Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.742
Revised: December 13, 2012
Accepted: December 25, 2012
Published online: February 7, 2013
Processing time: 168 Days and 19.3 Hours
AIM: To investigate the effect of probiotics on regulating T regulatory cells and reducing the severity of experimental colitis in mice.
METHODS: Forty C57/BL mice were randomly divided into four groups. Colitis was induced in the mice using 2,4,6-trinitrobenzene sulfonic acid (TNBS). After 10-d treatment with Bifico capsules (combined bifidobacterium, lactobacillus and enterococcus), body weight, colonic weight, colonic weight index, length of colon, and histological scores were evaluated. CD4+CD25+Foxp3+T cell in mesenteric lymph nodes were measured by flow cytometry, and cytokines in colonic tissue homogenates were analyzed by a cytometric bead array.
RESULTS: The colonic weight index and the colonic weight of colitis mice treated with Bifico were lower than that of TNBS-induced mice without treatment. However, colonic length and percent of body weight amplification were higher than in TNBS-induced mice without treatment. Compared with TNBS-induced mice without treatment, the level of CD4+CD25+Foxp3+T cells in mesenteric lymph nodes, the expression of interleukin (IL)-2, IL-4 and IL-10 in colonic tissues from colitis mice treated with Bifico were upregulated, and tumor necrosis factor-α and interferon-γ were downregulated.
CONCLUSION: Probiotics effectively treat experimental colitis by increasing CD4+CD25+Foxp3+T cell and regulating the balance of Th1 and Th2 cytokines in the colonic mucosa.
- Citation: Zhao HM, Huang XY, Zuo ZQ, Pan QH, Ao MY, Zhou F, Liu HN, Liu ZY, Liu DY. Probiotics increase T regulatory cells and reduce severity of experimental colitis in mice. World J Gastroenterol 2013; 19(5): 742-749
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/742.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.742
Ulcerative colitis (UC) belongs to the inflammatory bowel disease (IBD) group, which are chronic, relapsing-remitting gastrointestinal diseases of unknown etiology[1,2]. The primary treatments of UC (san azosulfamide, immunodepressants and surgery) are usually not well tolerated, have side effects and show a high relapse rate[3,4]. Many reports have indicated that the non-specific inflammation of UC is associated with bacterial overgrowth and dysbiosis[5,6]. As live microbial feed supplements, probiotics can beneficially affect the host by improving the balance of the intestinal microbial flora to effectively and securely treat UC in human and murids[1,7,8]. A pivotal event in the development of UC is that high sensitivity to intestinal symbiotic microbial flora induces serious autoimmunity enteritis, which inhibits the function or lowers the level of CD4+CD25+T cells. Re-import of CD4+CD25+T cells may prevent the occurrence or aggravation of IBD[9]. This could be accomplished by secreting suppressor cytokines to recovering immunological tolerance and maintain the stability of internal environment in the intestines[10,11].
To explore the effects of probiotics on regular intestinal mucosal internal environment, we observed the level of CD4+CD25+T cells in mesenteric lymph nodes and the expression of related cytokines in colonic mucosa from mice with colitis induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS).
TNBS (batch number: 2508-19-2) was purchased from Sigma (St. Louis, MO, United States). Bifico Capsules (batch number: 20101002), which combine bifidobacterium, lactobacillus and enterococcus, was purchased from Bifico Pharma (Shanghai, China). Mesalazine (batch number: 110201) was purchased from Sunflower Pharma (Jiamusi, China). The mouse regulatory T cell staining kit (batch number: 88-8111-40) was purchased from eBioscience CO (San Diego, CA, United States). The BD cytometric bead array (CBA) and mouse Th1/Th2/Th17 Cytokine Kit (batch number: 23-12380-00) was from BD Biosciences (San Jose, CA, United States).
