Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.721
Revised: October 25, 2012
Accepted: November 14, 2012
Published online: February 7, 2013
Processing time: 164 Days and 15.9 Hours
AIM: To evaluate the long-term treatment outcomes of entecavir monotherapy in treatment naive patients in an Australian tertiary care setting.
METHODS: A retrospective analysis of treatment naive patients receiving entecavir monotherapy through Westmead Hospital was performed. Patients were excluded if they had received previous treatment with another nucleoside or nucleotide analogue, were pregnant or less than 18 years old.
RESULTS: Out of 336 patients, 163 patients fulfilled the selection criteria. Range of follow up was 3-46 mo (mean 26 mo). 134 patients (82.2%) had pre-treatment biopsies, with 26 patients (16.0 %) demonstrating F3-4 fibrosis. In total, 153 patients (93.9%) achieved at least Partial Virological Suppression (PVS), with 134 patients (82.2%) achieving complete virological suppression. The cumulative CVS and PVS rates at 36 mo were 92.2% and 97.3%, respectively. 3 patients (1.8%) failed to achieve PVS, while 5 patients (3.0%) developed virological rebound. 128 patients (78.5%) maintained CVS throughout follow up. Predictors of CVS included lower baseline DNA level (P = 0.001), hepatitis B virus e antigen negative status (P = 0.001) and increasing age at treatment (log rank 0.001). No significant adverse effects were reported necessitating cessation of entecavir.
CONCLUSION: Entecavir monotherapy is efficacious and safe in an Australian tertiary care setting. Resistance and rebound rates are very low. This is similar to data from controlled and uncontrolled trials around the world.
- Citation: Fahrtash-Bahin F, Kariyawasam VC, Gray T, Byth K, George J, Douglas MW. Australian tertiary care outcomes of entecavir monotherapy in treatment naive patients with chronic hepatitis B. World J Gastroenterol 2013; 19(5): 721-726
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/721.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.721
An estimated 350 to 400 million people worldwide have chronic hepatitis B virus (HBV) infection, a leading cause of morbidity and mortality. Australia has an ethnically diverse population, with many migrants from the Asia-Pacific region, where the majority of hepatitis B related deaths occur[1].
The number of people living with chronic hepatitis B in Australia is estimated to be between 153 000 and 175 000, a prevalence of 0.7% to 0.8%[2,3]. In 2010, 228 incident cases of hepatitis B and 6878 notifications of hepatitis B were reported to the Australian government’s National Notifiable Diseases Surveillance System[4]. This reflects the major burden of disease in people with childhood acquired chronic hepatitis B.
In chronic HBV infection the goals of antiviral therapy are to suppress HBV DNA and reduce hepatic inflammation [alanine aminotransferase (ALT)], with the aim of preventing progression of liver fibrosis and achieving immune control [hepatitis B e antigen (HBeAg) and/or hepatitis B surface antigen (HBsAg) loss/conversion]. This reduces mortality due to cirrhosis, liver failure and HCC. Antiviral agents available include pegylated interferon, lamivudine, adefovir, entacavir, telbivudine and tenofovir.
Entecavir is an oral deoxyguanosine analog with potent activity against HBV[5]. In multiple clinical trials, entacavir has been found to be highly efficacious in treating nucleoside naive and lamividine refractory patients[6-8]. Emergence of drug resistance during antiviral therapy is well described for HBV, particularly with lamivudine, with resistance appearing in 20% of patients after 1 year and 70% after 5 years[9]. Entecavir has a higher genetic barrier to resistance than lamivudine, with quoted resistance rates of only 1%-2% in treatment naive patients after 5 years[10]. However, cross-resistance with lamivudine is a problem, and in patients who have failed lamivudine, entecavir resistance appears in up to 8% after 5 years[10,11].
The rates of entecavir response and resistance in Australia are unknown. Most published data are from large clinical trials, which do not necessarily reflect the rates seen in “real world” clinical practice. These trials were almost exclusively performed in the Northern Hemisphere, and do not reflect the unique, diverse migrant population seen in Australia. Furthermore, much of this data is based on a 1 mg dose of entecavir, rather than 0.5 mg now recommended for treatment naive patients.
The aim of this study was to evaluate the efficacy, rates of viral resistance and treatment outcomes of entacavir monotherapy in an Australian tertiary referral centre, outside of a clinical trial environment.
Data was entered retrospectively into a database incorporating all patients receiving entecavir through the Westmead Hospital pharmacy (Sydney, Australia) between 1 November 2006 and 31 July 2010.
All patients receiving entecavir through the Westmead Hospital pharmacy were considered for analysis. Patients were included if they met the following criteria: (1) eligible for entecavir 0.5 mg daily according to AASLD guidelines; (2) HBV infection for greater than 6 mo based on serology; and (3) deranged liver function tests or at least mild fibrosis on liver biopsy (> F2 on Scheuer classification).
Exclusion criteria were: (1) previous treatment with other nucleoside analogues, nucleotide analogues or trial medication (except for interferon); (2) pregnancy; (3) age less than 18 years; (4) hepatitis delta co-infection; and (5) Prophylactic entecavir during immunosuppression (e.g., chemotherapy).
Viral load was measured in international units/mL, using the Cobas Taqman assay (12 IU/mL) (Roche Diagnostics, Branchburg, NJ).
Complete and Partial Virologic Suppression were defined as HBV DNA < 12 IU/mL and 12-2000 IU/mL, respectively. Virologic rebound was defined as an increase in viral load > 1 log10 from the previous value, in a patient with initial virologic suppression[12]. Cumulative rates of suppression were calculated by the formula P = 1 - (1 - n1⁄N1) (1- n2⁄N2). . .(1 - nx⁄Nx), where P is the cumulative probability that the event will occur, nx is the number of cases at year x, and Nx is the number of patients still followed up at year x[13].
The Scheuer classification system for grading and staging of chronic hepatitis was used for patients that underwent liver biopsy.
All statistical analyses were performed using SPSS (version 16, Chicago IL). The study was approved by the Westmead Hospital Human Research Ethics Committee.
A total of 336 patients were included in the database, which collected data from 2006 to 2010. 89 patients were excluded due to past or current treatment with another agent. A further 83 patients were excluded as they received treatment for less than 3 mo, were less than 18 years old, received prophylactic entecavir, had hepatitis delta co-infection or were pregnant. Thus, 163 patients were included for analysis. The patient demographics are summarised in Table 1.
| Age (yr) | |
| Mean age | 52.0 (24-86) |
| Mean age at start | 47.4 (20-81) |
| Gender | |
| Males | 113 (69.3) |
| Females | 50 (30.7) |
| Pre-treatment Biopsies | 134 (82.2) |
| Duration of therapy (mo) | |
| Range | 3-46 |
| Mean | 25.63 |
| Median | 23 |
| Patients > 24 mo | 79 (48.5) |
| Patients > 36 mo | 49 (30.1) |
| Co-infection | |
| Hepatitis C | 1 (0.61) |
| HBV DNA load (IU/mL) | |
| > 100 000 | 95 (58.3) |
| 2000-100 000 | 45 (27.6) |
| 12-2000 | 23 (14.1) |
| HBe antigen status | 41 (25.2) |
| e antigen positive | 112 (68.7) |
| e antigen negative | 10 (6.1) |
| Unknown | |
| ALT (U/L) | |
| 10-40 | 39 (23.9) |
| 40-400 | 118 (72.4) |
| > 400 | 5 (3.01) |
| Unknown | 1 (0.61) |
| Fibrosis score | 0-10 (6.13) |
| 135 (82.82%) | 1-61 (37. 4) |
| 2-38 (23.3) | |
| 3-17 (10.4) | |
| 4-9 (5.52) | |
| Unknown-27 (16.6) |
All patients were reviewed at least four-monthly by one of six hepatologists at Westmead Hospital Liver Clinic, with assessment of HBV DNA, viral serology, liver function tests and routine haematological and renal laboratory evaluation. Relevant 6-monthly HCC surveillance was also performed, with serum alpha-fetoprotein and hepatic ultrasound.
