Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.727
Revised: October 10, 2012
Accepted: November 14, 2012
Published online: February 7, 2013
Processing time: 152 Days and 17.1 Hours
AIM: To evaluate the possibility of reducing the volume of polyethylene glycol (PEG)-electrolyte solution using adjunctive mosapride citrate for colonoscopy preparation.
METHODS: This was a single-center, prospective, randomized, investigator-blinded, non-inferiority study involving 252 patients of both sexes, aged from 20 to 80 years, scheduled for screening or diagnostic colonoscopy in our department. A total of 126 patients was randomized to receive 1.5 L PEG-electrolyte solution plus 15 mg of mosapride (1.5 L group), and 126 received 2 L PEG-electrolyte solution plus 15 mg of mosapride (2 L group). Patients completed a questionnaire on the acceptability and tolerability of the bowel preparation process. The efficacy of bowel preparation was assessed using a 5-point scale based on the Aronchick scale. The primary end point was adequate bowel preparation rates (score of excellent/good/fair) vs (poor/inadequate). Acceptability and tolerability, as well as disease detection, were secondary end points.
RESULTS: A total of 244 patients was included in the analysis. There were no significant differences between the 2 L and 1.5 L groups in age, sex, body mass index, number of previous colonoscopies, and the preparation method used previously. The adequate bowel preparation rates were 88.5% in the 2 L group and 82.8% in the 1.5 L group [95% lower confidence limit (LCL) for the difference = -14.5%, non-inferiority P = 0.019] in the right colon. In the left colon, the adequate bowel preparation rates were 89.3% in the 2 L group and 81.1% in the 1.5 L group (95% LCL = -17.0%, non-inferiority P = 0.066). Compliance, defined as complete (100%) intake of the PEG solution, was significantly higher in the 1.5 L group than in the 2 L group (96.8% vs 85.7%, P = 0.002). The proportion of abdominal distension (none/mild/moderate/severe) was significantly lower in the 1.5 L group than in the 2 L group (36/65/22/3 vs 58/48/18/2, P = 0.040). Within the subgroup who had undergone colonoscopy previously, a significantly higher number of patients in the 1.5 L group than in the 2 L group felt that the current preparation was easier than the previous one (54.1% vs 28.0%, P = 0.001). The disease detection rate was not significantly different between the two groups.
CONCLUSION: Although the 1.5 L group had better acceptability and tolerability, 15 mg of mosapride may be insufficient to compensate for a 0.5-L reduction of PEG solution.
- Citation: Tajika M, Niwa Y, Bhatia V, Kondo S, Tanaka T, Mizuno N, Hara K, Hijioka S, Imaoka H, Komori K, Yamao K. Can mosapride citrate reduce the volume of lavage solution for colonoscopy preparation? World J Gastroenterol 2013; 19(5): 727-735
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.727
Polyethylene glycol (PEG)-electrolyte solution is widely used worldwide for bowel cleansing. By consensus of the American Society for Gastrointestinal Endoscopy, the American Society of Colon and Rectal Surgeons, and the Society of American Gastrointestinal and Endoscopic Surgeons, PEG-electrolyte solution is the gold standard for colonoscopic bowel preparation (Grade IA), and aqueous sodium phosphate (NaP) is an alternative regimen to PEG-electrolyte solutions (Grade IA)[1]. Several meta-analyses on the available bowel preparations have favored NaP, concluding that it was effective and better tolerated by patients than PEG-electrolyte solution[2-4]. However, the disadvantages of NaP are its associated side effects. Significant changes in serum electrolyte levels[5], even in patients without renal failure, have prompted recommendations for serum electrolyte evaluation prior to the administration of NaP[6,7].
On the other hand, osmotically balanced electrolyte lavage solutions offer safe and effective cleansing, but volume-related discomfort and adverse experiences have decreased the percentage of patients completing the pre-examination preparation[1,8,9]. This is mainly due to the large volumes of fluid required for bowel preparation, the unpleasant taste, and an increase in the incidence of side effects[10]. Although 3-4 L of this solution is used in Western countries, approximately 2 L of this solution, along with a laxative, is usually considered adequate for bowel preparation in Japan. Despite the lower volume in Japan, the need to drink such large volumes of liquid with an unpalatable taste has a negative impact on patient compliance[11]. Therefore, more effective bowel preparation regimens for colonoscopy are required to improve the acceptability and tolerability of the procedure.
Mosapride citrate (mosapride) is a selective 5-hydroxytryptamine 4 (5-HT4) receptor agonist. Mosapride enhances gastric emptying and motility by facilitating acetylcholine release from the enteric cholinergic neurons, without blocking the dopaminergic D2 receptors[12]. It is known to be effective in gastroesophageal reflux disease[13], functional gastrointestinal disorders, such as functional dyspepsia[14], chronic gastritis with delayed gastric emptying, and diabetic gastroparesis[15]. Since 5-HT4 receptors are also located in the human colon and rectum[16,17], mosapride is also expected to have a prokinetic effect on the colo-rectum. A few clinical studies have reported that mosapride in combination with PEG-electrolyte solution may enhance bowel cleansing and improve patient acceptability and tolerability[18,19]. Furthermore, we previously conducted a randomized, double-blind, placebo-controlled study with mosapride in addition to PEG-electrolyte solution and demonstrated that co-administration of mosapride with PEG-electrolyte solution improved the quality of bowel preparation for colonoscopy, especially in patients without severe constipation[20]. Among the subgroup that had undergone previous colonoscopy, a significantly higher number of mosapride-group patients than placebo-group patients felt that the current preparation was easier. However, mosapride could not improve symptoms such as nausea, abdominal distension, abdominal pain, and willingness to repeat the same regimen compared with placebo. In short, mosapride did not sufficiently improve patient acceptability and tolerability. Therefore, it appears that it is necessary to reduce the volume of PEG-electrolyte solution to improve patient acceptability and tolerability.