Forty C57/BL mice (half male and half female, weighing 22-26 g, the animal certificate number was SCXK 2009-0004) were provided from Sino-British Sippr/BK Lab Animal Co. Ltd. (Changsha, China), and randomly divided into four groups of ten animals each: the normal group (mice were challenged and administrated with physiological saline), the model group (mice were induced by TNBS without treatment), the Bifico group (mice were induced by TNBS and treated with 345 mg/kg Bifico capsules), the Mesalazine group (mice were induced by TNBS and treated with 300 mg/kg mesalazine). All animals were housed at 20 ± 1 °C with a humidity of 50% ± 5% in a 12 h light/dark cycle and fed ad libitum with standard mouse feed and water throughout the experiments. The experimental protocols were performed according to the Guidelines of the Jiangxi University of Traditional Chinese Medicine Animal Research Committee.
Experimental colitis in mice was induced with TNBS Solution (100 mg·kg-1 body TNBS dissolved in 0.15 mL 30% ethanol) by enema[12]. The mice were maintained in a head-down position for 1 min. After 24 h, mice in the treated groups were administrated Bifico capsules (345 mg/kg) or mesalazine (300 mg/kg) by lavage for 10 d, while mice in the normal and model group received the equivalent volume of physiological saline.
To evaluate the therapeutic effect of probiotics treatment on experimented colitis, body weight, colonic weigh index [colonic weigh index (%) = colonic weigh/body weigh × 100%], colonic length and histological score were analyzed. The histological scoring was performed by Professor Chun Xiao of the pathological department from Jiangxi University of Traditional Chinese Medicine, and was a total score of inflammatory cell infiltration and tissue damage, according to the study of Schmidt and his colleagues[13]. Infiltration scores were as follows: 0, no infiltration; 1, increased number of inflammatory cells in the lamina propia; 2, inflammatory cells extending into the submucosa; 3, transmural inflammatory infiltrates; and for tissue damage: 0, no mucosal damage; 1, discrete epithelial lesions; 2, erosions or focal ulcerations; 3, severe mucosal damage with extensive ulceration extending into the bowel wall[13].
On day 11, mice were euthanized, and mesenteric lymph nodes were separated and triturated with cold physiological saline in a mortar into a cell suspension. The cell suspension was filtered through a 300-micron screen mesh to measure CD4+CD25+Foxp3+T cells. The colon from the ileocecal junction to the anus was excised, its length was measured, and then it was cut open longitudinally at the mesenteric attachment, washed with 5 mL of 0.01 mol/L phosphate buffered solution (PBS) (pH 7.4) to remove the fecal remnants and weighted to compute the colonic weigh index. Partial colons were fixed in PBS-buffered 4% paraformaldehyde, and then processed for paraffin embedding and cut into 5-μm thick sections. The sections were stained with hematoxylin and eosin. Other colons were made into tissue homogenates to analyze the expressions of the cytokines.
Cells from mesenteric lymph nodes were resuspended in eFluor® NC Flow Cytometry Staining Buffer at a final cell concentration of 2 × 107/mL. fluorescein isothiocyanate anti-mouse CD4+Ab (RM4-5, 0.125 μg/sample; eBioscience) and APC anti-mouse CD25+Ab (PC61.5, 0.25ug/sample; eBioscience) were added to the cell suspension. Cells were centrifuged at 300 ×g at 4 °C for 5 min, fixed using Fix/Perm Buffer (eBioscience) for at least 12 h at 4 °C, and then incubated with PE-anti-mouse Foxp3+Ab (FJK-16s, 0.5 μg/sample; eBioscience) for 30 min in the dark at 4 °C. Cells labeled with PE rat IgG2a were used as the isotype negative control. Fluorescence-activated cell sorting analysis was performed on a FACSCalibur (BD Biosciences)[14].
The concentrations of cytokines in colonic tissue homogenates were measured using a BD CBA Mouse Th1/Th2/Th17 Cytokine Kit according to the manufacturer’s protocol. Fifty microliters of homogenates or standards were incubated with 50 μL of capture beads for 1h at room temperature, and then mixed with 50 μL of phycoerythrobilin (PE)-labeled tumor necrosis factor (TNF)-α, interleukin (IL)-4, interferon (IFN)-γ, IL-10, IL-6, IL-17 and IL-2 detection antibodies and incubated for 2 h at room temperature to form a sandwich complex. Following incubation, 1 mL of washing buffer (BD Biosciences Pharmingen, United States) was added to each tube, and the mean fluorescence intensity was detected using flow cytometry (FACSCalibur, B. D. Co, United States). Data were analyzed using BD Cytometric BeadArray analysis software[15].
Statistical analyses were performed using SPSS for Windows, version 15.0. Data were presented as means ± SD. Statistical analysis was performed using ANOVA and the F-test. P < 0.05 or P < 0.01 were considered significant.
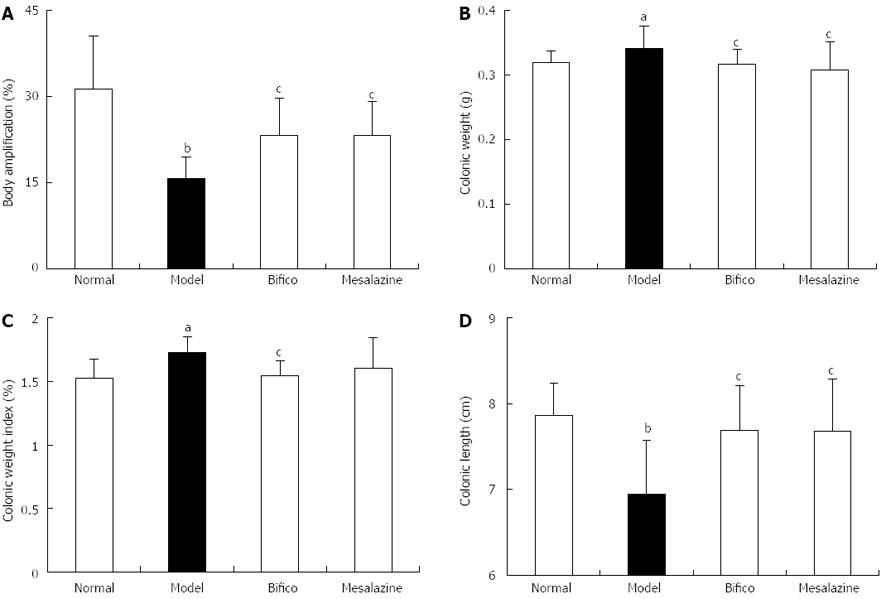
Body weight amplifications of mice in the normal, Bifico and mesalazine groups were higher than that of the model group (31.28 ± 9.23, 23.20 ± 6.51, 23.17 ± 6.05, vs 15.73 ± 3.73, P < 0.05) (Figure 1A); however, the colonic weight index (CWI) and colonic weight (CW) in the Bifico group decreased compared with the model group (CWI: 1.53 ± 0.15, 1.54 ± 0.12 vs 1.72 ± 0.13, P < 0.05; CW: 0.32 ± 0.02, 0.32 ± 0.02 vs 0.36 ± 0.03, P < 0.05) (Figure 1B and C). The colonic lengths of mice in the normal, Bifico and mesalazine groups were significantly higher compared with those in the model group (7.86 ± 0.37, 7.68 ± 0.53, 7.68 ± 0.61, vs 6.94 ± 0.63, P < 0.05) (Figure 1D).
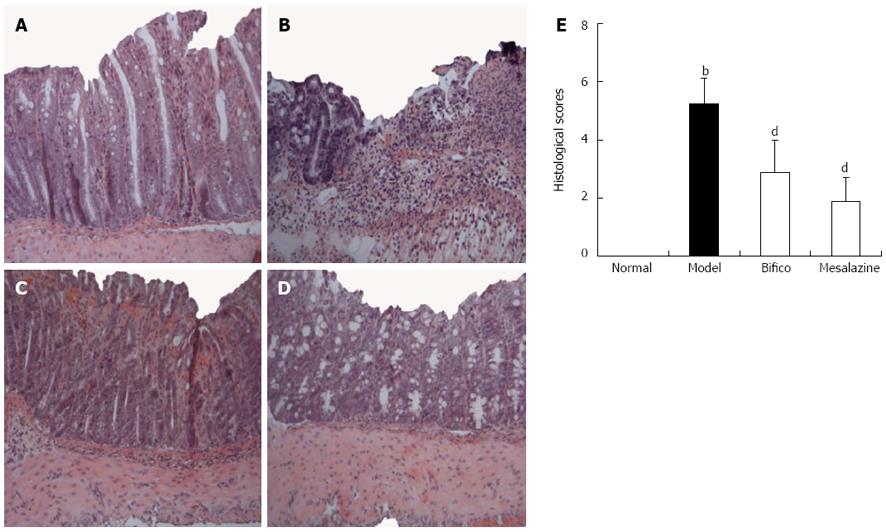
As shown in Figure 2A, integral colonic epithelium and no inflammatory cell infiltration were observed in the slice from the normal mice. However, ulceration, colonic epithelium loss, incrassate intestinal wall and abundant inflammatory cell infiltration were observed in the colon of mice in the model group (Figure 2B). Meanwhile, the above histological symptoms were remarkably relieved with fewer infiltrated inflammatory cell and restored epithelium in slices from colitis mice treated with Bifico and mesalazine (Figure 2C and D). Compared with the model group, the histological score in the Bifico and mesalazine groups decreased statistically significantly (2.88 ± 1.33, 1.88 ± 0.83 vs 5.25 ± 0.89, P < 0.01) (Figure 2E). The results indicated that Bifico effectively restrained colonic injury of experimented colitis mice.
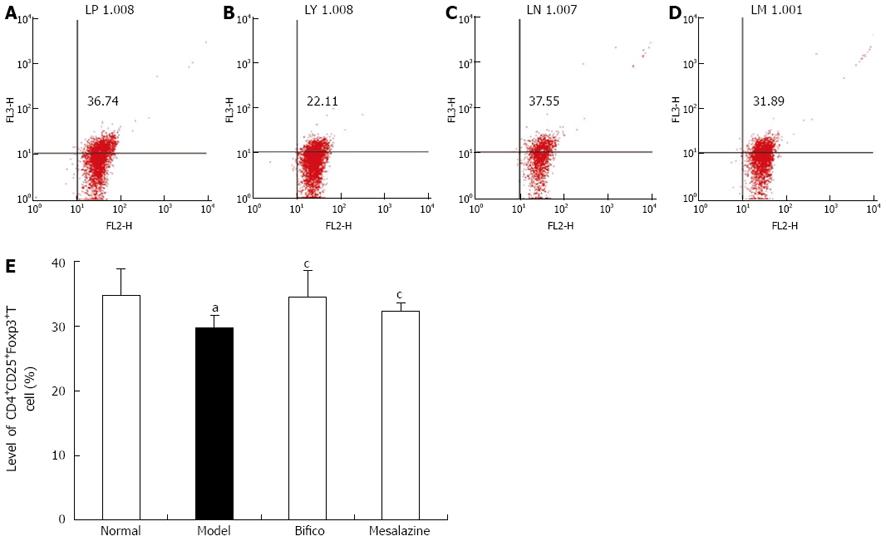
To identify the developmental capacity of CD4+CD25+T regulatory cells, the level of CD4+CD25+Foxp3+T cells in mesenteric lymph nodes was analyzed by flow cytometry. As shown in Figure 3, the level of CD4+CD25+Foxp3+T cells in the model group was decreased compared with the normal group (29.70 ± 1.87 vs 34.73 ± 4.04, P < 0.05). But in the Bifico and mesalazine groups, the levels of CD4+CD25+Foxp3+T cells were higher than in the model group (34.46 ± 4.11, 32.18 ± 1.44 vs 29.70 ± 1.87, P < 0.05).
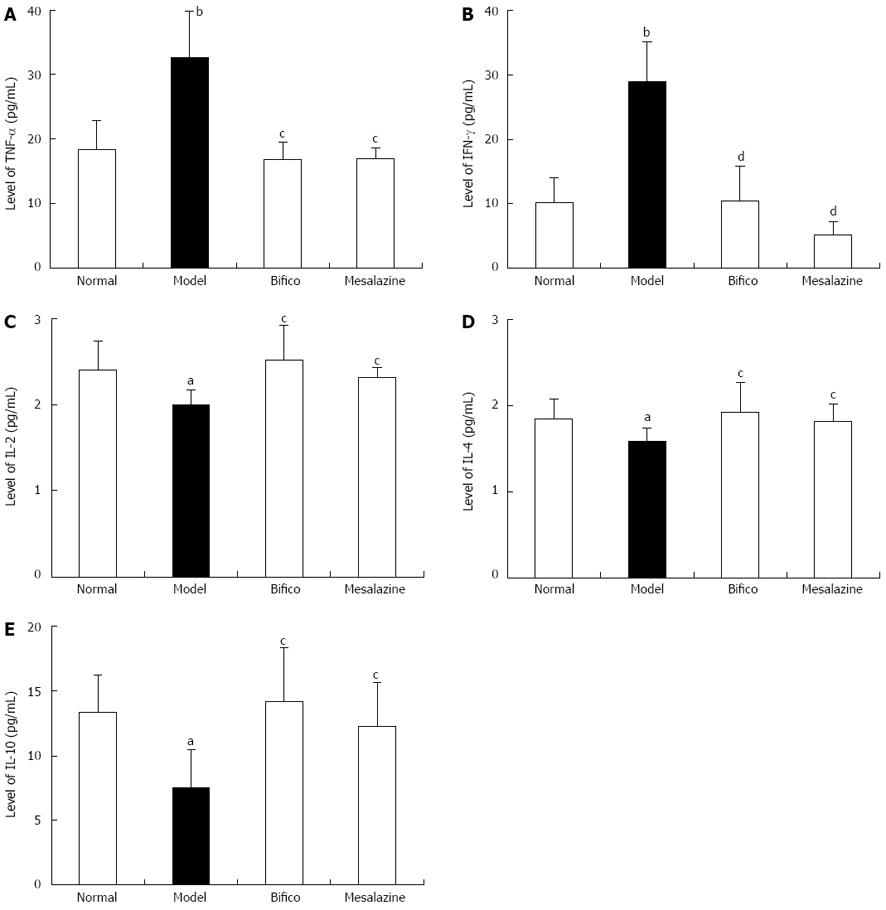
Compared with the normal group, the expressions of TNF-α and IFN-γ in colonic tissues from mice in the model group were significantly increased (TNF-α: 32.67 ± 7.12 vs 18.33 ± 4.55, P < 0.01; IFN-γ: 28.90 ± 6.13 vs 10.24 ± 3.74, P < 0.01) (Figure 4A and B), and they were significantly decreased in the Bifico and mesalazine groups compared with the model (TNF-α: 16.87 ± 2.70, 16.96 ± 1.72 vs 32.67 ± 7.12, P < 0.05; IFN-γ: 10.46 ± 5.44, 5.20 ± 2.10 vs 28.90 ± 6.13, P < 0.01) (Figure 4A and B). Meanwhile, the expressions of IL-2, IL-4 and IL-10 in the model group were lower than in the normal group (IL-2: 2.00 ± 0.17 vs 2.40 ± 0.34, P < 0.05; IL-4: 1.58 ± 0.16 vs 1.84 ± 0.24, P < 0.05; IL-10: 7.50 ± 2.96 vs 13.35 ± 2.89, P < 0.05) (Figure 4C-E). Compared with the model group, the three cytokines were upregulated in the Bifico and mesalazine groups (IL-2: 2.52 ± 0.40, 2.32 ± 0.11 vs 2.00 ± 0.17, P < 0.05; IL-4: 1.92 ± 0.34, 1.82 ± 0.20 vs 1.58 ± 0.16, P < 0.05; IL-10: 14.19 ± 4.14, 12.25 ± 3.41 vs 7.50 ± 2.96, P < 0.05) (Figure 4C-E). The expressions of IL-6 and IL-17 in colonic tissues from colitis mice without treatment were higher than in the normal group (IL-6: 4.69 ± 1.47 vs 2.35 ± 0.19, P < 0.01; IL-17: 5.94 ± 1.54 vs 3.37 ± 0.96, P < 0.01); however, in the two treated groups, they were no different compared with the model.
In the present study, the curative effect of probiotics treatment on experimental colitis was demonstrated by the increased body weight, the increased length of the colon, and decreasing histological scores followed by restored pathological characters. Probiotics treatment for 10 d inhibited the tendency to decrease CD4+CD25+T cells in mesenteric lymph nodes, downregulated the expression of TNF-α and IFN-γ, and upregulated the products of IL-2, IL-4 and IL-10 in colonic mucosa from TNBS-induced colitis mice.
Many studies have indicated that dysregulation of intestinal microflora plays an important role in initiating and perpetuating colonic inflammation in the development of human UC[16-18]. Findings from animal dextran sulfate sodium (DSS)- or TNBS-colitis models suggest that the intestinal microflora plays a key role in the abnormal immune response leading to mucosal injury in experimental colitis, because germ-free animals do not mount an intestinal inflammatory response[1,19-20]. Recently, probiotic therapy has been recognized as a safe and effective treatment for patients with UC. Lin-Lin Chen and their colleagues demonstrated that every strain of three probiotics in Bifico Capsules (combined bifidobacterium, lactobacillus and enterococcus) had a beneficial effect on DSS-induced experimental colitis in mice[21].
CD4+CD25+T regulatory (Treg) cells are critical for maintaining immune homeostasis and establishing tolerance to foreign, non-pathogenic antigens, including commensal bacteria and food, and are identified by their constitutive expression of Foxp3. CD4+CD25+T cell secretes suppressive cytokines, such as IL-2, IL-10 and TGF-β, to regulate the balance of Th1/Th2 cells and maintain mucosal immunity in the intestine[22-24]. Evidence has shown that non-functional, absent Tregs or genetic mutations in Foxp3 induces hypersensitivity to bacterial antigens[10,11], and destroys the balance of intestinal mucosal immunity to inflammatory injury followed by lymphocytic infiltration of the intestinal mucosa[25,26]. CD4+CD25+Foxp3+ T cells increased in the colon of paracmasis patients with UC[22]. These data indicated that Tregs would be a promising target for treating UC, because increasing the activity of appropriate Tregs in the gut should help to restore inflamed colonic tissues[27].
In the present study, we found that the level of CD4+CD25+Foxp3+ T cells was downregulated in mesocolic lymph nodes from experimented colitis mice without treatment, while IL-2, IL-10 and IL-4 were hypo-expressed in colonic tissues in the model group. The results showed that the suppressed function of CD4+CD25+Foxp3+T cell induced disturbance of Th1/Th2 cytokines in the colonic mucosa, which leads to increased susceptibility to symbiotic bacteria, overexpression of proinflammatory factors (IL-1, TNF-α, IFN-γ), finally resulting in inflammatory injury and ulceration. After 10-d of treatment by probiotics, impaired colonic mucosa was restored with epithelial hyperplasia, intact colonic epithelia and fewer infiltrating inflammatory cells infiltration. Probiotics effectively treated mice with colitis. CD4+CD25+Foxp3+T cells were upregulated, with high expression of IL-10, IL-4 and IL-2, and TNF-α and IFN-γ were decreased in colonic mucosa from colitis mice treated by probiotics. In the study, probiotics improved the quantity of CD4+CD25+Foxp3+T cells, and restored the suppressed function of CD4+CD25+Foxp3+T cells. The Treg cells secreted more IL-10 and IL-4, competitively bound with IL-2, and enhanced the immune tolerance of intestinal mucosa to resist bacterial antigens and decreased the activity of proinflammatory factors (as TNF-α and IFN-γ), thereby renewing the immunological barrier of the colonic mucosa. Probiotics alleviated colonic inflammatory injury of colitis mice by alleviating the suppression of or increasing the quantity of CD4+CD25+Foxp3+T cell.
In summary, Probiotics effectively treated experimental colitis by improving CD4+CD25+Foxp3+T cell and regulating the balance of Th1/Th2 cytokines in the colonic mucosa.
Ulcerative colitis (UC) is a chronic, inflammatory disease of the colonic mucosa, characterized by a relapsing-remitting course; however, the cause is largely unknown. Dysregulation of intestinal microflora and hypofrontality of T regulatory cell play important roles in pathogenicity of UC.
The barrier of intestinal mucosal immunity may become damaged, which may be primarily caused by decreased inhibition of T regulatory cell by bacterial antigens. This suggested that regulating the function of T regulatory cells and/or the balance of intestinal microflora could potentially be used to treat UC.
Previous studies have highlighted that re-imported T regulatory cells may prevent occurrence or aggravation of UC. In the present study, probiotics (Bifico) inhibited the decreased tendency of CD4+CD25+Foxp3+T cell induced by 2,4,6-trinitrobenzene sulfonic acid to effectively treat experimental colitis, followed by regulation of the balance of Th1/Th2 cytokines in the colonic mucosa.
By understanding the effects of probiotics in the treatment experimental colitis, which was mediated by inhibiting the decreased level of T regulatory cell and regulating the balance of Th1/Th2 cytokines, the study provided some evidence of the effect of probiotics on maintaining stable intestinal mucosal immunity in the treatment of UC.
The authors investigated the effects of probiotics on treating experimental colitis and explored its potential mechanism by observing the level of T regulatory cell and expression of Th1/Th2 cytokines in colonic mucosa. It has revealed that probiotics effectively treated experimental colitis, which was accomplished by improving CD4+CD25+Foxp3+T cell and regulating balance Th1 and Th2 cytokines in colonic mucosa. The results are valuable for exploring the mechanism of treating UC with probiotics.
P- Reviewer Rosen MJ S- Editor Jiang L L- Editor Stewart GJ E- Editor Zhang DN
| 1. | Tsuda Y, Yoshimatsu Y, Aoki H, Nakamura K, Irie M, Fukuda K, Hosoe N, Takada N, Shirai K, Suzuki Y. Clinical effectiveness of probiotics therapy (BIO-THREE) in patients with ulcerative colitis refractory to conventional therapy. Scand J Gastroenterol. 2007;42:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Ochsenkühn T, D’Haens G. Current misunderstandings in the management of ulcerative colitis. Gut. 2011;60:1294-1299. [PubMed] [DOI] [Full Text] |
| 3. | Lewis JD, Gelfand JM, Troxel AB, Forde KA, Newcomb C, Kim H, Margolis DJ, Strom BL. Immunosuppressant medications and mortality in inflammatory bowel disease. Am J Gastroenterol. 2008;103:1428-1435; quiz 1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Maul J, Zeitz M. Ulcerative colitis: immune function, tissue fibrosis and current therapeutic considerations. Langenbecks Arch Surg. 2012;397:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Gionchetti P, Rizzello F, Lammers KM, Morselli C, Sollazzi L, Davies S, Tambasco R, Calabrese C, Campieri M. Antibiotics and probiotics in treatment of inflammatory bowel disease. World J Gastroenterol. 2006;12:3306-3313. [PubMed] |
| 6. | Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, Dunne WM, Allen PM, Stappenbeck TS. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9:390-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Rust C, Brand S. PSC: Protect and serve with colitis: does it help the liver to have severe ulcerative colitis? Gut. 2011;60:1165-1166. [PubMed] [DOI] [Full Text] |
| 8. | Lagranderie M, Kluge C, Kiefer-Biasizzo H, Abolhassani M, Nahori MA, Fitting C, Huerre M, Bandeira A, Bercovier H, Marchal G. Mycobacterium bovis Bacillus Calmette-Guérin killed by extended freeze-drying reduces colitis in mice. Gastroenterology. 2011;141:642-652, 652.e1-4. [PubMed] [DOI] [Full Text] |
| 9. | Sujino T, Kanai T, Ono Y, Mikami Y, Hayashi A, Doi T, Matsuoka K, Hisamatsu T, Takaishi H, Ogata H. Regulatory T cells suppress development of colitis, blocking differentiation of T-helper 17 into alternative T-helper 1 cells. Gastroenterology. 2011;141:1014-1023. [PubMed] [DOI] [Full Text] |
| 10. | Singh B, Read S, Asseman C, Malmström V, Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 375] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939-3943. [PubMed] |
| 12. | Segain JP, Raingeard de la Blétière D, Sauzeau V, Bourreille A, Hilaret G, Cario-Toumaniantz C, Pacaud P, Galmiche JP, Loirand G. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn’s disease and experimental colitis. Gastroenterology. 2003;124:1180-1187. [PubMed] [DOI] [Full Text] |
| 13. | Schmidt N, Gonzalez E, Visekruna A, Kühl AA, Loddenkemper C, Mollenkopf H, Kaufmann SH, Steinhoff U, Joeris T. Targeting the proteasome: partial inhibition of the proteasome by bortezomib or deletion of the immunosubunit LMP7 attenuates experimental colitis. Gut. 2010;59:896-906. [PubMed] [DOI] [Full Text] |
| 14. | Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol. 2005;175:3075-3083. [PubMed] |
| 15. | Sun K, Wang CS, Guo J, Horie Y, Fang SP, Wang F, Liu YY, Liu LY, Yang JY, Fan JY. Protective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Life Sci. 2007;81:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Berrebi D, Languepin J, Ferkdadji L, Foussat A, De Lagausie P, Paris R, Emilie D, Mougenot JF, Cezard JP, Navarro J. Cytokines, chemokine receptors, and homing molecule distribution in the rectum and stomach of pediatric patients with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2003;37:300-308. [PubMed] |
| 17. | Hegazy SK, El-Bedewy MM. Effect of probiotics on pro-inflammatory cytokines and NF-kappaB activation in ulcerative colitis. World J Gastroenterol. 2010;16:4145-4151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Koh SJ, Kim JS. Prebiotics: germinated barley foodstuff for the prevention of colitis-associated colon cancer? J Gastroenterol Hepatol. 2011;26:1219-1220. [PubMed] [DOI] [Full Text] |
| 19. | Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 457] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 20. | Campieri M, Gionchetti P. Bacteria as the cause of ulcerative colitis. Gut. 2001;48:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Chen LL, Wang XH, Cui Y, Lian GH, Zhang J, Ouyang CH, Lu FG. Therapeutic effects of four strains of probiotics on experimental colitis in mice. World J Gastroenterol. 2009;15:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 22. | Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852-5860. [PubMed] |
| 23. | Liu B, Tonkonogy SL, Sartor RB. Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice. Gastroenterology. 2011;141:653-662, 662.e1-4. [PubMed] [DOI] [Full Text] |
| 24. | Kameyama K, Nemoto Y, Kanai T, Shinohara T, Okamoto R, Tsuchiya K, Nakamura T, Sakamoto N, Totsuka T, Hibi T. IL-2 is positively involved in the development of colitogenic CD4+ IL-7R alpha high memory T cells in chronic colitis. Eur J Immunol. 2010;40:2423-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 384] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 26. | McMurchy AN, Di Nunzio S, Roncarolo MG, Bacchetta R, Levings MK. Molecular regulation of cellular immunity by FOXP3. Adv Exp Med Biol. 2009;665:30-46. [PubMed] |
| 27. | Himmel ME, Yao Y, Orban PC, Steiner TS, Levings MK. Regulatory T-cell therapy for inflammatory bowel disease: more questions than answers. Immunology. 2012;136:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |