ASVS is particularly important. The accuracy of ASVS in localizing insulinomas has been reported to range from 94% to 100%[23,28]. Using ASVS, insulinomas are seen as well-defined, round or oval vascular blushes that are of increased vascularity compared with the surrounding normal pancreatic parenchyma (Figure 4). Insulinomas are visualized during the early arterial phase and persist for a variable length of time into the venous phase of the run. The localization of an insulinoma by ASVS relies on the fact that a hyperosmolar concentration of calcium in the vessels supplying the tumor will cause degranulation of cells within the neoplasm, releasing insulin into the portal venous system, which results in a detectable rise in insulin in venous samples obtained from the hepatic vein[27]. The splenic, gastroduodenal, superior mesenteric, and proper hepatic arteries are the vessels most commonly studied during ASVS; an increase in insulin concentrations in the hepatic vein will localize the insulinoma to the body/tail of the pancreas, an anterosuperior site of the pancreatic head, and postero-inferior site of the pancreatic head, respectively. An increase in insulin concentrations after injection of calcium into the proper hepatic artery suggests that hepatic metastases may be present. Changes in serum insulin levels plotted as a function of time after calcium injection indicate that insulin concentrations are markedly elevated only in the feeding arteries of the insulinoma (Figure 5).
The range of imaging modalities now available means that blind resection for insulinoma can be avoided because of accurate preoperative and intraoperative localization of the tumor[53]. Manual palpation of the pancreas by an experienced surgeon and ultrasonography are both sensitive methods for the intraoperative detection of the site of insulinomas[29-31]. The sensitivity of these two methods is clinically acceptable and has been reported as 75%-95% and 80%-100%, respectively[29,32,54].
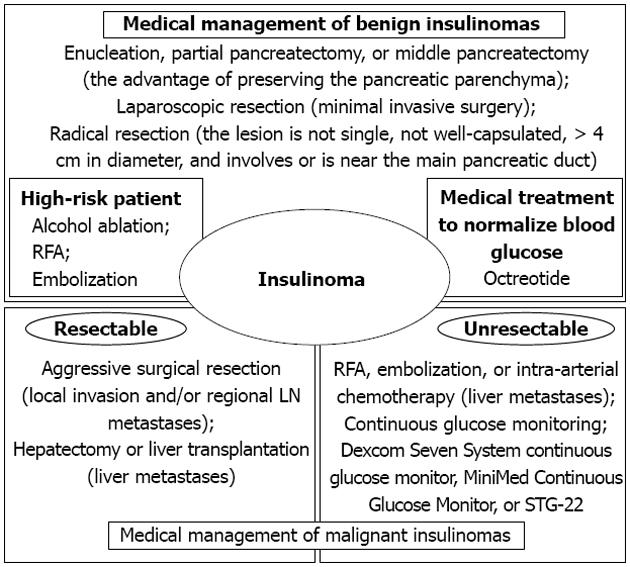
Most patients with benign insulinomas can be cured with surgery, although other techniques for the management of insulinomas, including injection of octreotide, EUS-guided alcohol ablation, radiofrequency ablation (RFA), or embolization of an insulinoma of the pancreas, have been described[55-61].
After identification of an insulinoma, surgery is indicated for all localized tumors. The choice of procedure will depend on the features of the tumor mass, such as type, size, and localization. Atypical resection, including enucleation, partial pancreatectomy, or middle pancreatectomy, has the advantage of preserving the pancreatic parenchyma as much as possible, thereby reducing the risk of late exocrine/endocrine insufficiency[62]. To date, laparoscopic resection has often been performed for insulinomas that are benign, small, and/or located in the body or tail of the pancreas[63]. Radical resection should be considered for patients in whom the lesion is not single, not well-capsulated, > 4 cm in diameter, and involves or is near the main pancreatic duct. Lymphadenectomy is not usually performed. Although the cure rate after resection for insulinoma is very high, it is necessary to be aware of the potential for postoperative complications after pancreatic surgery, especially postoperative pancreatic fistula[64-66].
