Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.682
Revised: December 11, 2012
Accepted: December 15, 2012
Published online: February 7, 2013
Processing time: 145 Days and 19.2 Hours
AIM: To investigate the role of interleukin (IL)-17 in small bowel allograft rejection.
METHODS: We detected the expression of helper T cell 17 (Th17) cells in biopsy specimens from 3 cases of living small bowel transplantation in our department through immunofluorescence stain. We then established a rat heterotopic small bowel transplantation model. The rats were sacrificed on the 1st, 2nd, 3rd, 5th, and 7th d after small bowel transplantation. The degrees of transplantation rejection in rat intestine graft were examined through hematoxylin eosin (HE) stain, and the expression of Th17 cells in rat intestine graft were detected through immunofluorescence stain. In addition, the recipient rats undergoing intestinal transplantation were administrated with mouse-anti-rat IL-17 monoclonal antibody (mAb), and the survival of rats was analyzed. The recipient rats which received mouse-anti-rat IL-17 mAb treatment were sacrificed on the 1st, 2nd, 3rd, 5th, and 7th d after small bowel transplantation. The degrees of transplantation rejection and the expression of Th17 cells in rat intestine graft were detected through HE and immunofluorescence stain. The expression of IL-17, IL-1β, tumor necroses factor receptor-α (TNF-α), IL-6, and IL-8 in the intestine graft or serum were also detected.
RESULTS: The expressions of Th17 cells ran parallel with the degree of acute rejection in human intestine grafts. The intestine graft rejection of rats was aggravated with prolonged duration after intestinal transplantation, and the expressions of Th17 cells were also correlated with the degree of acute rejection in rat intestine grafts. Administration of mouse-anti-rat IL-17 mAb prolonged the survival of rats after small bowel transplantation (P < 0.001). Furthermore, we found that the administration of mouse-anti-rat IL-17 mAb significantly decreased the intensity of CD4+IL-17+ Th17 cells in intestine grafts on the 2nd, 3rd, 5th, and the 7th d (97.22 ± 4.05 vs 12.45 ± 2.02 on the 7th d, P < 0.0001), and suppressed the severity of acute rejection. The expression of IL-17 in the intestine graft declined after mouse-anti-rat IL-17 mAb administration on the 2nd, 3rd, 5th, and the 7th d (0.88 ± 0.03 vs 0.35 ± 0.02 on the 7th d, P < 0.0001). We also detected the IL-17 serum level and found that the IL-17 level reduced from the 1st d to the 7th d (6.52 ± 0.18 ng/mL vs 2.04 ± 0.15 ng/mL on the 7th d, P < 0.0001). No significant difference in the level of IL-17 mRNA in the intestine graft was identified between the two groups. The levels of IL-1β, TNF-α, IL-6, and IL-8 mRNA in the intestine graft after the administration of mouse-anti-rat IL-17 mAb were also tested. We found that on the 3rd, 5th, and 7th d after intestinal transplantation, administration of mouse-anti-rat IL-17 mAb significantly inhibited the levels of IL-1β (12.11 ± 1.16 vs 1.27 ± 0.15 on the 7th d, P < 0.001), TNF-α (27.37 ± 2.60 vs 1.06 ± 0.26 on the 7th d, P < 0.001), IL-6 (21.43 ± 1.79 vs 1.90 ± 0.32 on the 7th d, P < 0.001), and IL-8 (20.44 ± 1.44 vs 1.34 ± 0.20 on the 7th d, P < 0.001) mRNA in the intestine graft.
CONCLUSION: IL-17 may act as a promising and potent target for inhibiting acute rejection after small bowel transplantation.
- Citation: Yang JJ, Feng F, Hong L, Sun L, Li MB, Zhuang R, Pan F, Wang YM, Wang WZ, Wu GS, Zhang HW. Interleukin-17 plays a critical role in the acute rejection of intestinal transplantation. World J Gastroenterol 2013; 19(5): 682-691
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/682.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.682
Small bowel transplantation is a prevailing therapy for short bowel syndrome[1-3]. However, compared with liver[4,5], kidney[6,7], and heart transplantation[8,9], small bowel transplantation has less satisfying effects. The small bowel and mesentery involve redundant lymphoid tissue, which are organs with high immunogenicity prone to inducing severe transplantation rejection. FK506 (Tacrolimus) may inhibit the activation of T cells through suppressing the production of rejection related cytokines, calcium-dependent phosphatase calcineurin, and JNK/p38 pathways[10,11]. FK506 can prevent the aggregation of lymphocytes in the early rejection and chemotaxis of inflammatory cells. But the usage of FK506 after clinical small bowel transplantation may result in severe side effects, such as renal toxicity and neurotoxicity[12].