The aim of this study was to evaluate the reduction of PEG-electrolyte solution volume when combined with mosapride citrate for colonoscopy preparation.
This was a prospective, randomized, investigator-blinded study, comparing 1.5 L PEG plus mosapride (1.5 L group), with 2 L PEG-electrolyte solution plus mosapride (2 L group) dosing for patients who underwent colonoscopy. All patients provided written, informed consent prior to entering the study. The study was conducted at Aichi Cancer Center Hospital (ACCH), Nagoya, from January 2010 to June 2010, and was reviewed and approved by the ethics committee of ACCH. This trial was registered in an international clinical trial registry (UMIN000001556).
All consecutive outpatients of both sexes, aged 20 to 80 years, who were scheduled for screening or diagnostic colonoscopy at ACCH were evaluated for inclusion in the study. Patients with the following clinical features were excluded: significant cardiac, renal, hepatic, or metabolic co-morbidities, ascites, severe constipation (< 2 bowel movements a week), known allergy to PEG-electrolyte solution, history of gastric stapling or bypass procedure, or a history of prior colonic or rectal surgery. Patients were excluded if there was a suspected diagnosis of intestinal obstruction because of advanced colorectal cancer.
Patients were randomly allocated to receive one of two different bowel preparation regimens using a computer-generated random-number list. Patients were randomized in block sizes of two, with serially numbered, sealed, opaque envelopes. Concealed allocation was accomplished through non-research personnel who were not involved in this study. Patients were instructed not to discuss their bowel preparation with anyone other than the unblinded research assistant. With the exceptions of the patient and the unblinded research assistant, all other individuals participating in this study, including the endoscopists and endoscopy nurses, were blinded to the allocated treatment group. Comparisons between the 1.5 L group and the 2 L group were made in an investigator-blind fashion.
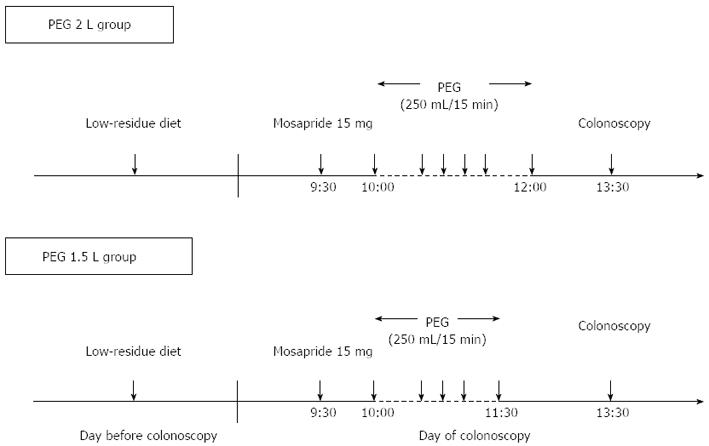
The day before colonoscopy, all patients were instructed to eat a pre-packaged, low residue diet (Enimaclin CS; Horii Pharmaceutical Ind., Ltd., Osaka, Japan) that consisted of a lunch, snack, and dinner, and asked to drink more than 2 liters of clear liquid. On the day of the colonoscopy, all participants reported to the endoscopy room at 9:00 am and received in-hospital bowel preparation. In-hospital preparation was important to ensure uniformity and remove any confounding caused by poor patient adherence. More than 10 toilet facilities were made available in the endoscopy unit for patient comfort. Six mosapride tablets (15 mg) (Gasmotin; Dainippon Sumitomo Pharma Co., Ltd. Osaka, Japan) were administered orally with water at half past nine. After 30 min, both groups were instructed to drink 0.25 L of PEG-electrolyte solution (Niflec; Ajinomoto Pharmaceuticals Co., Ltd. Tokyo, Japan) every 15 min (Figure 1). Colonoscopies were performed from half past thirteen, and the start times were recorded for each patient.
The efficacy of bowel preparation was assessed using the Aronchick scale[21]. Participating endoscopists were trained to use the Aronchick scale to achieve a good level of agreement. Investigators performed calibration exercises involving more than 20 colonoscopies prior to study commencement, based on their interpretation of scale anchors, to ensure that their findings agreed. The final assessment of bowel preparation was divided into two categories, adequate and failure. Bowel preparation rated as fair, good, or excellent, based on the Aronchick scale, was considered adequate; poor or inadequate ratings were considered failure. After colonoscopy, two observers, one who was the operator and the other who was a fellow in the procedure room, decided the score by mutual agreement. They scored the quality of the preparation on the right side (proximal to splenic flexure) colon and on the left side (distal to splenic flexure) colon and rectum separately. If the decision was discordant, a third expert reviewer graded and scored the recorded images later, and this evaluation was used in the final analysis. Twelve experienced colonoscopists carried out all colonoscopy procedures, each of whom had performed more than 1000 colonoscopies.
During or immediately following the colonoscopy, the investigator completed a physician questionnaire regarding assessment of bowel preparation, amount of irrigation fluid used, time needed to reach the cecum, and ease of insertion to the cecum and difficulty in observing the lumen of the colo-rectum because of peristalsis.
The nursing staff recorded the time required to drink the indicated volume of lavage solution. They also recorded the time and number of bowel movements from the start of ingestion to the appearance of clear excretion. Until one hour after finishing the PEG-electrolyte solution plus mosapride, the nursing staff checked patients’ excretions. If there was a solid stool with muddy excretions or no excretion at that time, the patient was given an additional preparation, such as additional PEG-electrolyte solution or enemas. The patients who received an additional preparation were defined by the Aronchick scale as inadequate. The patient questionnaire consisted of 20 questions[20]. The adverse events were scored using a 4-point scale, where 1 = none, 2 = mild, 3 = moderate, and 4 = severe. The patients completed the questionnaire form before undergoing colonoscopy and submitted it to the nursing staff.
The primary end point was the difference in the rate of adequate colon cleansing between the 1.5 L PEG-electrolyte solution plus 15 mg of mosapride (1.5 L group) and the 2 L PEG-electrolyte solution plus 15 mg of mosapride (2 L group). Secondary end points included differences in patients’ acceptability and tolerance of solutions, time to first defecation, frequency of defecation, complete time for colonic preparation, time needed to reach the cecum, amount of irrigation fluid used, subjective difficulty in colonoscopy insertion to the cecum and in observing the lumen of the colo-rectum because of peristalsis, and disease detection rates.
Based on a previous study[20], the adequate bowel preparation rate for the PEG-electrolyte solution plus mosapride was expected to be less than 80%. It was expected that about 80% of the 1.5 L group would be judged adequate, and the non-inferiority margin was set at -15%. This study was designed to have 80% power to establish non-inferiority (using a one-sided significance level of 0.025 and a target sample size of 250).
The primary efficacy analysis was based on an intent-to-treat analysis and included patients who were randomized and received any treatment. In patients in this group, the preparation was classified as adequate or inadequate based on the colonoscopists’ score of cleansing. Patients who did not undergo colonoscopy because of preparation-related adverse events, or preparation failure, or in whom the right colon could not be reached because of bowel obstruction or technical reasons were excluded. The rates of adequate preparation were compared between the groups by χ2 test or Fisher’s exact test for categorical variables.
For the secondary end points, the Mann-Whitney U test was used to compare continuous variables. Categorical variables were tested using the corrected χ2 test or 2-sided Fisher’s exact tests as appropriate. The criterion for significance was P < 0.05.
All statistical analyses were performed using Statistical Analysis Software (SAS Ver. 9.2 for the PC, SAS Institute Inc., Cary, NC, United States).
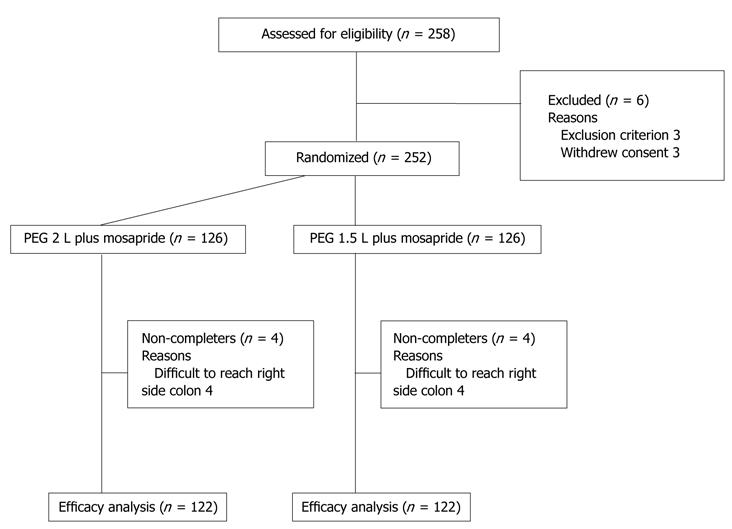
A total of 252 patients was randomized into two groups (Figure 2). Although 252 patients were analyzed, colonoscope insertion to the right colon failed in 4 patients in each group (advanced stenosing cancer in two, and patient refusal in six because of pain). These eight patients were excluded from the efficacy analysis. The baseline characteristics of the patients are shown in Table 1. There was no significant difference in age, sex, body mass index, number of previous colonoscopies, and the preparation method used previously between the 2.0 L and 1.5 L groups.
| Variable | 2.0 L | 1.5 L | P value |
| Patients (n) | 126 | 126 | |
| Age (yr, mean ± SD) | 65.3 ± 9.9 | 66.3 ± 9.6 | NS |
| < 60 | 30 | 25 | NS |
| 60 - < 70 | 51 | 44 | |
| ≥ 70 | 45 | 53 | |
| Male | 77 | 67 | NS |
| Female | 49 | 59 | |
| Indication (n) | |||
| Screening | 40 | 41 | |
| Surveillance | 67 | 63 | NS |
| Diagnostic | 19 | 22 | |
| Body mass index (kg/m2) | 22.5 + 3.2 | 22.3 + 2.7 | NS |
| Previous colonoscopy | |||
| None (first time) | 44 | 41 | NS |
| ≥ 2 | 82 | 85 | |
| Previous preparation for colonoscopy (n) | |||
| 2L PEG | 82 | 85 | NS |
The efficacy of bowel preparation is shown in Table 2. The adequate bowel preparation rates were 88.5% in the 2 L group and 82.8% in the 1.5 L group (95% lower confidence limit, lower confidence limit (LCL), for the difference = -14.5%, non-inferiority P = 0.019) in the right side colon. In the left colon, the adequate bowel preparation rates were 89.3% in the 2 L group and 81.1% in the 1.5 L group (95% LCL = -17.0%, non-inferiority P = 0.066). In the right side colon, there were significant differences in the proportion of the overall colon-cleansing score between the two groups (P = 0.006). Eleven patients (8.7%) required additional preparation in the 2 L group. On the other hand, 18 patients (14.3%) required additional preparation in the 1.5 L group. However, there was no significant difference in required additional preparation between the two groups.
| Variable | Right side colon | Left side colon and rectum | P value1 | |||
| 2.0 L | 1.5 L | 2.0 L | 1.5 L | Right | Left | |
| Patients (n) | 122 | 122 | 122 | 122 | ||
| Overall score (n) | ||||||
| Excellent | 36 | 22 | 48 | 37 | 0.006 | NS |
| Good | 52 | 38 | 49 | 45 | ||
| Fair | 20 | 41 | 12 | 17 | ||
| Poor | 3 | 3 | 2 | 5 | ||
| Inadequate | 11 | 18 | 11 | 18 | ||
| No. adequate | 108 (88.5) | 101 (82.8) | 109 (89.3) | 99 (81.1) | NS | NS |
As shown in Table 3, there were no differences in frequency of defecation, time needed to reach the cecum, elapsed time from last fluid intake to colonoscopy, amount of irrigation fluid used, and subjective difficulties in insertion to the cecum and in observing the lumen of the colo-rectum between the two groups.
| Variable | 2.0 L | 1.5 L | P value |
| Patients | 126 | 126 | |
| Time to first defecation (min, mean ± SD) | 55.7 ± 27.4 | 56.4 ± 27.8 | NS |
| Frequency of defecation (times, median, quartile) | 7 (4-15) | 7 (4-15) | NS |
| Time to preparation (min, mean ± SD) | 157.3 ± 51.9 | 159.6 ± 57.1 | NS |
| Elapsed time from last fluid intake to colonoscopy (min, mean ± SD) | 169.4 ± 56.5 | 179.7 ± 61.1 | NS |
| Cecal intubation rate | 122 (96.8) | 122 (96.8) | NS |
| Insertion time (min, median, quartile)1 | 11.4 (3-76) | 10.1 (3-47) | NS |
| Feel of peristalsis | 20 (16.4) | 25 (20.5) | NS |
| Amount of irrigation fluid | |||
| None | 38 | 41 | NS |
| < 50 mL | 74 | 74 | |
| 50-100 mL | 9 | 9 | |
| > 100 mL | 5 | 2 | |
| Compliance > 80% | 121 (96.0) | 125 (99.2) | NS |
| 100% intake | 108 (85.7) | 122 (96.8) | 0.002 |
| Any symptom | |||
| Nausea (none/mild/moderate/severe) | 109/13/3/1 | 117/5/3/1 | NS |
| Vomiting (none/mild/moderate/severe) | 0/0/1/0 | 0/0/0/0 | NS |
| Distension (none/mild/moderate/severe) | 36/65/22/3 | 58/48/18/2 | 0.04 |
| Abdominal pain (none/mild/moderate/severe) | 98/26/2/0 | 107/18/1/0 | NS |
| Circulatory reactions (none/mild/moderate/severe) | 0 | 0 | NS |
| Willingness to repeat | |||
| The same preparation regimen (much/fair/never) | 78/19/29 | 97/12/17 | 0.034 |
| How easy/difficult to take preparation compared to previous | |||
| (easy/no difference/difficult) | 23/54/5 | 46/36/3 | 0.001 |
There was no significant difference in compliance as defined by > 80% intake of the prescribed PEG-electrolyte solution volume. However, complete (100%) intake of the PEG solution was significantly higher in the 1.5 L group than in the 2 L group (P = 0.002) (Table 3). The proportion of abdominal distension was significantly less in the 1.5 L group than in the 2 L group (P = 0.040), but symptoms such as nausea, vomiting, abdominal pain, and circulatory reactions were similar in both groups. The proportion of patients willing to repeat the same preparation regimen was significantly higher in the 1.5 L group (P = 0.034). Furthermore, among the subgroup of patients who had undergone colonoscopy more than twice previously, a significantly higher number of patients in the 1.5 L group than in the 2 L group felt that the current preparation was easier than in the past (P = 0.001).
In this study, 11 colorectal cancers were detected in 11 patients (4.4%), 4 (3.2%) in the 2 L group and 7 (5.6%) in the 1.5 L group (Table 4). A total of 177 polyps was detected in 74 patients (58.7%) in the 2 L group, and 187 polyps were detected in 73 patients (57.9%) in the 1.5 L group. The proportions of polyps by size and location were similar in the two groups.
| Variable | 2.0 L | 1.5 L | P value (2.0 L vs 1.5 L) | |||
| Right side colon | Left side colon | Right side colon | Left side colon | Right | Left | |
| Patients | 126 | 126 | ||||
| Cancer patients | 3 | 1 | 5 | 21 | NS | NS |
| Polyp patients | 74 (58.7) | 73 (57.9) | ||||
| Proportion of polyps | ||||||
| < 5 mm | 60 | 62 | 65 | 70 | NS | NS |
| 5-10 mm | 15 | 29 | 20 | 19 | ||
| > 10 mm | 6 | 5 | 8 | 5 | ||
| Total polyps per study arm | 81 | 96 | 93 | 94 | NS | NS |
| Polyps per patient, mean ± SD | 0.71 ± 1.19 | 0.79 ± 1.20 | 0.78 ± 1.19 | 0.78 ± 1.11 | NS | NS |
| Diverticulosis | 30 | 37 | ||||
In this study, 1.5 L PEG-electrolyte solution plus mosapride was found to be non-inferior to 2 L PEG-electrolyte solution plus mosapride with respect to adequate bowel preparation rates only in the right colon, not in the entire colo-rectum. On the other hand, patient tolerability, especially abdominal distension, and acceptability were superior in the 1.5 L group compared to the 2 L group.
This is the first study, to the best of our knowledge, that has evaluated the effect of mosapride when used in conjunction with reduced dose, 1.5 L PEG-electrolyte solution for colonoscopy preparation. We previously conducted a randomized, double-blind, placebo-controlled study with mosapride in addition to PEG-electrolyte solution and demonstrated that co-administration of mosapride with PEG-electrolyte solution improved the quality of bowel preparation for colonoscopy, especially in patients without severe constipation[20]. On the other hand, the beneficial effect of mosapride on gastric emptying[22] was expected to ameliorate nausea, vomiting, and fullness of the abdomen during bowel preparation. Mishima et al[19] showed that administration of mosapride prior to PEG-electrolyte solution significantly decreased the incidence of uncomfortable abdominal symptoms. However, there were no significant differences in the frequencies of these symptoms between the mosapride group and the placebo group in the previous study[20]. Therefore, we think that there is a need to reduce the volume of PEG-electrolyte solution to improve patients’ acceptability and tolerability. In the present study, it was assumed that 2 L PEG-electrolyte solution plus mosapride was the standard regimen for bowel preparation based on the results of the previous study. Thus, the study was designed to compare a 1.5 L PEG group with a 2 L PEG group.
In the present study, the patients’ acceptability and tolerability were superior in the 1.5 L group. The 0.5 L reduction of PEG-electrolyte solution significantly improved patients’ acceptability and tolerability; 100% intake of PEG-electrolyte solution was significantly higher in the 1.5 L group than in the 2 L group. For Japanese patients with relatively smaller physiques than Western patients, 2 L PEG-electrolyte solution may be too much to drink. With respect to adverse events, abdominal distension was more common than nausea, vomiting, and abdominal pain. The proportion of abdominal distension was significantly improved in the 1.5 L group compared with the 2 L group. This may be the reason why willingness to repeat the same preparation regimen was significantly higher in the 1.5 L group, and a significantly higher number of patients in the 1.5 L group than in the 2 L group felt that the current preparation was easier.
Although 0.5-L volume reduction improved patient acceptability and tolerability, it would not make sense to decrease the volume of solution if the adequate bowel preparation rates were worse, and it prolonged the time to preparation. In the present study, 1.5 L PEG was non-inferior to 2 L PEG with respect to adequate bowel preparation rates in the right colon, but the proportion for the overall colon-cleansing score was significantly higher in the 2 L PEG group than in the 1.5 L PEG group. Furthermore, although there was no significant difference between the two groups, 18 patients required additional preparation in the 1.5 L group compared with 11 patients in the 2 L group. One of the reasons why the times to preparation were similar in the two groups is that it took longer for the patients who required additional preparation in the 1.5 L group compared with the 2 L group. From these results, we cannot help but recognize that the dose of 15 mg may be insufficient to compensate for the 0.5-L reduction in PEG solution with respect to cleansing efficacy. In the present study, 15 mg of mosapride was given for colonoscopy preparation. The dose of 15 mg per day is the recommended usual dosage of mosapride citrate for adult patients with chronic gastritis. Since the effects of mosapride are reported to be dose-dependent[23], additional studies that address the optimal dosage are required to clarify the best bowel preparation method for colonoscopy.
Over the years, many researchers have investigated several different combinations and dosages of prokinetic agent or laxatives in search of acceptable, tolerable, and efficacious low-volume bowel preparation that may lead to a better experience for the patient and a more thorough colonoscopic examination[24-26]. Cisapride has been used as a prokinetic agent along with lavage solution for bowel preparation and has been demonstrated to shorten the required time period for precolonoscopic bowel preparation and to decrease the lavage solution volume[27,28], although these results have been difficult to reproduce[29]. However, cisapride was withdrawn from the market because of severe cardiac effects[30]. Other prokinetic agents, including metoclopramide and tegaserod, have been co-administered with oral lavage solution in an attempt to improve the quality of bowel preparation and patient tolerance to lavage solution through increasing the amplitude of gastric contraction and peristalsis of small intestine, and shortening transit time[31,32]. However, the effect of these agents had not yet been clearly established, and the results of studies that have evaluated these agents have thus far been contradictory[33]. The effects of prokinetic agents with the reported timings and doses may not be enough to compensate for the large volume of PEG solution.
Stimulant laxatives such as bisacodyl and magnesium citrate have been used as adjuncts to low-volume PEG-electrolyte solution, achieving results similar to those with full-volume PEG-electrolyte solution[8,34]. Recently, Cohen et al[35] compared a reduced-dose 2 L PEG formulation plus ascorbic acid with 2 L PEG formulation plus bisacodyl. The authors found that the use of PEG plus ascorbic acid resulted in better colon cleansing and higher adenoma detection rates than PEG plus bisacodyl. Moreover, Repici et al[36] compared a new iso-osmotic sulphate-free formulation (2 L formulation of PEG-citrate-simethicone) in combination with bisacodyl with 2 L formulation PEG plus ascorbic acid. The authors reported that low-volume PEG-citrate-simethicone with bisacodyl provided better bowel cleansing and similar tolerability and acceptance compared with PEG plus ascorbic acid. Unfortunately, these low-volume formulations are currently not available in Japan. In the previous study[20], we selected mosapride from among several prokinetic agents because it is a highly selective agonist for 5-HT4 receptors and does not affect other receptors, including dopamine D2 receptors. However, the results of this study did not demonstrate the efficacy of mosapride in reducing lavage solution volume. A combination with some laxatives may improve the cleansing efficacy of our low-volume 1.5 L PEG formulation plus mosapride.
Few studies designed to assess the quality of bowel preparation for colonoscopy have also examined the disease detection rates, including adenoma detection rates[37-39]. Previous studies demonstrated that a better bowel preparation led to a higher rate of colon lesion detection, enhancing the ability to discern smaller lesions and thus improving the thoroughness of colonoscopy[37,38]. In the present study, there were no differences between the two groups in the polyp detection rate, the proportion of the size of polyps, total polyps per study arm, and polyps per patient. These findings may lead to the conclusion that the efficacy of bowel preparation with 1.5 L PEG is non-inferior to 2 L PEG with respect to bowel cleansing. However, polyp detection rates are indeed affected by several variables, such as patients’ background, colonoscopy indication, and endoscopist technique, as well as endoscopy technology, that would introduce uncertainly into the results of this study. Additional studies are necessary to demonstrate the relationship between bowel preparation and the adenoma detection rate.
There are several limitations to consider in interpreting the present results. First, the study was conducted in a single hospital with a small number of patients. Although we hypothesized that the non-inferiority margin was -15%, that margin might be inappropriate. The sample size may have been too small to elucidate the non-inferiority of 1.5 L PEG, which may explain why non-inferiority in only a limited part of colon could be demonstrated. Second, for the evaluation of bowel preparation, the Aronchick scale was used. The merit of the Aronchick scale is that in cases in which additional bowel preparation was needed, such cases could be defined as “inadequate” using the Aronchick scale. However, the Aronchick scale was designed to assess bowel preparation of the entire colon. In the present study, it was scored separately on the right side colon and left side colon; a different scoring system, such as the Ottawa scale[40] and the Boston scale[41], that evaluates different colon segments individually and generates a summary score may have been more appropriate. Finally, biochemical parameters were not evaluated in the two groups. However, co-administration of mosapride at a dose of 40 mg and PEG-electrolyte solution is already approved in Japan for barium enema examination preparation[42], based on its excellent safety profile.
In conclusion, co-administration of mosapride with 1.5 L PEG-electrolyte solution was non-inferior to mosapride with 2.0 L PEG-electrolyte solution for adequate bowel preparation rates only in the right colon, although better acceptability and tolerability compared to the larger PEG-electrolyte solution volumes were found. A mosapride dose of 15 mg may provide insufficient cleansing efficacy to compensate for a 0.5-L reduction in PEG-electrolyte solution.
Although polyethylene glycol (PEG) electrolyte solution has been used for colonoscopy preparation since 1980, the need to drink large volumes is a limiting factor that affects patient tolerance. Low-volume bowel preparation regimens for colonoscopy are reported to improve patients’ acceptance and compliance.
Mosapride citrate (mosapride) is a selective 5-hydroxytryptamine 4 (5-HT4) receptor agonist. Mosapride enhances gastric emptying and motility by facilitating acetylcholine release from enteric cholinergic neurons, without blocking the dopaminergic D2 receptors. Since 5-HT4 receptors are also located in the human colon and rectum, mosapride is also expected to have a prokinetic effect on the colo-rectum.
The present randomized trial compared 1.5 L PEG plus mosapride with 2 L PEG plus mosapride dosing for patients who underwent colonoscopy in terms of cleansing effectiveness, patient compliance, tolerability, and disease detection rates.
The low-volume preparation (1.5 L PEG) represents a valid alternative to high-volume preparations (2 L PEG) with regard to patient compliance and tolerability. However, optimal visualization of colonic wall seems to be one of the primary advantages of high-volume preparations. A mosapride dose of 15 mg may provide insufficient cleansing efficacy to compensate for a 0.5-L reduction in PEG solution.
PEG-electrolyte solution: PEG-electrolyte solution is used worldwide for bowel cleansing. Approximately 2 L of this oral solution with some laxatives are usually required for adequate bowel preparation in Japan.
This randomized trial compared 1.5 L PEG plus mosapride with 2 L PEG plus mosapride in terms of cleansing effectiveness, patient acceptability, physical tolerability, and endoscopic findings. This is an interesting and well written study. The conclusion sounds good and useful for the general practice.
P- Reviewers Bechtold ML, Martinek J, Castro FJ S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63:894-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 2. | Young CJ, Simpson RR, King DW, Lubowski DZ. Oral sodium phosphate solution is a superior colonoscopy preparation to polyethylene glycol with bisacodyl. Dis Colon Rectum. 2000;43:1568-1571. [PubMed] |
| 3. | Lee J, McCallion K, Acheson AG, Irwin ST. A prospective randomised study comparing polyethylene glycol and sodium phosphate bowel cleansing solutions for colonoscopy. Ulster Med J. 1999;68:68-72. [PubMed] |
| 4. | Hsu CW, Imperiale TF. Meta-analysis and cost comparison of polyethylene glycol lavage versus sodium phosphate for colonoscopy preparation. Gastrointest Endosc. 1998;48:276-282. [PubMed] |
| 5. | Verghese VJ, Ayub K, Qureshi W, Taupo T, Graham DY. Low-salt bowel cleansing preparation (LoSo Prep) as preparation for colonoscopy: a pilot study. Aliment Pharmacol Ther. 2002;16:1327-1331. [PubMed] |
| 6. | DiPalma JA, Buckley SE, Warner BA, Culpepper RM. Biochemical effects of oral sodium phosphate. Dig Dis Sci. 1996;41:749-753. [PubMed] |
| 7. | Sharma VK, Schaberg JW, Chockalingam SK, Vasudeva R, Howden CW. The effect of stimulant laxatives and polyethylene glycol-electrolyte lavage solution for colonoscopy preparation on serum electrolytes and hemodynamics. J Clin Gastroenterol. 2001;32:238-239. [PubMed] |
| 8. | Belsey J, Epstein O, Heresbach D. Systematic review: adverse event reports for oral sodium phosphate and polyethylene glycol. Aliment Pharmacol Ther. 2009;29:15-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007;39:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Gili M, Roca M, Ferrer V, Obrador A, Cabeza E. Psychosocial factors associated with the adherence to a colorectal cancer screening program. Cancer Detect Prev. 2006;30:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Harewood GC, Wiersema MJ, Melton LJ. A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol. 2002;97:3186-3194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 241] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Yoshida N, Omoya H, Oka M, Furukawa K, Ito T, Karasawa T. AS-4370, a novel gastrokinetic agent free of dopamine D2 receptor antagonist properties. Arch Int Pharmacodyn Ther. 1989;300:51-67. [PubMed] |
| 13. | Ruth M, Finizia C, Cange L, Lundell L. The effect of mosapride on oesophageal motor function and acid reflux in patients with gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2003;15:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Mizuta Y, Shikuwa S, Isomoto H, Mishima R, Akazawa Y, Masuda J, Omagari K, Takeshima F, Kohno S. Recent insights into digestive motility in functional dyspepsia. J Gastroenterol. 2006;41:1025-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Asakawa H, Hayashi I, Fukui T, Tokunaga K. Effect of mosapride on glycemic control and gastric emptying in type 2 diabetes mellitus patients with gastropathy. Diabetes Res Clin Pract. 2003;61:175-182. [PubMed] |
| 16. | McLean PG, Coupar IM. Stimulation of cyclic AMP formation in the circular smooth muscle of human colon by activation of 5-HT4-like receptors. Br J Pharmacol. 1996;117:238-239. [PubMed] |
| 17. | Sakurai-Yamashita Y, Yamashita K, Kanematsu T, Taniyama K. Localization of the 5-HT(4) receptor in the human and the guinea pig colon. Eur J Pharmacol. 1999;383:281-285. [PubMed] |
| 18. | Nakashima M, Okumura S, Iizuka H, Ohmae Y, Sagawa T, Kudo T, Masuo T, Kobayashi R, Marubashi K, Ishikawa T. Mosapride Citrate for Colonoscopy Preparation with Lavage. Kitakanto Med J. 2002;52:111-115. |
| 19. | Mishima Y, Amano Y, Okita K, Takahashi Y, Moriyama N, Ishimura N, Furuta K, Ishihara S, Adachi K, Kinoshita Y. Efficacy of prokinetic agents in improving bowel preparation for colonoscopy. Digestion. 2008;77:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Tajika M, Niwa Y, Bhatia V, Kawai H, Kondo S, Sawaki A, Mizuno N, Hara K, Hijioka S, Matsumoto K. Efficacy of mosapride citrate with polyethylene glycol solution for colonoscopy preparation. World J Gastroenterol. 2012;18:2517-2525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 318] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | Kanaizumi T, Nakano H, Matsui Y, Ishikawa H, Shimizu R, Park S, Kuriya N. Prokinetic effect of AS-4370 on gastric emptying in healthy adults. Eur J Clin Pharmacol. 1991;41:335-337. [PubMed] |
| 23. | Mine Y, Morikage K, Oku S, Yoshikawa T, Shimizu I, Yoshida N. Effect of mosapride citrate hydrate on the colon cleansing action of polyethylene glycol electrolyte lavage solution (PEG-ELS) in guinea pigs. J Pharmacol Sci. 2009;110:415-423. [PubMed] |
| 24. | Sharma VK, Chockalingham SK, Ugheoke EA, Kapur A, Ling PH, Vasudeva R, Howden CW. Prospective, randomized, controlled comparison of the use of polyethylene glycol electrolyte lavage solution in four-liter versus two-liter volumes and pretreatment with either magnesium citrate or bisacodyl for colonoscopy preparation. Gastrointest Endosc. 1998;47:167-171. [PubMed] |
| 25. | Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum. 1994;37:229-233; discussion 233-234. [PubMed] |
| 26. | DiPalma JA, Wolff BG, Meagher A, Cleveland Mv. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol. 2003;98:2187-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 27. | Lazarczyk DA, Stein AD, Courval JM, Desai D. Controlled study of cisapride-assisted lavage preparatory to colonoscopy. Gastrointest Endosc. 1998;48:44-48. [PubMed] |
| 28. | Ueda S, Iishi H, Tatsuta M, Oda K, Osaka S. Addition of cisapride shortens colonoscopy preparation with lavage in elderly patients. Aliment Pharmacol Ther. 1994;8:209-214. [PubMed] |
| 29. | Martínek J, Hess J, Delarive J, Jornod P, Blum A, Pantoflickova D, Fischer M, Dorta G. Cisapride does not improve precolonoscopy bowel preparation with either sodium phosphate or polyethylene glycol electrolyte lavage. Gastrointest Endosc. 2001;54:180-185. [PubMed] |
| 30. | Tonini M, De Ponti F, Di Nucci A, Crema F. Review article: cardiac adverse effects of gastrointestinal prokinetics. Aliment Pharmacol Ther. 1999;13:1585-1591. [PubMed] |
| 31. | Rhodes JB, Engstrom J, Stone KF. Metoclopramide reduces the distress associated with colon cleansing by an oral electrolyte overload. Gastrointest Endosc. 1978;24:162-163. [PubMed] |
| 32. | Sanaka MR, Super DM, Mullen KD, Ferguson DR, McCullough AJ. Use of tegaserod along with polyethylene glycol electrolyte solution for colonoscopy bowel preparation: a prospective, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2006;23:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Brady CE, DiPalma JA, Pierson WP. Golytely lavage--is metoclopramide necessary? Am J Gastroenterol. 1985;80:180-184. [PubMed] |
| 34. | Ell C, Fischbach W, Bronisch HJ, Dertinger S, Layer P, Rünzi M, Schneider T, Kachel G, Grüger J, Köllinger M. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol. 2008;103:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 35. | Cohen LB, Sanyal SM, Von Althann C, Bodian C, Whitson M, Bamji N, Miller KM, Mavronicolas W, Burd S, Freedman J. Clinical trial: 2-L polyethylene glycol-based lavage solutions for colonoscopy preparation - a randomized, single-blind study of two formulations. Aliment Pharmacol Ther. 2010;32:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Repici A, Cestari R, Annese V, Biscaglia G, Vitetta E, Minelli L, Trallori G, Orselli S, Andriulli A, Hassan C. Randomised clinical trial: low-volume bowel preparation for colonoscopy - a comparison between two different PEG-based formulations. Aliment Pharmacol Ther. 2012;36:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 559] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 38. | Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378-384. [PubMed] |
| 39. | Chiu HM, Lin JT, Wang HP, Lee YC, Wu MS. The impact of colon preparation timing on colonoscopic detection of colorectal neoplasms--a prospective endoscopist-blinded randomized trial. Am J Gastroenterol. 2006;101:2719-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482-486. [PubMed] |
| 41. | Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 930] [Cited by in RCA: 924] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 42. | Futei S, Sugino Y, Kuribayashi S, Imai Y, Ueno F, Hibi T, Mitsushima T. [New preparation method for barium enema: efficacy and administration of oral intestinal lavage solution with gastrointestinal prokinetic agent]. Nihon Igaku Hoshasen Gakkai Zasshi. 2004;64:22-30. [PubMed] |