However, there is a considerable risk of morbidity and mortality associated with the surgical management of insulinomas, which precludes surgery in high-risk patients. Alcohol ablation and RFA have been established as minimally invasive procedures in the treatment of primary liver tumors and hepatic metastases. Recently, successful EUS-guided alcohol ablation and CT-guided RFA of pancreatic insulinomas have been reported in humans[56,57]. These two patients were in poor general condition and were experiencing recurrent symptomatic episodes of hypoglycemia. Because it was considered that surgical management for benign insulinoma of the pancreas was impossible in both cases, ablation of the solitary mass was performed. Both patients were discharged without any complications and reported no further hypoglycemic episodes. Embolization of an insulinoma of the pancreas is another non-surgical alternative[55,67,68]. Because angiographically the insulinoma is demonstrated in the arterial phase as a hypervasculated mass, embolization could be performed using flow to direct particles exclusively into the tumor. Although it remains contentious as to whether these procedures are a viable treatment for patients with an insulinoma, they may be offered as an alternative for certain patients, such as those who refuse surgery, those who are of advanced age, those with a poor general condition, those who have already undergone multiple abdominal surgeries, or those with an increased risk of postoperative complications due to other reasons.
Insulinomas are rare endocrine tumors, most of which can be cured by surgery. Medical treatment to normalize blood glucose is useful during the preoperative period, as well as for patients who cannot be cured by surgery, such as those with diffuse β-cell disease, multiple insulinomas, unresectable malignant insulinoma, those in whom surgery is contraindicated, or patients who refuse surgery[59]. Octreotide is a somatostatin analog that inhibits insulin secretion and the peripheral action of many gastrointestinal hormones, primarily via activation of somatostatin sst2 receptors. Octreotide has been used for the treatment of insulinoma, with successful control of blood glucose levels[60,61]. In addition, ocreotide may have an antiproliferative effect, as well as a moderate antitumoral action, on pancreatic endocrine tumors[69]. Therapy may be initiated with short-acting ocreotide two to four times daily, or 20-30 mg long-acting ocreotide every 4 wk[70]. Initiation of therapy with short-acting ocreotide can be used to assess systemic tolerability, particularly any gastrointestinal side-effects[71]. Thus, pharmacotherapy with somatostatin to control hypoglycemia represents a feasible option for the non-surgical management of insulinomas.
To be considered malignant, insulinomas must show evidence of local invasion into the surrounding soft tissue or there must be verification of lymph node or liver metastasis[72]. The reported incidence of malignant insulinomas ranges between 7% and 10%[72-74], and the 10-year survival has been reported to be 29%[2]. The major sites of metastasis or recurrence are the liver and regional lymph nodes. Aggressive surgical resection is recommended because these tumors are much less virulent than their malignant ductal exocrine counterparts, in which there are severe hormonal symptoms that cannot be controlled by medical treatment. RFA can be used to reduce the tumor mass in the liver, thereby reducing hormonal symptoms[58,75]. Selective embolization alone or in combination with intra-arterial chemotherapy is an established procedure to reduce both hormonal symptoms and liver metastases. Although experience is limited, liver transplantation for multiple liver metastases of malignant insulinomas may be considered in patients with no extrahepatic metastases[76,77]. Aggressive sequential multimodal therapy (chemoembolization, RFA, liver resection, liver transplantation) can prolong the survival of patients with sporadic malignant insulinoma, even in the presence of liver metastases.