Recently, the helper T cell (Th)1/Th2 paradigm has been expanded, following the discovery of a third subset of effecter T helper cells that produce interleukin (IL)-17 (Th17) and exhibit effecter functions[13-15]. On the basis of these studies, investigators proposed that IL-17-producing T cells serve as a distinct T helper cell subset, which are called Th17 cells[16-18]. The primary function of Th17 cells appears to be the clearance of pathogens that are not adequately handled by Th1 or Th2 cells[19]. Th17 cells, as potent inducers of tissue inflammation, have a proven association with the pathogenesis of many experimental autoimmune diseases and human inflammatory conditions[20,21].
In the present study, we reveal that Th17 cells and IL-17 cytokine are expressed in the intestine graft after small bowel transplantation. Furthermore, we found that the level of Th17 cells ran parallel with the degree of rejection. The data clearly indicates that Th17 cells and IL-17 cytokine may play an important role in transplant rejection.
This study was approved by the Ethics Committee of the Fourth Military Medical University. Biopsy specimens embedded in paraffin from 3 cases of living-related small bowel transplantation were collected from Xijing Hospital of Digestive Diseases, Fourth Military Medical University (FMMU) from 1999 to 2003. All clinical information was available. The sections were stained for pathological examination. The brief clinical characteristics of these patients are listed as follows: Patient No. 1 was an 18-year-old boy with a 40 cm intestine who received a 150 cm segment of distal ileum from his father; Patient No. 2 was a 17-year-old boy with a 8 cm intestine who received a 170-cm graft of distal ileum from his father and; Patient No. 3 was a 15-year-old boy with a 10 cm intestine who received a 160-cm graft of distal ileum from his mother. All the recipients underwent different degrees of graft rejection after operation. The first two patients survived, while the third died from acute graft rejection and severe infection.
Forty inbred male F344/NCrl BR and forty LEW/Crl rats (age: 8 to 12 wk old, weight: 180 to 230 g) were purchased from Vital River Lab Animal Technology Co., Ltd (Beijing, China). Animals were maintained in specific pathogen-free conditions. All animal experiments were approved by the Animal Experiment Administration Commission of FMMU.
Donors and recipients were intraperitoneally anesthetized with pentobarbital (5 g/L). The small intestine 5 cm distal to the ligament of Treitz was harvested from the donor rats and 20 cm of isolated jejunum, along with mesenteric blood vessels, was prepared for transplantation. The left kidney of each recipient was removed and the infrarenal abdominal aorta isolated. An end-to-side vascular anastomosis was performed between the recipient and donor’s abdominal aortae. The cuffed portal vein was inserted into the left renal vein. The two ends of the small bowel graft were constructed as separate stomas through the left abdominal wall. Detailed procedures were followed as previously described[22]. In treatment experiments, the recipient rats in the control group were administrated with mouse-immunoglobulin G (IgG), while the recipient rats in the anti-IL-17 monoclonal antibody (mAb) group were administrated intravenously with 200 μg mouse-anti-rat IL-17 mAb[23] daily during the operation, and on the 1st, 2nd, 3rd, 5th, and 7th d afterwards.
Specimens were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned at 4 μm thickness, and stained with hematoxylin and eosin, following standard methods for routine morphological analysis.
The biopsy specimens of human and rat intestine grafts were stained with hematoxylin eosin (HE), and analyzed by two independent pathologists. The histological degrees for acute intestine graft rejection were divided into four grades: indeterminate for acute rejection, mild acute rejection, moderate acute rejection, and severe acute rejection. The details of the diagnostic criteria for acute intestine graft rejection have been described previously[24].