The mean and median duration of follow up were 26 and 23 mo, respectively. The range of follow up was 3-46 mo, with 50% of patients followed for greater than 24 mo.
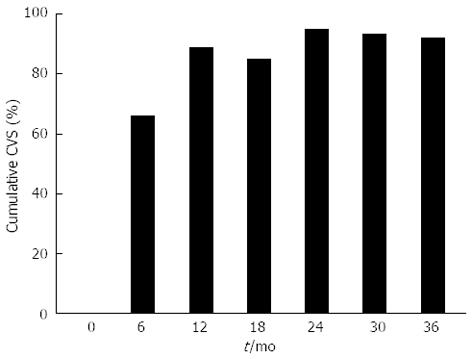
The response to entecavir treatment over time is detailed in Table 2, with a graphical summary of virological suppression in Figure 1. In total, 153 patients (93.9%) achieved partial virological suppression (PVS), with 134 patients (82.2%) achieving complete virological suppression (CVS). The cumulative CVS and PVS rates at 36 mo were 92.2% and 97.3%, respectively. Three patients (1.84%) failed to achieve partial or complete suppression (mean duration of therapy 8 mo). Five patients (3.01%) developed virological rebound (mean duration of therapy 29 mo, 3 females, 4 eAg negative, median fibrosis score 1 (biopsies in 4 patients), mean baseline DNA 9.71 × 106 IU/mL). Four of the 5 patients that developed virological rebound had achieved CVS. Reasons for rebound included non-compliance in 4 patients and suspected entecavir resistance in 1 patient. Specific viral resistance testing was not performed. Four patients (2.45%) were changed to another agent due to failed virologic suppression or virologic rebound. Of these, 3 patients (1.84%) were changed to tenofovir and 1 patient (0.61%) to adefovir, with CVS and PVS achieved in 2 patients each, respectively.
| Month | 0 | 6 | 12 | 18 | 24 | 30 | 36 | 42 |
| Patients with DNA | 163 (100) | 158 (96.9) | 141 (86.5) | 121 (74.2) | 105 (64.4) | 76 (46.6) | 56 (34.4) | 24 (14.7) |
| Complete virological suppression | 0/169 (0) | 104/158 (65.8) | 117/141 (83.0 ) | 96/121 (74.4) | 94/105 (89.5) | 65/76 (85.5) | 46/56 (82.1) | 19/24 (79.2) |
| Cumulative CVS | 0% | 66.7% | 88.8% | 85.1% | 94.8% | 93.2% | 92.2% | 91.6% |
| Partial virological suppression | 22/163 (13.5) | 141/158 (89.2) | 133/141 (94.3) | 117/121 (90.7) | 100/105 (95.2) | 75/76 (98.7) | 54/56 (96.4) | 23/24 (95.8) |
| Cumulative PVS | 13.5% | 89.2% | 94.9% | 92.1% | 96.3% | 99.0% | 97.3% | 96.6% |
One hundred and thirty three patients (94.3%) maintained CVS throughout follow up. One hundred and twenty nine patients (79.14%) achieved PVS at 12 mo. The annual HBeAg positive to negative seroconversion rate was 14.3%. One patient (HBeAg negative) had loss of surface antigen, resulting in an annual surface antigen loss rate of 0.3% for HBeAg negative patients and 0.2% for all patients, respectively.
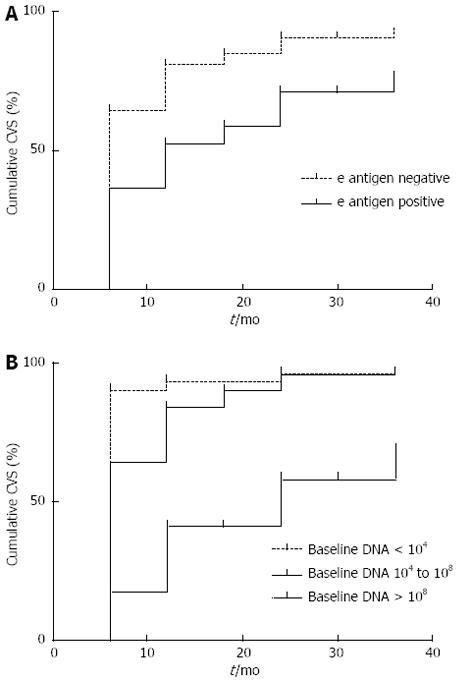
The time to achieve CVS was related to baseline DNA levels (P = 0.001) and HBeAg status at baseline (P = 0.001) (Figure 2).
The mean and median times to CVS were 11.4 (9.77-13.09) and 6 (5.32-6.69) mo, respectively. For HBeAg negative patients the mean time was 6 mo (5.35-6.65), while for HBeAg positive patients it was 12 mo (3.42-20.58) (log rank < 0.001).
There was a statistically significant difference in median time taken to achieve CVS based on age and HBeAg status (Table 3). There was no significant difference in loss of HBeAg based on age (log rank 0.559).
| Age-log rank 0.001 (median) | ||
| < 40 yr- | 40-55 yr- | > 55 yr- |
| 12 mo (9.25-14.75) | 6 mo (5.01-6.99) | 6 mo (5.24-6.76) |
| Hepatitis B e antigen negative status-log rank 0.19 (median) | ||
| < 40 yr- | 40-55 yr- | > 55 yr- |
| 6 mo (4.05-7.95) | 6 mo (4.99-7.02) | 6 mo (5.59-6.41) |
| Hepatitis B e antigen positive status-log rank 0.19 (median) | ||
| < 40 yr- | 40-55 yr- | > 55 yr- |
| 18 mo (7.37-28.63) | 18 mo (14.17-26.47) | 6 mo (6.5-17.5) |
| Hepatitis B e antigen seroconversion rate based on age-log rank 0.559 (median) | ||
| < 40 yr- | 40-55 yr- | > 55 yr- |
| 36 mo (20.55-51.45) | 30 mo (18.46-41.54) | 6 mo (9.8-32.2) |
There was no significant effect of duration of therapy, fibrosis score or gender on time to CVS, HBeAg conversion or ALT normalisation. There was a trend towards faster ALT normalisation based on increased age (log rank 0.053).
Among the 163 patients in the study, 4 patients (2.45%) developed HCC (mean time to development 19.9 mo), while in 6 (3.68%) cases the diagnosis preceded commencement of therapy. Two patients with HCC had a pre-treatment biopsy which did not show cirrhosis (F2 and F3, respectively).
Twenty one patients (12.88%) failed to follow up in clinic during the study period and thus did not receive ongoing entecavir through the hospital pharmacy. There were no reports of entecavir being ceased due to adverse effects.
This study gives an important insight into outcomes of entecavir monotherapy for treatment naive patients with chronic hepatitis B in an Australian tertiary referral setting, with up to 3.5 years follow-up. To our knowledge it is the first study to describe the efficacy, tolerability and resistance rates of entecavir in treatment naive patients in the Australian tertiary care setting. Daily monotherapy with 0.5 mg entecavir was efficacious, with 94% of patients achieving at least partial suppression, and 82% achieving undetectable HBV DNA levels. Only 2% of patients failed to reach any significant reduction in viral load, while 3% developed virologic rebound. Viral resistance testing was not performed. No significant adverse events were reported, and treatment discontinuation was not due to adverse events.
These results are consistent with earlier registration trials, which demonstrated suppression of HBV-DNA to undetectable levels in 67% of HBeAg-positive patients after 12 mo, and in 90% of HBeAg-negative patients[7,8]. Subsequent studies confirmed durable and increasing viral suppression on entecavir, with undetectable HBV DNA achieved by 94% of HBeAg-positive patients over 5 years of treatment, and in 95% of HBeAg-negative patients over 3 years of treatment[14,15]. Clinical trials have also demonstrated minimal emergence of viral resistance (1.2%), after up to 6 years of entecavir treatment[10,16].
Pol et al[17] analysed the efficacy and tolerability of entecavir in routine clinical practice settings, by comparing data from 5 international cohorts. In these studies CVS was achieved in 76%-96% of patients, with virologic rebound in 0.6%-4%, and excellent overall tolerability and safety. Therefore the findings from our study are consistent with international data from other “real life” clinical settings, as well as earlier registration trials, which applied more stringent selection criteria.
As has been shown in previous studies, we found that the likelihood of achieving CVS was increased in patients with lower baseline DNA levels and e antigen negative status[18,19]. Amongst patients with baseline HBV DNA > 108 the CVS was only 67.5% compared to 92% for patients with baseline HBV DNA < 104. This is most likely explained by the high proportion of eAg positive cases (82%) in the high baseline DNA group compared to the low baseline DNA group (10.5%). The time to achieve CVS was also significantly related to these factors. An interesting and novel finding was that the time taken to achieve CVS was significantly related to age. In patients older than 40 years old the median time to achieve CVS was 6 mo, whereas in patients younger than 40 years old it was 12 mo. This relationship was maintained following HBeAg status stratification, and may reflect better compliance in the older population groups.
A strength of this study is the high proportion of patients in whom pre-treatment liver biopsies were performed (82%). For the duration of this study, liver biopsy was a requirement under the Australian Pharmaceutical Benefit Scheme to access subsidised entacavir treatment. Somewhat surprisingly the degree of fibrosis was not significantly associated with patients achieving CVS, or the time to achieve CVS.
Although there was an association between DNA suppression and ALT normalisation, our study did not take into account other variables that could influence transaminase changes, including anthropomorphic measurements, alcohol intake and intercurrent illness.
The study is limited by its retrospective analysis of medical records and the lack of sufficient follow up data for 13% of patients in the pharmacy registry. Furthermore only a minority of the patients included in the analysis (15%) had completed more than 36 mo of therapy. Finally, viral resistance testing was not performed for patients that failed to achieve PVS or had virologic rebound, as it was not routinely available in our clinic during the study period. The wide range in follow up is mainly due to late commencement in the study period with less time for follow up, drop out of patients and limited treatment duration.
In conclusion, entecavir monotherapy is efficacious and well tolerated for the treatment of patients with chronic hepatitis B infection in Australia. In our cohort baseline HBV DNA levels and HBeAg status influenced time to complete virologic suppression, consistent with previous studies. These factors are not independent, however, as HBeAg negative status most likely correlates with lower baseline HBV DNA levels. There was a very low incidence of failure to suppress DNA, and no apparent emergence of viral resistance on therapy. Our study supports the recommendations that 0.5 mg daily entecavir monotherapy is an appropriate first line agent for patients with treatment naive chronic hepatitis B in Australia.
An estimated 350 to 400 million people worldwide have chronic hepatitis B virus (HBV) infection, a leading cause of morbidity and mortality. Australia has an ethnically diverse population, with many migrants from the Asia-Pacific region, where the majority of hepatitis B related deaths occur.
Entecavir is an oral antiviral agent with potent activity against HBV. The rates of entecavir response and resistance in Australia are unknown. Most published data are from large clinical trials, which do not necessarily reflect the rates seen in “real world” clinical practice.
Out of 163 patients, 153 patients (93.9%) achieved at least partial suppression of HBV DNA, with 134 patients (82.2%) achieving complete suppression. Predictors of complete suppression included lower baseline DNA level, HBV e antigen negative status and increasing age at treatment. No significant adverse effects were reported necessitating cessation of entecavir.
The study results suggest that entecavir monotherapy is efficacious and safe in an Australian tertiary care setting. Resistance and rebound rates are very low. This is similar to data from controlled and uncontrolled trials around the world.
Hepatitis B is a viral infection that can lead to persistent inflammation and scarring of the liver. This has significant adverse outcomes including cirrhosis of the liver and liver cancer. Entecavir is a antiviral agent that prevents proliferation of the hepatitis B virus.
Well described cohort of entecavir treated patients in clinical practice from Australia; This is an interesting retrospective study from Australia confirms the efficacy and safety of entecavir in clinical practice.
P- Reviewer Lampertico P S- Editor Jiang L L- Editor A E- Editor Zhang DN
| 1. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1750] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 2. | Gidding HF, Warlow M, MacIntyre CR, Backhouse J, Gilbert GL, Quinn HE, McIntyre PB. The impact of a new universal infant and school-based adolescent hepatitis B vaccination program in Australia. Vaccine. 2007;25:8637-8641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | O’Sullivan BG, Gidding HF, Law M, Kaldor JM, Gilbert GL, Dore GJ. Estimates of chronic hepatitis B virus infection in Australia, 2000. Aust N Z J Public Health. 2004;28:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | NNDSS Annual Report Writing Group. Australia's notifiable disease status, 2010: Annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell. 2012;36:1-69. [PubMed] |
| 5. | Innaimo SF, Seifer M, Bisacchi GS, Standring DN, Zahler R, Colonno RJ. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob Agents Chemother. 1997;41:1444-1448. [PubMed] |
| 6. | Chang TT, Gish RG, Hadziyannis SJ, Cianciara J, Rizzetto M, Schiff ER, Pastore G, Bacon BR, Poynard T, Joshi S. A dose-ranging study of the efficacy and tolerability of entecavir in Lamivudine-refractory chronic hepatitis B patients. Gastroenterology. 2005;129:1198-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1090] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 8. | Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 910] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 9. | Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 590] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 10. | Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 633] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 11. | Sherman M, Yurdaydin C, Simsek H, Silva M, Liaw YF, Rustgi VK, Sette H, Tsai N, Tenney DJ, Vaughan J. Entecavir therapy for lamivudine-refractory chronic hepatitis B: improved virologic, biochemical, and serology outcomes through 96 weeks. Hepatology. 2008;48:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, Liaw YF, Mizokami M, Kuiken C. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology. 2007;46:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 364] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 13. | Pawlotsky JM, Dusheiko G, Hatzakis A, Lau D, Lau G, Liang TJ, Locarnini S, Martin P, Richman DD, Zoulim F. Virologic monitoring of hepatitis B virus therapy in clinical trials and practice: recommendations for a standardized approach. Gastroenterology. 2008;134:405-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Shouval D, Lai CL, Chang TT, Cheinquer H, Martin P, Carosi G, Han S, Kaymakoglu S, Tamez R, Yang J. Relapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: the case for continuous antiviral therapy. J Hepatol. 2009;50:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 466] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 16. | Tenney DJ, Pokornowski KA, Rose RE, Baldick CJ, Eggers BJ, Fang J, Wichroski MJ, Diva UA, Xu D, Wilber RB. W1805 Entecavir Maintains a High Genetic Barrier to HBV Resistance Through 6 Years in NaiVE Patients. Gastroenterol. 2009;136:A-865. [DOI] [Full Text] |
| 17. | Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in ‘real-life’ settings: from clinical trials to clinical practice. J Viral Hepat. 2012;19:377-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Wong GL, Wong VW, Chan HY, Tse PC, Wong J, Chim AM, Yiu KK, Chu SH, Chan HL. Undetectable HBV DNA at month 12 of entecavir treatment predicts maintained viral suppression and HBeAg-seroconversion in chronic hepatitis B patients at 3 years. Aliment Pharmacol Ther. 2012;35:1326-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Yao GB, Ren H, Xu DZ, Zhou XQ, Jia JD, Wang YM, Chen CW. Virological, serological and biochemical outcomes through 3 years of entecavir treatment in nucleoside-naive Chinese chronic hepatitis B patients. J Viral Hepat. 2010;17 Suppl 1:51-58. [PubMed] |