Malignant insulinomas remain extremely rare tumors. In many patients with malignant insulinomas, the tumors are unresectable and medical treatment therapy is limited in its ability to prevent hypoglycemic episodes[78,79]. Continuous glucose monitoring in patients with insulinomas can detect hypoglycemia, monitor responses to medical therapy, and confirm a cure postoperatively. In the literature, continuous glucose monitoring has been reported using a Dexcom Seven System continuous glucose monitor (Dexcom, San Diego, CA, United States) or the MiniMed Continuous Glucose Monitor (CGM Medtronic MiniMed, Northridge, CA, United States)[78,79]. These studies reported that continuous glucose monitoring is a useful addition to the armamentarium for the prevention of hypoglycemia. These techniques are considered an effective adjunct to therapy to reduce hypoglycemic episodes by alerting patients to low glucose concentrations before they develop neuroglycopenic symptoms; however, patients should respond promptly to oral glucose intake after hypoglycemia has been detected by these machines. In patients with a poorer general condition, malignant insulinomas that are unresectable, and uncontrolled hypoglycemia, it is proposed that blood glucose concentrations are monitored using the STG-22 (Nikkiso Co., Tokyo, Japan). The STG-22 is a reliable and accurate device for the measurement of blood glucose concentrations compared with the ABL 800FLEX machine (Radiometer Medical ApS, Brønshøj, Denmark) that is recommended by the National Committee for Clinical Laboratory Standards[80,81]. The STG-22 closed-loop glycemic control system is composed of a glucose sensor for the detection and/or monitoring of glucose and pumps for infusing an appropriate amount of insulin or glucose. The insulin and glucose pumps are computer regulated based on a target blood glucose value that is defined prior to initiation of the system[82,83]. It is has been proven clinically that the STG-22 device is safe and beneficial for maintaining glycemic control without hypoglycemic episodes in surgical patients[84-86] (Figure 6).
Insulinomas are the most common neuroendocrine tumors of the pancreas and cause hypoglycemia related to endogenous hyperinsulinism. More than 90% of insulinomas are benign and usually small, well-encapsulated, solitary tumors. Surgical resection is the treatment of choice for insulinomas and offers the only chance for cure. Most insulinomas can be identified intraoperatively by an experienced surgeon. However, what should be done in an operating theater when an insulinoma cannot be identified? Based on information presented in this review, we recommended the use of various non-invasive and invasive imaging modalities when the tumor cannot be detected using conventional diagnostic procedures. Blind surgical resection of the pancreas should not be undertaken in any patient who suffers from hypoglycemic episodes due to an insulinoma. Identifying the location of the insulinoma enables the surgeon to proceed with surgery uninterrupted, minimizing time in the operating theater, reducing the likelihood for re-operation, limiting perioperative complications, and ensuring, in most cases, a successful outcome[38].
In patients with malignant insulinomas, aggressive surgical resection, including extended pancreatic resection, liver resection, and/or liver transplantation, should be attempted when possible to improve patient survival[58,75-77]. Furthermore, aggressive secondary treatments may be indicated, such as chemoembolization or RFA for liver metastases from malignant insulinomas of the pancreas to control hypoglycemia. In patients who have unresectable or uncontrollable malignant insulinomas of the pancreas, several strategies need to be considered to both control hypoglycemic episodes and improve quality of life, including administration of ocreotide and continuous glucose monitoring. Insulinomas of the pancreas remain rare and may occur simply by chance. To refine the diagnosis and management of these tumors, epidemiologic and pathologic data should continue to be collected.
This study was made possible with the support of Satoshi Ito and Kazuhiro Hanazaki (Kochi Medical School).