The paraffin-embedded human intestine mucosa specimens were sectioned at 4 μm thickness. Immunofluorescence was performed by standard procedures, with rabbit-anti-human IL-17 polyclonal antibody (1:50 dilution; Santa Cruz Biotechnology) and mouse-anti-human CD4 mAb (1:100 dilution; Santa Cruz Biotechnology) as the primary antibodies. Goat-anti-rabbit IgG-TR (1:50 dilution; Santa Cruz Biotechnology) and goat-anti-mouse IgG-FITC (1:50 dilution; Santa Cruz Biotechnology) were taken as secondary antibodies. We did not compare the staining for IL-17 before and after transplantation, but only selected the paraffin-embedded human intestine mucosa specimens at different transplantation rejection degrees in order to detect the expressions of Th17 cells.
The negative control sections were used in our immunofluorescence method. We took phosphate buffered solution (PBS) instead of the primary antibodies as a negative control, and adopted rat cardiac allograft specimen sections as a positive control (data not shown). The methods of the immunofluorescence stain of Th17 in our present study were in accordance with our previous report[25].
The total proteins of the intestine graft were extracted and determined according to the manufacturer’s manuals. Proteins were electrophoresed in 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted on a nitrocellulose membrane. The membrane was incubated with rabbit anti-rat IL-17 polyclonal antibody (1:500 dilutions). After three washes for 15 min in PBS-T, the membrane was incubated with the HRP-conjugated goat-anti-rabbit IgG antibody (1:2000 dilutions). Blots were developed by using an enhanced chemiluminescence system (ECL, Amersham, Little Chalfort, United Kingdom). β-actin was considered as an internal control.
Blood from the heart was extracted when the rat was sacrificed. Serum was collected and adopted in order to detect the level of IL-17 with a rat IL-17 enzyme-linked immunosorbent assay kit (ELISA) (Rapidbio, America) by standard procedures. A450 nm was recorded by a spectrophotometer, and was compared between groups.
Total RNA was extracted from rat intestine grafts with a homogenizer by using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Complementary DNA was prepared with a reverse-transcription kit from TOYOBO (Osaka, Japan). Real-time reverse-transcription polymerase chain reaction (RT-PCR) was performed by using a kit (SYBR Premix EX Taq, Takara) and the ABI PRISM 7500 real-time PCR system, with β-actin as an internal control. Primers used in real-time PCR were as follows, β-actin, forward: CATCCGTAAAGACCTCTATG CCAAC, reverse: ATGGAGCCACCGATCCACA; IL-17, forward: GGGAAGTTGGA CCACCACAT, reverse: TTCTCCACCCGGAAAGTGAA; IL-1β, forward: CTTCAAATCTCACAGCAGC ATCTCG, reverse: TCCACGGGCAAGACATAGGT AGC, tumor necroses factor receptor-α (TNF-α), forward: CTGTGCC TCAGCCTCTTCTCATTC, reverse: TTGGGA ACTTCTCCTCCTTGTTGG; IL-6, forward: GACTGATGTTGTTGACAGCCACTGC, reverse: TAGCCACTCCTTCTGT GACTCTAACT, IL-8, forward: GCCAACAC AGAAATTATTGTAAAGCTT, reverse: CCTCTGCACCCAGT TTTCCTT.
Statistical analysis was performed with the SPSS 16.0 program. Results were expressed as means ± SD. Comparisons between groups were made by the unpaired Student’s t-test. For survival studies, Kaplan-Meier survival curves were generated and a statistical analysis was performed via the log-rank test. P < 0.05 was considered statistically significant.
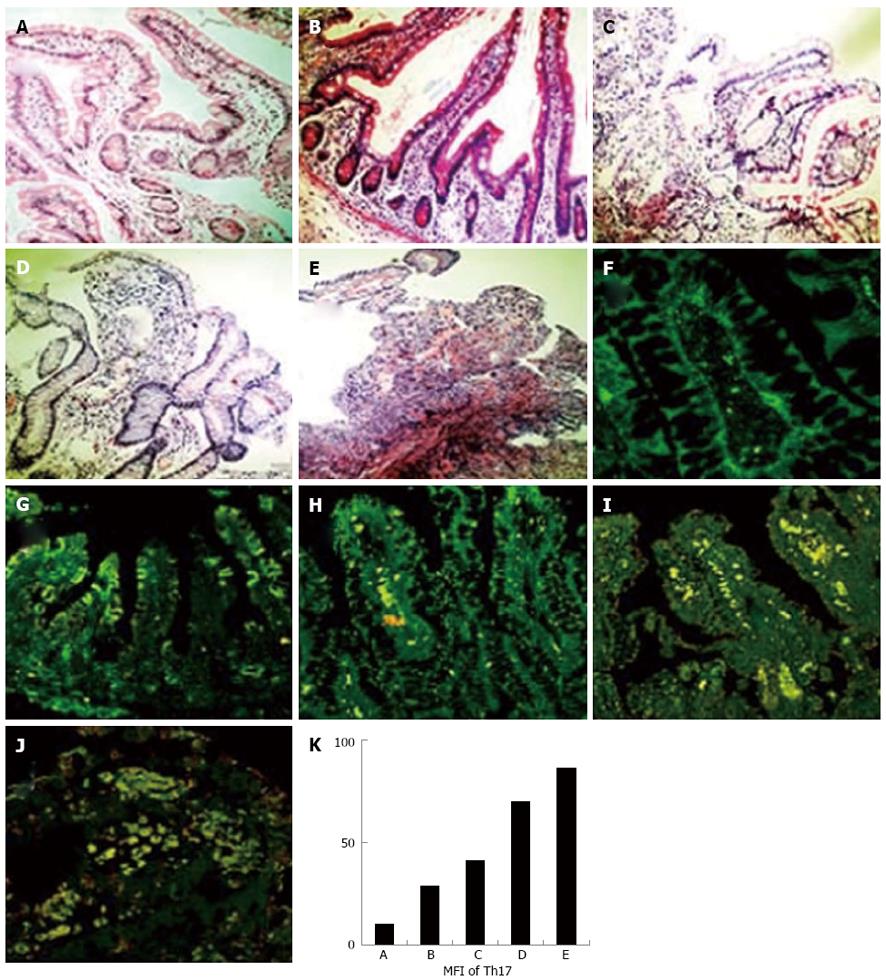
To examine the expression of Th17 cells on a human intestine graft, biopsy specimens embedded in paraffin from 4 cases of living small bowel transplantation in our department were collected and stained with HE (Figure 1A-E) and antibodies against IL-17 and CD4 (Figure 1F-J). The density of IL-17+CD4+ Th17 cells was calculated and analyzed by Image-Pro-Plus 5.1 software. As shown in Figure 1K, the density of IL-17+CD4+ Th17 cells increased when the rejection degrees aggravated.
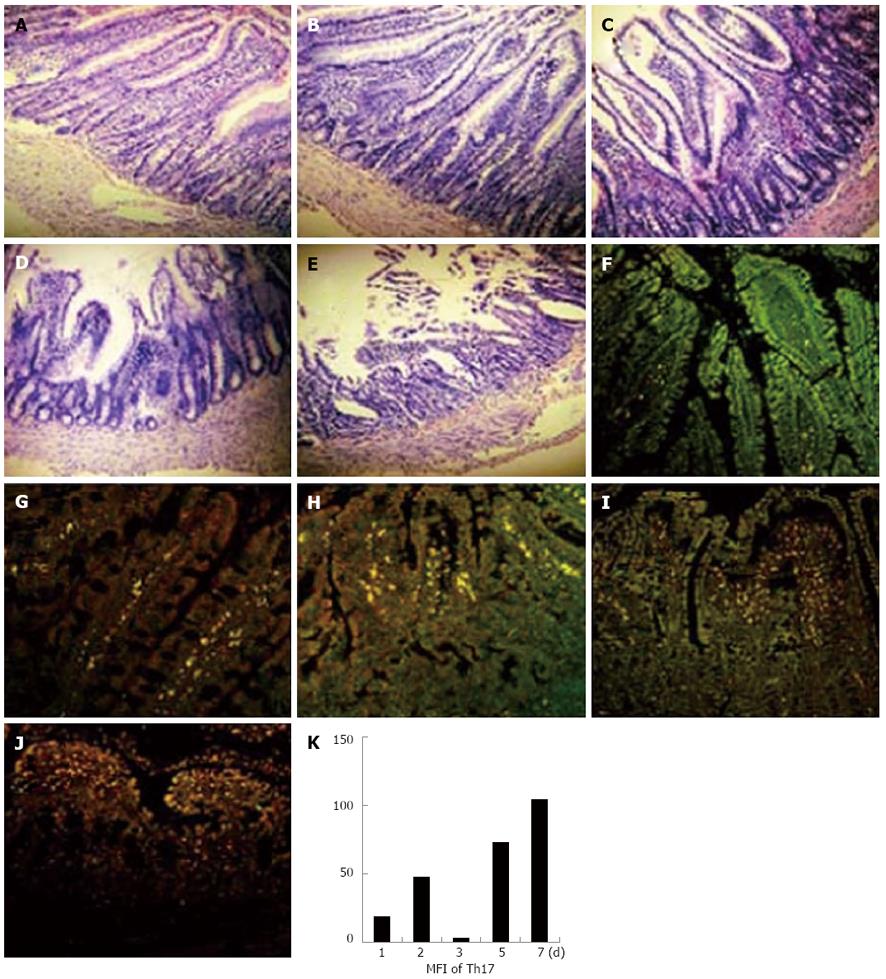
In order to investigate whether Th17 cells exist in rat intestine grafts after transplantation, intestines from F344/NCrl BR were grafted to LEW/Crl rats; the recipient rats were sacrificed on the 1st, 2nd, 3rd, 5th, and 7th d after transplantation, and the expression of Th17 cells in rat intestine grafts were analyzed (Figure 2A-J). In accordance with the findings in human intestine grafts, we found that Th17 cells were located in rat intestine grafts and the degree of Th17 cells corresponded to the severity of transplant rejection (Figure 2K). Taken together, Th17 cells did exist in intestine grafts, and the levels of Th17 cells might relate to the transplant rejection degrees in both human and rat recipients.
Since IL-17 may play a role in small bowel transplantation rejections, we hypothesized that IL-17 could be considered as a potential target for the treatment of graft rejection. In order to further demonstrate our hypothesis, the recipients were administrated with mouse-anti-rat IL-17 monoclonal antibody intravenously during, and after, small bowel transplantation, and the survival of recipients were analyzed. We found that administration of anti-IL-17 mAb could significantly prolong the survival of rats after small bowel transplantation (Figure 3).
We further analyzed the effect of anti-IL-17 administration on graft rejections. The administration of anti-IL-17 mAb was proved to effectively inhibit transplant rejection of intestinal grafts in rats post-transplantation by HE staining (Figure 4A-E). Furthermore, we found that the expression of Th17 cells in intestine grafts also dramatically declined (Figure 4F-K), indicating that anti-IL-17 mAb could possibly prolong the survival of recipient rats by inhibiting transplant rejection.
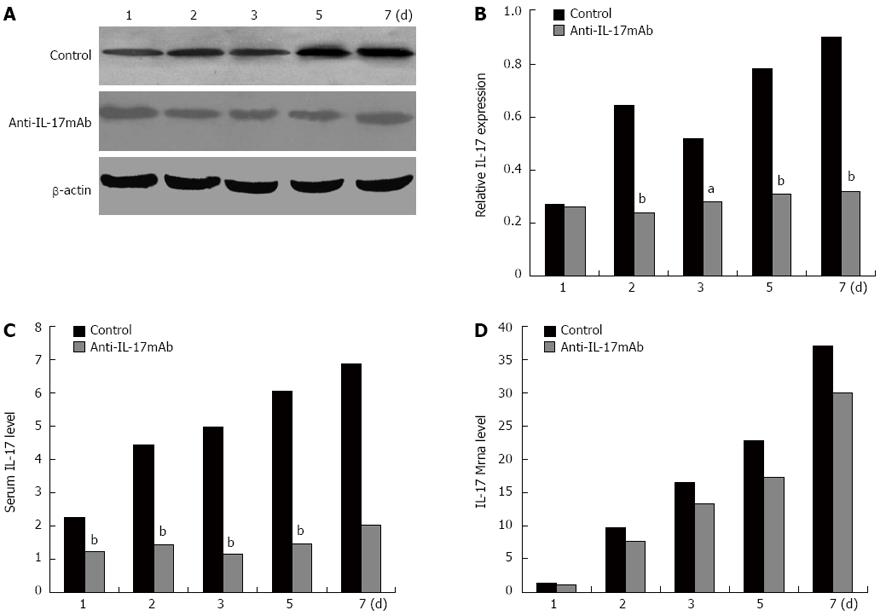
During and after allotransplantation, recipient rats were administrated with anti-IL-17 mAb. The recipient rats were sacrificed on the 1st, 2nd, 3rd, 5th, and 7th d after transplantation. The expressions of IL-17 in intestine grafts and serums were further detected by western blot and ELISA (Figure 5). In the control group which only received saline administration, the levels of IL-17 protein in the intestine graft and serum were found to have increased post-allotransplantation and run parallel with transplant rejection degrees. In the anti-IL-17 mAb administration group, the expression of IL-17 could also be detected, but the degrees were remarkably lower than the control group. The levels of IL-17 mRNA in the intestine graft in two groups were not significantly different. Therefore, the administration of anti-IL-17 mAb could decrease the IL-17 level in intestine grafts and serum.
As shown in Figure 6, the expressions of IL-1β, IL-6, IL-8, and TNF-α mRNA of intestine grafts in the control group were found to increase post-allotransplantation and correlate to transplant rejection degrees. Administration of anti-IL-17 mAb could dramatically decrease the expression of mRNA levels of these cytokines in intestine grafts, compared with the control group.
Th17, known as a distinct lineage of helper T cells from Th1 and Th2, has both a defense role against microbe infection and a pathogenic role in several autoimmune diseases[26]. Th17 cells have been shown to be implicated in allograft rejection of solid organs, such as lung and cardiac transplantation[25,27,28]. Vanaudenaerde et al[27] observed that IL-17 expression increased in bronchoalveolar lavages in patients with acute lung transplantation rejection. The disease-promoting role of Th17 in cardiac allograft rejection was also confirmed, especially in the absence of a Th1 response[25]. Th17 cells not only function in host-versus-graft disease, but also participate in graft-versus-host disease[29-31]. So far, the relationship between Th17 cells and small bowel transplantation has been unclear. In this study, we demonstrated that Th17 cells participated in the development of human and rat small bowel transplantation rejection.
Here we found that Th17 cells existed in intestine grafts with different degrees of acute rejection. Furthermore, we indicated that the density of Th17 cells increased when the rejection degree aggravated, suggesting that it might be related to the transplant rejection degrees in human recipients. Th17 cells were also found to be located in rat intestine grafts, and the degree of Th17 cells correlated with transplant rejection degrees, which was in accordance with the findings in human intestine grafts. These demonstrate that Th17/IL-17 may participate and play a critical role in the graft rejections of small bowel transplantation.
IL-17, secreted by Th17 cells, is a highly inflammatory cytokine with robust effects on stromal cells in many tissues[32,33]. Hsieh et al[34] reported that IL-17 could serve as a predictive parameter for borderline subclinical renal allograft rejection in the future. Itoh et al[35] found that IL-17-deficient recipient mice had decreased allograft inflammatory cell recruitment, and demonstrated that IL-17 contributed to the pathogenesis of chronic allograft rejection. In order to demonstrate the key role of IL-17 in the graft rejection of small bowel transplantation, we utilized mouse-anti-rat IL-17 mAb to treat recipient rats undergoing small bowel transplantation for the first time. Surprisingly, after administration of anti-IL-17 mAb, the acute rejection degrees of recipient rats significantly decreased compared to the control group. The levels of Th17 cells that had infiltrated the intestine graft during anti-IL-17 mAb administration also fell greatly. Administration with anti-IL-17 mAb could extend the survival time of rats undergoing small bowel transplantation. To sum up, IL-17 cytokine could probably be taken as a potent target to treat acute rejection in small bowel transplantation.
The differentiation factors (transforming growth factor beta plus IL-6 or IL-21), the growth and stabilization factor (IL-23), and the transcription factors (signal transducer and activator of transcription 3, related orphan receptor-γt (RORγt), and RORα) were involved in the development of Th17 cells. Mice reconstituted with the bone marrow of RORγt deficient mice showed an impaired Th17 differentiation. The combinations of IL-1β plus IL-6[36] or IL-1β plus IL-23[37] were proposed to be the differentiation factors for human Th17 cells. We found that the expression of IL-1β and IL-6 was significantly decreased after anti-IL-17 mAb administration. Therefore, anti-IL-17 mAb might suppress the expression of IL-1β and IL-6 in rat intestine grafts, and then inhibit the development and activation of Th17 cells. TNF is also induced by IL-17 cytokine. Its expression was found to be reduced after anti-IL-17 mAb administration. The migration and infiltration of inflammatory cells into intestine grafts requires the expression of chemokines. Chemokine (C-X-C motif) ligand 8 (IL-8), a target of IL-17, is involved in transplant rejections. Compared to the control group, the expression of IL-8 was found to be obviously decreased in intestine grafts treated with anti-IL-17 mAb. This suggests that anti-IL-17 mAb administration might suppress the migration and infiltration of Th17 cells into intestine grafts by decreasing the expression of chemokines.
In conclusion, we have illustrated that Th17 cells might play an important role in human and rat small bowel acute transplantation rejection. The administration of anti-IL-17 mAb could significantly suppress the acute rejection degree of rat intestine grafts and prolong the survival time of recipient rats. IL-17 could be considered a promising and potent target for inhibiting acute rejection after small bowel transplantation.
Small bowel transplantation is a widespread therapy for short bowel syndrome. However, the efficacy of small bowel transplantation is not satisfactory due to severe transplantation rejection. Although administration of FK506 may inhibit the activation of T cells, prevent the aggregation of lymphocytes in early rejection, and restrain the chemotaxis of inflammatory cells, its administration after human small bowel transplantation may cause severe side effects such as renal toxicity and neurotoxicity.
The helper T cell (Th) 17 cell, as a distinct lineage of helper T cells from Th1 and Th2, has a proven implication in the allograft rejection of solid organs, such as lung and cardiac transplantation. Interleukin (IL)-17 expression was increased in bronchoalveolar lavages in patients with acute lung transplantation rejection. The disease-promoting role of Th17 in cardiac allograft rejection was also confirmed. Th17 cells are involved not only in host-versus-graft disease, but graft-versus-host disease as well.
So far, the relationship between Th17 cells and small bowel transplantation has been unclear. Authors demonstrated the presence of Th17 cells in the intestine grafts of humans and rats, and further found that the expression levels of Th17 cells in intestine grafts correlated with the degree of rejection. The authors hypothesized that Th17/IL-17 might play a critical role in small bowel transplantation rejection and could be regarded as a potential target for the treatment of graft rejection. They then treated recipient rats with mouse-anti-rat IL-17 monoclonal antibody (mAb) and found that its administration could significantly prolong the survival of rats after small bowel transplantation. Furthermore, the authors found that the expression of Th17 cells in intestine grafts also dramatically declined, indicating that mouse-anti-rat IL-17 mAb may prolong the survival of recipient rats by inhibiting transplant rejections. In the present study, the authors demonstrated that Th17 cells participated in the development of human and rat small bowel transplantation rejection.
The administration of mouse-anti-rat IL-17 mAb could significantly suppress the acute rejection degree of rat intestine grafts and prolong the survival time of recipient rats. IL-17 could be considered as a promising and potent target for inhibiting acute rejection after small bowel transplantation.
Small bowel transplantation: an operation to replace a diseased or shortened small bowel with a healthy bowel from a donor and a valuable therapy for short bowel syndrome. Transplant rejection: immune system attacks between the transplant recipient and the transplanted organ or tissue, which include host-versus-graft disease and graft-versus-host disease. Th17 cell: known as a distinct lineage of helper T cells from Th1 and Th2 that has both a defense role against microbe infection and a pathogenic role in several autoimmune diseases.
This is an investigation of the role of IL-17 in acute intestinal transplantation rejection and the administration of anti-IL-17 monoclonal antibodies for the suppression of IL-17 production by Th17 cells in the intestine graft and, as a result, suppression of acute rejection of the intestine graft in both human and rat models. It is a very good work and I hope it is applicable in human subjects undergoing intestinal transplantation.
P- Reviewers Vorobjova T, Eghtesad B S- Editor Gou SX L- Editor Rutherford A E- Editor Zhang DN
| 1. | Donohoe CL, Reynolds JV. Short bowel syndrome. Surgeon. 2010;8:270-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Tzvetanov IG, Oberholzer J, Benedetti E. Current status of living donor small bowel transplantation. Curr Opin Organ Transplant. 2010;15:346-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Li M, Ji G, Feng F, Song W, Ling R, Chen D, Liu X, Li J, Shi H, Wang W. Living-related small bowel transplantation for three patients with short gut syndrome. Transplant Proc. 2008;40:3629-3633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Kamath BM, Olthoff KM. Liver transplantation in children: update 2010. Pediatr Clin North Am. 2010;57:401-414, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Starzl TE, Fung JJ. Themes of liver transplantation. Hepatology. 2010;51:1869-1884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Freitas MC. Kidney transplantation in the US: an analysis of the OPTN/UNOS registry. Clin Transpl. 2011;1-16. [PubMed] |
| 7. | Huang E, Segev DL, Rabb H. Kidney transplantation in the elderly. Semin Nephrol. 2009;29:621-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Everly MJ. Cardiac transplantation in the United States: an analysis of the UNOS registry. Clin Transpl. 2008;35-43. [PubMed] |
| 9. | Kruse CD, Helvind M, Jensen T, Gustafsson F, Mortensen SA, Andersen HO. Good long-term survival after paediatric heart transplantation. Dan Med J. 2012;59:A4367. [PubMed] |
| 10. | Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. 1995;17:584-591. [PubMed] |
| 11. | Kochel I, Strzadała L. [FK506 - binding proteins in the regulation of transcription factors activity in T cells]. Postepy Hig Med Dosw (Online). 2004;58:118-127. [PubMed] |
| 12. | Zhu Y, Wei W, Li Y. FK506 treatment in a Long-term chronic rejection rat model of small bowel transplantation. Clin Invest Med. 2010;33:E168-E173. [PubMed] |
| 13. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5473] [Article Influence: 288.1] [Reference Citation Analysis (0)] |
| 14. | Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2372] [Cited by in RCA: 2489] [Article Influence: 131.0] [Reference Citation Analysis (0)] |
| 15. | Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1566] [Cited by in RCA: 1701] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 16. | Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2961] [Cited by in RCA: 3167] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 17. | Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3074] [Cited by in RCA: 3358] [Article Influence: 167.9] [Reference Citation Analysis (0)] |
| 18. | Wynn TA. T(H)-17: a giant step from T(H)1 and T(H)2. Nat Immunol. 2005;6:1069-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67 Suppl 3:iii26-iii29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3754] [Cited by in RCA: 3787] [Article Influence: 236.7] [Reference Citation Analysis (0)] |
| 21. | Miossec P. Diseases that may benefit from manipulating the Th17 pathway. Eur J Immunol. 2009;39:667-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Yang J, Li M, Zhang H, Hong L, Feng F, Pan F, Wang Y, Wang W. Application of a self-made swivel intravenous transfusion device in constructing allogenic small bowel transplantation rejection model in rats. Transplant Proc. 2009;41:4397-4400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Yang J, Hong L, Wang W, Zhang H. A novel monoclonal antibody specific to mouse IL-17. Hybridoma (Larchmt). 2011;30:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Wu T, Abu-Elmagd K, Bond G, Nalesnik MA, Randhawa P, Demetris AJ. A schema for histologic grading of small intestine allograft acute rejection. Transplantation. 2003;75:1241-1248. [PubMed] |
| 25. | Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D’Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133-3144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 250] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 26. | Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1417] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 27. | Vanaudenaerde BM, Dupont LJ, Wuyts WA, Verbeken EK, Meyts I, Bullens DM, Dilissen E, Luyts L, Van Raemdonck DE, Verleden GM. The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J. 2006;27:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Shilling RA, Wilkes DS. Role of Th17 cells and IL-17 in lung transplant rejection. Semin Immunopathol. 2011;33:129-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 30. | Mauermann N, Burian J, von Garnier C, Dirnhofer S, Germano D, Schuett C, Tamm M, Bingisser R, Eriksson U, Hunziker L. Interferon-gamma regulates idiopathic pneumonia syndrome, a Th17+CD4+ T-cell-mediated graft-versus-host disease. Am J Respir Crit Care Med. 2008;178:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Chen H, Wang W, Xie H, Xu X, Wu J, Jiang Z, Zhang M, Zhou L, Zheng S. A pathogenic role of IL- 17 at the early stage of corneal allograft rejection. Transpl Immunol. 2009;21:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 903] [Cited by in RCA: 861] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 33. | Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 2009;11:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 34. | Hsieh HG, Loong CC, Lui WY, Chen A, Lin CY. IL-17 expression as a possible predictive parameter for subclinical renal allograft rejection. Transpl Int. 2001;14:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Itoh S, Nakae S, Axtell RC, Velotta JB, Kimura N, Kajiwara N, Iwakura Y, Saito H, Adachi H, Steinman L. IL-17 contributes to the development of chronic rejection in a murine heart transplant model. J Clin Immunol. 2010;30:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1385] [Cited by in RCA: 1574] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 37. | Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 1566] [Article Influence: 87.0] [Reference Citation Analysis (0)] |