P- Reviewers Matthews V, Iwasaki Y S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Moldow RE, Connelly RR. Epidemiology of pancreatic cancer in Connecticut. Gastroenterology. 1968;55:677-686. [PubMed] |
| 2. | Service FJ, McMahon MM, O’Brien PC, Ballard DJ. Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc. 1991;66:711-719. [PubMed] |
| 3. | Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig Dis Sci. 1991;36:933-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 163] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Lam KY, Lo CY. Pancreatic endocrine tumour: a 22-year clinico-pathological experience with morphological, immunohistochemical observation and a review of the literature. Eur J Surg Oncol. 1997;23:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Eriksson B, Oberg K. Neuroendocrine tumours of the pancreas. Br J Surg. 2000;87:129-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 6. | Gumbs AA, Moore PS, Falconi M, Bassi C, Beghelli S, Modlin I, Scarpa A. Review of the clinical, histological, and molecular aspects of pancreatic endocrine neoplasms. J Surg Oncol. 2002;81:45-53; discussion 54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Mansour JC, Chen H. Pancreatic endocrine tumors. J Surg Res. 2004;120:139-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 320] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Chang F, Chandra A, Culora G, Mahadeva U, Meenan J, Herbert A. Cytologic diagnosis of pancreatic endocrine tumors by endoscopic ultrasound-guided fine-needle aspiration: a review. Diagn Cytopathol. 2006;34:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Sotoudehmanesh R, Hedayat A, Shirazian N, Shahraeeni S, Ainechi S, Zeinali F, Kolahdoozan S. Endoscopic ultrasonography (EUS) in the localization of insulinoma. Endocrine. 2007;31:238-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Doi R, Komoto I, Nakamura Y, Kawamura J, Fujimoto K, Wada M, Saga T, Imamura M. Pancreatic endocrine tumor in Japan. Pancreas. 2004;28:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Mittendorf EA, Liu YC, McHenry CR. Giant insulinoma: case report and review of the literature. J Clin Endocrinol Metab. 2005;90:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Kondo T, Tomita S, Adachi H, Motoshima H, Taketa K, Matsuyoshi A, Tokunaga H, Miyamura N, Araki E. A case of hyperinsulinemia of undetermined origin, successfully treated with long-acting octreotide. Endocr J. 2005;52:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Chatziioannou A, Kehagias D, Mourikis D, Antoniou A, Limouris G, Kaponis A, Kavatzas N, Tseleni S, Vlachos L. Imaging and localization of pancreatic insulinomas. Clin Imaging. 2001;25:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Sheth S, Hruban RK, Fishman EK. Helical CT of islet cell tumors of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2002;179:725-730. [PubMed] |
| 16. | Noone TC, Hosey J, Firat Z, Semelka RC. Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract Res Clin Endocrinol Metab. 2005;19:195-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Owen NJ, Sohaib SA, Peppercorn PD, Monson JP, Grossman AB, Besser GM, Reznek RH. MRI of pancreatic neuroendocrine tumours. Br J Radiol. 2001;74:968-973. [PubMed] |
| 18. | Thoeni RF, Mueller-Lisse UG, Chan R, Do NK, Shyn PB. Detection of small, functional islet cell tumors in the pancreas: selection of MR imaging sequences for optimal sensitivity. Radiology. 2000;214:483-490. [PubMed] |
| 19. | Anaye A, Mathieu A, Closset J, Bali MA, Metens T, Matos C. Successful preoperative localization of a small pancreatic insulinoma by diffusion-weighted MRI. JOP. 2009;10:528-531. [PubMed] |
| 20. | Kann PH, Ivan D, Pfützner A, Forst T, Langer P, Schaefer S. Preoperative diagnosis of insulinoma: low body mass index, young age, and female gender are associated with negative imaging by endoscopic ultrasound. Eur J Endocrinol. 2007;157:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | McLean AM, Fairclough PD. Endoscopic ultrasound in the localisation of pancreatic islet cell tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Kann PH, Rothmund M, Zielke A. Endoscopic ultrasound imaging of insulinomas: limitations and clinical relevance. Exp Clin Endocrinol Diabetes. 2005;113:471-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Tseng LM, Chen JY, Won JG, Tseng HS, Yang AH, Wang SE, Lee CH. The role of intra-arterial calcium stimulation test with hepatic venous sampling (IACS) in the management of occult insulinomas. Ann Surg Oncol. 2007;14:2121-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Morita S, Machida H, Kuwatsuru R, Saito N, Suzuki K, Iihara M, Obara T, Mitsuhashi N. Preoperative localization of pancreatic insulinoma by super selective arterial stimulation with venous sampling. Abdom Imaging. 2007;32:126-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Guettier JM, Kam A, Chang R, Skarulis MC, Cochran C, Alexander HR, Libutti SK, Pingpank JF, Gorden P. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab. 2009;94:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Chung JC, Choi SH, Jo SH, Heo JS, Choi DW, Kim YI. Localization and surgical treatment of the pancreatic insulinomas. ANZ J Surg. 2006;76:1051-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Jackson JE. Angiography and arterial stimulation venous sampling in the localization of pancreatic neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Gimm O, König E, Thanh PN, Brauckhoff M, Karges W, Dralle H. Intra-operative quick insulin assay to confirm complete resection of insulinomas guided by selective arterial calcium injection (SACI). Langenbecks Arch Surg. 2007;392:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Wong M, Isa SH, Zahiah M, Azmi KN. Intraoperative ultrasound with palpation is still superior to intra-arterial calcium stimulation test in localising insulinoma. World J Surg. 2007;31:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Hashimoto LA, Walsh RM. Preoperative localization of insulinomas is not necessary. J Am Coll Surg. 1999;189:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Shin LK, Brant-Zawadzki G, Kamaya A, Jeffrey RB. Intraoperative ultrasound of the pancreas. Ultrasound Q. 2009;25:39-48; quiz 48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Ravi K, Britton BJ. Surgical approach to insulinomas: are pre-operative localisation tests necessary? Ann R Coll Surg Engl. 2007;89:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Kalafat H, Mihmanli I, Saribeyoglu K, Belli A. Intraoperative doppler ultrasound: a reliable diagnostic method in insulinoma. Hepatogastroenterology. 2007;54:1256-1258. [PubMed] |
| 34. | Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. 2005;54:3592-3601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 35. | Suzuki K, Miyamoto M, Miyamoto T, Hirata K. Insulinoma with early-morning abnormal behavior. Intern Med. 2007;46:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Service FJ. Hypoglycemic disorders. N Engl J Med. 1995;332:1144-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 289] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 37. | de Herder WW, Niederle B, Scoazec JY, Pauwels S, Kloppel G, Falconi M, Kwekkeboom DJ, Oberg K, Eriksson B, Wiedenmann B. Well-differentiated pancreatic tumor/carcinoma: insulinoma. Neuroendocrinology. 2006;84:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 38. | Rostambeigi N, Thompson GB. What should be done in an operating room when an insulinoma cannot be found? Clin Endocrinol (Oxf). 2009;70:512-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Service FJ, Natt N. The prolonged fast. J Clin Endocrinol Metab. 2000;85:3973-3974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Lebowitz MR, Blumenthal SA. The molar ratio of insulin to C-peptide. An aid to the diagnosis of hypoglycemia due to surreptitious (or inadvertent) insulin administration. Arch Intern Med. 1993;153:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | van Bon AC, Benhadi N, Endert E, Fliers E, Wiersinga WM. Evaluation of endocrine tests. D: the prolonged fasting test for insulinoma. Neth J Med. 2009;67:274-278. [PubMed] |
| 42. | Vezzosi D, Bennet A, Fauvel J, Caron P. Insulin, C-peptide and proinsulin for the biochemical diagnosis of hypoglycaemia related to endogenous hyperinsulinism. Eur J Endocrinol. 2007;157:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Hirshberg B, Livi A, Bartlett DL, Libutti SK, Alexander HR, Doppman JL, Skarulis MC, Gorden P. Forty-eight-hour fast: the diagnostic test for insulinoma. J Clin Endocrinol Metab. 2000;85:3222-3226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Marks V. Recognition and differential diagnosis of spontaneous hypoglycaemia. Clin Endocrinol (Oxf). 1992;37:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Kar P, Price P, Sawers S, Bhattacharya S, Reznek RH, Grossman AB. Insulinomas may present with normoglycemia after prolonged fasting but glucose-stimulated hypoglycemia. J Clin Endocrinol Metab. 2006;91:4733-4736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Goh BK, Ooi LL, Cheow PC, Tan YM, Ong HS, Chung YF, Chow PK, Wong WK, Soo KC. Accurate preoperative localization of insulinomas avoids the need for blind resection and reoperation: analysis of a single institution experience with 17 surgically treated tumors over 19 years. J Gastrointest Surg. 2009;13:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Lo CY, Lam KY, Kung AW, Lam KS, Tung PH, Fan ST. Pancreatic insulinomas. A 15-year experience. Arch Surg. 1997;132:926-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Tucker ON, Crotty PL, Conlon KC. The management of insulinoma. Br J Surg. 2006;93:264-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | McAuley G, Delaney H, Colville J, Lyburn I, Worsley D, Govender P, Torreggiani WC. Multimodality preoperative imaging of pancreatic insulinomas. Clin Radiol. 2005;60:1039-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Balci NC, Semelka RC. Radiologic features of cystic, endocrine and other pancreatic neoplasms. Eur J Radiol. 2001;38:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Kurosaki Y, Kuramoto K, Itai Y. Hyperattenuating insulinoma at unenhanced CT. Abdom Imaging. 1996;21:334-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Gouya H, Vignaux O, Augui J, Dousset B, Palazzo L, Louvel A, Chaussade S, Legmann P. CT, endoscopic sonography, and a combined protocol for preoperative evaluation of pancreatic insulinomas. AJR Am J Roentgenol. 2003;181:987-992. [PubMed] |
| 53. | Davì MV, Falconi M. Pancreas: Insulinoma--new insights into an old disease. Nat Rev Endocrinol. 2009;5:300-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Ritzel RA, Isermann B, Schilling T, Knaebel HP, Büchler MW, Nawroth PP. Diagnosis and localization of insulinoma after negative laparotomy by hyperinsulinemic, hypoglycemic clamp and intra-arterial calcium stimulation. Rev Diabet Stud. 2004;1:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Rott G, Biggemann M, Pfohl M. Embolization of an insulinoma of the pancreas with trisacryl gelatin microspheres as definitive treatment. Cardiovasc Intervent Radiol. 2008;31:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Jürgensen C, Schuppan D, Neser F, Ernstberger J, Junghans U, Stölzel U. EUS-guided alcohol ablation of an insulinoma. Gastrointest Endosc. 2006;63:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 57. | Limmer S, Huppert PE, Juette V, Lenhart A, Welte M, Wietholtz H. Radiofrequency ablation of solitary pancreatic insulinoma in a patient with episodes of severe hypoglycemia. Eur J Gastroenterol Hepatol. 2009;21:1097-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Gillams A, Cassoni A, Conway G, Lees W. Radiofrequency ablation of neuroendocrine liver metastases: the Middlesex experience. Abdom Imaging. 2005;30:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Vezzosi D, Bennet A, Courbon F, Caron P. Short- and long-term somatostatin analogue treatment in patients with hypoglycaemia related to endogenous hyperinsulinism. Clin Endocrinol (Oxf). 2008;68:904-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Kishikawa H, Okada Y, Hirose A, Tanikawa T, Kanda K, Tanaka Y. Successful treatment of insulinoma by a single daily dose of octreotide in two elderly female patients. Endocr J. 2006;53:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Katabami T, Kato H, Shirai N, Naito S, Saito N. Successful long-term treatment with once-daily injection of low-dose octreotide in an aged patient with insulinoma. Endocr J. 2005;52:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Falconi M, Bettini R, Boninsegna L, Crippa S, Butturini G, Pederzoli P. Surgical strategy in the treatment of pancreatic neuroendocrine tumors. JOP. 2006;7:150-156. [PubMed] |
| 63. | España-Gómez MN, Velázquez-Fernández D, Bezaury P, Sierra M, Pantoja JP, Herrera MF. Pancreatic insulinoma: a surgical experience. World J Surg. 2009;33:1966-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Okabayashi T, Maeda H, Nishimori I, Sugimoto T, Ikeno T, Hanazaki K. Pancreatic fistula formation after pancreaticooduodenectomy; for prevention of this deep surgical site infection after pancreatic surgery. Hepatogastroenterology. 2009;56:519-523. [PubMed] |
| 65. | Okabayashi T, Kobayashi M, Nishimori I, Sugimoto T, Onishi S, Hanazaki K. Risk factors, predictors and prevention of pancreatic fistula formation after pancreatoduodenectomy. J Hepatobiliary Pancreat Surg. 2007;14:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Okabayashi T, Hanazaki K, Nishimori I, Sugimoto T, Yoshioka R, Dabanaka K, Kobayashi M, Onishi S. Pancreatic transection using a sharp hook-shaped ultrasonically activated scalpel. Langenbecks Arch Surg. 2008;393:1005-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Moore TJ, Peterson LM, Harrington DP, Smith RJ. Successful arterial embolization of an insulinoma. JAMA. 1982;248:1353-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Uflacker R. Arterial embolization as definitive treatment for benign insulinoma of the pancreas. J Vasc Interv Radiol. 1992;3:639-644; discussion 644-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Shojamanesh H, Gibril F, Louie A, Ojeaburu JV, Bashir S, Abou-Saif A, Jensen RT. Prospective study of the antitumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinoma. Cancer. 2002;94:331-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 70. | Oberg K, Kvols L, Caplin M, Delle Fave G, de Herder W, Rindi G, Ruszniewski P, Woltering EA, Wiedenmann B. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 367] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 71. | Healy ML, Dawson SJ, Murray RM, Zalcberg J, Jefford M. Severe hypoglycaemia after long-acting octreotide in a patient with an unrecognized malignant insulinoma. Intern Med J. 2007;37:406-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19:783-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 73. | Hirshberg B, Cochran C, Skarulis MC, Libutti SK, Alexander HR, Wood BJ, Chang R, Kleiner DE, Gorden P. Malignant insulinoma: spectrum of unusual clinical features. Cancer. 2005;104:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 74. | La Rosa S, Klersy C, Uccella S, Dainese L, Albarello L, Sonzogni A, Doglioni C, Capella C, Solcia E. Improved histologic and clinicopathologic criteria for prognostic evaluation of pancreatic endocrine tumors. Hum Pathol. 2009;40:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Scott A, Hinwood D, Donnelly R. Radio-frequency ablation for symptom control in a patient with metastatic pancreatic insulinoma. Clin Endocrinol (Oxf). 2002;56:557-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Le Treut YP, Delpero JR, Dousset B, Cherqui D, Segol P, Mantion G, Hannoun L, Benhamou G, Launois B, Boillot O. Results of liver transplantation in the treatment of metastatic neuroendocrine tumors. A 31-case French multicentric report. Ann Surg. 1997;225:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 173] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 77. | Olausson M, Friman S, Cahlin C, Nilsson O, Jansson S, Wängberg B, Ahlman H. Indications and results of liver transplantation in patients with neuroendocrine tumors. World J Surg. 2002;26:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Sawyer AM, Schade DS. Use of a continuous glucose monitor in the management of inoperable metastatic insulinoma: a case report. Endocr Pract. 2008;14:880-883. [PubMed] |
| 79. | Munir A, Choudhary P, Harrison B, Heller S, Newell-Price J. Continuous glucose monitoring in patients with insulinoma. Clin Endocrinol (Oxf). 2008;68:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K. The accuracy of a continuous blood glucose monitor during surgery. Anesth Analg. 2008;106:160-163, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 81. | Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K. Accuracy and reliability of continuous blood glucose monitor in post-surgical patients. Acta Anaesthesiol Scand. 2009;53:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Hanazaki K, Nosé Y, Brunicardi FC. Artificial endocrine pancreas. J Am Coll Surg. 2001;193:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 83. | Okabayashi T, Hnazaki K, Nishimori I, Sugimoto T, Maeda H, Yatabe T, Dabanaka K, Kobayashi M, Yamashita K. Continuous post-operative blood glucose monitoring and control using a closed-loop system in patients undergoing hepatic resection. Dig Dis Sci. 2008;53:1405-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Maeda H, Yatabe T, Kohsaki T, Kobayashi M, Hanazaki K. Continuous postoperative blood glucose monitoring and control by artificial pancreas in patients having pancreatic resection: a prospective randomized clinical trial. Arch Surg. 2009;144:933-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 85. | Okabayashi T, Nishimori I, Maeda H, Yamashita K, Yatabe T, Hanazaki K. Effect of intensive insulin therapy using a closed-loop glycemic control system in hepatic resection patients: a prospective randomized clinical trial. Diabetes Care. 2009;32:1425-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 86. | Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Yatabe T, Maeda H, Kobayashi M, Hanazaki K. Risk factors and predictors for surgical site infection after hepatic resection. J Hosp Infect. 2009;73:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |