Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.607
Revised: September 3, 2012
Accepted: September 12, 2012
Published online: February 7, 2013
Serrated polyps have been an area of intense focus for gastroenterologists over the past several years. Contrary to what was thought before, a growing body of literature indicates that these polyps can be precursors of colorectal cancer (CRC). Most of these lesions, particularly those in the proximal colon, have so far been under-recognized and missed during colonoscopy, qualifying these lesions to be the main cause of interval cancers. It is estimated that 10%-20% of CRCs evolve through this alternative, serrated pathway, with a distinct genetic and epigenetic profile. Aberrant DNA methylation plays a central role in the development of this CRC subtype. This characteristic molecular background is reflected in a unique pathological and clinical manifestation different from cancers arising via the traditional pathway. In this review we would like to highlight morphological, molecular and clinical features of this emerging pathway that are essential for gastroenterologists and may influence their everyday practice.
- Citation: Patai &V, Molnár B, Tulassay Z, Sipos F. Serrated pathway: Alternative route to colorectal cancer. World J Gastroenterol 2013; 19(5): 607-615
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/607.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.607
It is being increasingly recognized that colorectal cancer (CRC) is not a single disease, but rather a heterogeneous disorder including a collection of many distinct diseases with diverse molecular background and clinicopathological manifestations. According to the adenoma-carcinoma sequence proposed by Vogelstein et al[1] adenomatous polyps have long been considered as the sole preneoplastic lesions leading to CRC. On the other hand, hyperplastic polyps (HP) often found in the distal colon, until recently have been considered innocuous lesions, despite some contradictory opinions[2,3]. This common view has recently been challenged, as it turned out that these polyps along with other similar lesions commonly termed “serrated polyps” can be precursors to CRC[4,5].
The aim of this article is to provide a thorough clinicopathologic overview of this emerging pathway in colorectal carcinogenesis and help to understand how this accumulating data can be translated into clinical management strategies and better clinical outcomes.
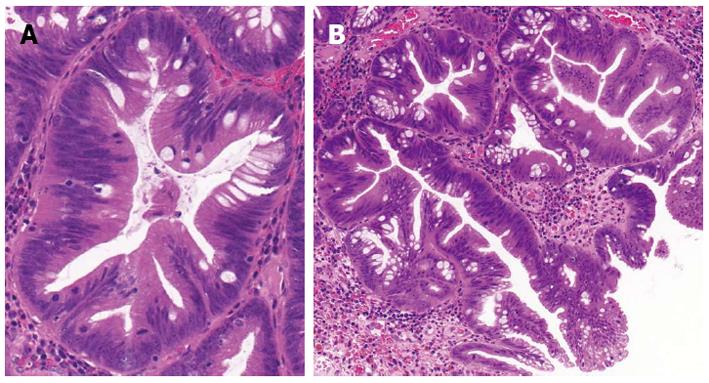
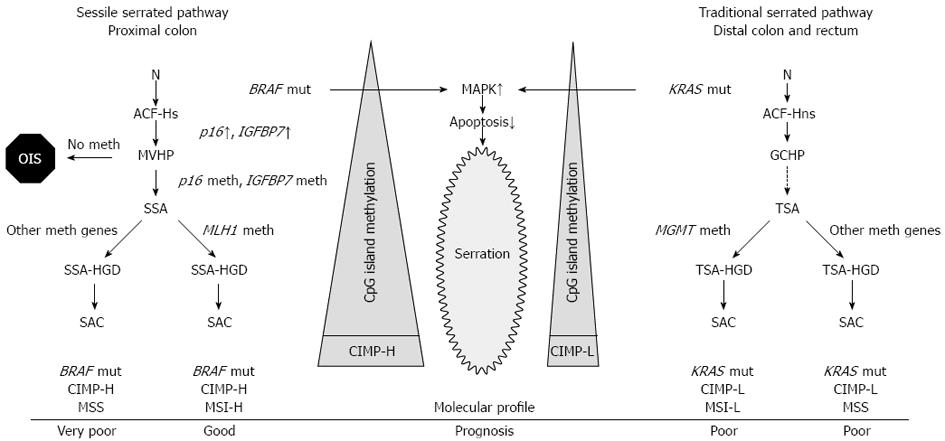
The term “serrated polyp” contains a wide variety of colonic lesions and broadly refers to HP and different serrated adenomas. The main histological feature of serrated polyps is the infolding of the crypt epithelium[5], that is represented as a serrated or saw-toothed appearance in longitudinal section and a stellate or starlike appearance on cross section (Figure 1). The molecular basis for this histological feature has been attributed to decreased apoptosis[6-8] that is caused by the activated mitogen activated protein kinase (MAPK)-ERK pathway that is induced by either BRAF or KRAS mutation (Figure 2). Inhibited apoptosis leads to the accumulation of non-proliferating cells. Serration is a general characteristics of this pathway from HP all the way to serrated adenocarcinoma (SAC)[9].
The earliest known microscopical precursors to CRC are mucosal abnormalities termed aberrant crypt foci (ACF). ACF can be further subclassified into two categories: dysplastic and hyperplastic[10,11] (also called as heteroplastic or non-dysplastic). Dysplastic ACFs, often termed as microadenomas, have been associated with sporadic adenomas arising via the traditional pathway[12,13]. Hyperplastic ACF may be serrated or non-serrated[11]. They are very frequent; almost every individual over 50 has at least one ACF in the distal colorectum[14]. Serrated hyperplastic ACF has a higher frequency of BRAF mutations, than non-serrated ACF, whereas non-serrated ACF has a higher frequency of KRAS mutations, than serrated ACF[11,15]. This finding supports the idea that these lesions are potentially initiating step on the serrated pathway to CRC[15], however their high frequency imply that only a small fragment progresses to HP or more advanced lesions of the serrated pathway[9]. BRAF and KRAS (mutually exclusive) mutations induce the activation of the MAPK-ERK pathway leading to decreased apoptosis and an initial burst of MAPK-ERK-dependent proliferation, leading to the formation of hyperplastic crypts. This uncontrolled proliferation is counteracted by a protective phenomenon called oncogene-induced senescence that is driven by telomere attrition, that triggers the induction of tumor suppressors including p16[16] or IGFBP7[17] (insulin-like growth factor binding protein 7), similarly as it was described in melanocytes. Hyperplastic crypts may remain dormant for prolonged periods due to the induction of crypt senescence[8] (Figure 2).
HPs are the most common (80%-90%) and the best described serrated polyps. They occur most frequently in the distal colon and the rectum; they are usually slightly elevated, diminutive polyps, less than 5 mm in size. Key morphological features include elongated crypts with serration limited to the upper half of the crypt, with lack of cytologic or architectural dysplasia. These alterations can be seen only in the upper third or only on the surface of the crypts[18]. The proliferative zone may be expanded, but usually confined to the crypt base. The nuclei are small, uniform and basally placed[18], the cytoplasm is eosinophilic. If surface epithelium is not present for histological evaluation, a thickened basal membrane and muscularis mucosae with short smooth muscle extensions into the basal part of the mucosa (“comb-like” appearance) can be helpful hints to identify HP[19] (Table 1).
| Sessile serrated adenoma | Traditional serrated adenoma | Hyperplastic polyps | |
| Location | Proximal | Distal | Distal |
| Macroscopic characteristics | Sessile, flat, covered with mucus, poorly defined borders | Protruding, pedunculated | Flat |
| Color | Normochromatic, pale | Reddish | Pale |
| Size | > 5 mm | > 5 mm | < 5 mm |
| Molecular features | BRAF mt | KRAS mt | |
| Histological characteristics | Dilated, branched serrated crypts at the bottom | Prominent crypt serration, ectopic crypt formation | Serrations at the top |
| Pit pattern | Open-shape (type II-O) | Fern or pinecone-like | Starlike (type II) |
| Precursor | MVHP | GCHP | ACF |
| Malignant potential | +++ | ++ | - |
| CIMP status | CIMP-H | CIMP-L | |
| MSI status | MSI-H or MSS | MSI-L or MSS | MSS |
| Gender predominance | Female | Male | Male |
| Dysplasia | Absent | Present | Absent |
| Ectopic crypt formation | Absent | Present | Absent |
HPs usually occur a decade earlier (in the fifth and the sixth decade) than adenomatous polyps[20]. Several risk factors have been linked with the prevalence of serrated polyps including cigarette smoking, alcohol consumption, obesity and low-folate intake[20,21], whereas regular nonsteroidal anti-inflammatory drug use, hormone replacement therapy, and high calcium intake have been associated with reduced risk[20]. It is of note that besides smoking, all other factors have also been linked to adenoma formation via the traditional pathway[9,20]. This observation gained further importance when it was discovered that smoking is only a strong risk factor for those CRCs that exhibit a unique molecular phenotype [CpG island methylator phenotype (CIMP)] linked to sessile serrated pathway[22,23].
Based on the epithelial mucin content, Torlakovic et al[19] histologically subclassified HPs into three categories: goblet cell-rich, microvesicular, and mucin-poor.
Microvesicular hyperplastic polyp (MVHP), also called type 2 HP, is the most common type and the typical representation of HPs encountered in the distal colon. It is characterized by large microvesicular mucin-containing epithelial cells in the upper half of the crypt, reduced goblet cells compared to normal colonic mucosa, and goblet cell abnormalities. MVHP shows prominent serration mostly in the upper half of the crypt and it has a large proliferative zone, which may take up the basal half of the mucosa. Nuclear stratification is present, but it is not prominent. The overall architecture is slightly distorted and minimal to mild crypt dilatation is present. Almost all MVHPs are slightly thicker than surrounding normal colonic mucosa. At the molecular level, MVHPs commonly (80%) exhibit BRAF V600E mutation[24], where valine is substituted for glutamic acid. As it was discussed above, this induces MAPK-ERK pathway that is followed by oncogene-induced senescence that includes overexpression of growth control genes p16 and IGFBP7 holding the cells in a dormant state. Aberrant CpG island methylation of the promoter region of p16 and IGFBP7 bypasses this dormant state[8] and drives MVHPs further to the next stage of serrated polyp progression, namely sessile serrated adenoma[9]. CpG island methylation is more pronounced in proximal MVHPs than in those located distally[25,26] (Figure 2).
Goblet cell-rich hyperplastic polyp (GCHP), also known as type 1 HP, is most commonly found in the distal colon, and is probably the most under-recognized variant. As its name implies this subtype is abundant of large, mature, distended goblet cells in the upper half of enlarged crypts and surface epithelial cells. Surface serrations are less prominent than in MVHP. Nuclear atypia is generally not present, but nuclei are slightly enlarged. KRAS mutations (in codon 12 and 13) were detected in almost half of these lesions[24,27], whereas BRAF mutations were rarely detected[27]. Successor lesions of GCHPs were rarely observed and it is open question whether they are self-limiting[9,24] or progress to advanced KRAS-mutated serrated polyps, presumably traditional serrated adenomas (TSA)[28] (Figure 2).
The mucin-poor hyperplastic polyp is the rarest form, almost absent of goblet cells. It has prominent nuclear atypia, hyperchromatic nuclei, lack of mucin, therefore it is considered to be a reactive version of MVHP with unknown clinical significance[29].
This distinction among these HP variants is primarily of theoretical importance, and an area of academic interest with little or no clinical importance at the moment. However, the distinction between HPs and more advanced lesions is of cardinal clinical importance[28] (Table 1).
In their landmark paper from 1990, Longacre and Fenoglio-Preiser[30] retrospectively overviewed 18 000 colorectal polyps and identified 110 (0.6%) as serrated adenomas. In 2003, Torlakovic et al[19] further divided serrated adenomas into two categories, TSA (those originally described by Longacre and Fenoglio-Preiser) and a new group identified as sessile serrated adenomas (SSAs), lesions with a serrated morphology without cytologic dysplasia.
SSAs are thought to be the second most common form of serrated polyps representing about 20% of all serrated polyps[18,19,31], however more recent studies have shown decreased prevalence (3%-8%)[32,33]. As mentioned above, before 2003 SSAs were labeled as “HP”[34]. It was demonstrated in a recent case series that according to the new WHO classification for serrated colonic polyps[35] a considerable proportion of HPs (especially those greater than 5 mm) were reclassified as SSAs[36].
Still today it is hard to distinguish SSAs from HPs, as there are only subtle differences, and SSAs lack the typical features (such as cytologic dysplasia) of traditional adenomas. SSAs tend to locate in the proximal colon, but they can also be encountered in the distal colon (Table 1).
The microscopical features of SSAs were first described by Torlakovic and Snover[37] in their landmark paper in 1996, then the term was reintroduced in 2003[19,31]. Microscopically, most characteristic features include horizontal crypt extensions (inverted T- or L- shape) at the crypt bases, crypt branching, crypt invaginations and inverted crypts beneath the mucosal muscle layer (pseudoinvasion), mature goblet cells at the crypt bases, dilation in the lower crypts, serration throughout the crypt length, extending into the lower third of the crypt as well[18,38]. The proliferation zone can extend to the basis of the crypt. SSAs can exhibit mild nuclear atypia, but always lack cytologic dysplasia.
Endoscopically, SSAs are flat or slightly elevated, malleable lesions with irregular borders and may be covered with a thin layer of yellowish mucus giving them a pale appearance[28,38]. They are usually larger than 5 mm in diameter. Their surface is smooth or granular[28], sometimes resembling a prominent mucosal fold. These features altogether make it difficult to detect and remove completely with conventional white-light endoscope, therefore advanced, image-enhanced endoscopy techniques including traditional or virtual magnifying chromoendoscopy are needed. Chromoendoscopy is an image-enhanced endoscopic technique that highlights differences in colonic mucosa based on structural patterns, so-called “pit patterns”. In a recent magnifying endoscopy study a new Type II open-shape pit pattern (Type II-O) was described and shown highly predictive of SSAs (with a sensitivity of 65.5% and a specificity of 97.3%). Progression of SSAs to more advanced lesions was associated with additional morphological changes, including the Type III, IV and V pit patterns[39] (Table 1).
On a molecular level, SSAs exhibit BRAF mutation and high level of CpG island methylation, supporting the hypothesis that they represent an intermediate stage between MVHPs and sporadic CIMP-H cancers. It was shown that SSAs can progress to dysplasia (SSA with high grade dysplasia, SSA-HGD) and then to CIMP-H cancers. Methylation and consequential loss of expression of MLH1 (a major DNA mismatch repair gene) is thought to drive this transformation. Impaired mismatch repair leads to high level of microsatellite instability (MSI-H) (Figure 2). It was hypothesized that this malignant progression can occur at faster rate than that observed in the lesions emerging via the traditional pathway[40]. This is based on the observation that SACs are more prevalent than SSA-HGD[31,32,38]. The exact time of progression from SSA to SAC is unknown. In a recent case report an untreated SSA was described to transform into an early submucosal invasive cancer in a period of 8 months[41]. These data further underline the need for improved detection of these lesions.
TSAs were first described by Longacre and Fenoglio-Presier[30] in 1990. As mentioned above, until 2003 they were termed serrated adenomas when Torlakovic et al[19] divided serrated adenomas to SSA and TSA. In 2008 the same group further characterized these lesions[42]. TSAs represent the rarest subtype of serrated lesions, with a frequency of 1%-6%[18]. Similar to the majority of serrated lesions (unlike SSAs), TSAs have a predilection for the distal colon and the rectum. Macroscopically they resemble traditional adenomas, as having a pedunculated, polypoid appearance. Cytologic dysplasia (90% low-grade and 10% high-grade[18]), as their name implies, is a major feature of TSAs (Table 1).
In 2008, Torlakovic et al[42] proposed ectopic crypt formation as decisive morphological criterion for diagnosing TSAs. Ectopic crypts are newly formed aberrant crypts that lost their anchoring to the underlying muscular layer of mucosa[18]. Other characteristics include diffuse eosinophilic cytoplasm, mucosal bridges and protrusions resembling tennis-racquets[42]. On a molecular level, TSAs are frequently KRAS mutants; however they can exhibit BRAF mutation, and also lack both mutations.
Filiform serrated adenoma is an unusual, less aggressive variant of traditional serrated adenoma, with morphological features similar to TSA[43]. Unlike TSA, filiform SA is composed predominantly of prominent, thin, elongated filiform projections lined by neoplastic epithelium with a serrated contour[44].
Mixed polyps are combinations of traditional adenomas and serrated lesions. They are postulated to be the result of collision tumors[45] and successors of SSAs. It is not encouraged to use this term as it does not disclose the preinvasive nature of these lesions[18]. Still, “mixed polyp” is a widely used term and when used it is recommended to describe the components of these lesions (e.g., TSA and traditional adenoma etc.)[18].
SAC, a special subtype of colorectal adenocarcinomas morphologically and histochemically resembling serrated polyps, was first described by Jass and Smith in 1992[46]. The relationship between serrated adenomas and SAC were further confirmed by Mäkinen et al[47], then histological characteristics of SAC were described[48] and reviewed by the same group[38]. Based on these seminal reports, most important diagnostic criteria of SAC include epithelial serrations, eosinophilic and abundant cytoplasm with vesicular nuclei, chromatin condensation and lack of necrosis. SAC was further classified into three major growth patterns. The most common (70%) serrated pattern contains mature, abundant, mucus-producing epithelium with well-preserved polarity, very similar to serrated polyps[38]. The mucinous pattern (43%) strongly overlaps with the first group, and is characterized by eosinophilic papillary rods (93%) and eosinophilic cell balls floating in the mucus[38]. The least common (7%) trabecular pattern is a feature of poorly differentiated SACs, where serrated structures are absent and cancer cells grow in a trabecular pattern, but still these cases show eosinophilic epithelium with vesicular nuclei, uncharacteristic of poorly differentiated traditional CRCs[38].
Gene expression profiling study by Laiho et al[49] provided molecular evidence that SAC is a biologically distinct subclass of CRC. Comparison of SAC and conventional CRCs revealed 201 differentially expressed genes. Three potential candidates were identified that can be involved in the oncogenesis of SAC: Ephrin receptor B2 (EPHB2), hypoxia-inducible factor 1-alpha (HIF1α) and patched (PTCH) appeared as genes important for the oncogenesis of serrated CRC. EPHB2 and PTCH expression are decreased in SAC compared to conventional CRC. On the other hand, constitutive overexpression of HIF1α, a major proangiogenic factor, can be the cause of infrequent necrosis seen in SAC[50]. Activating mutations of oncogenic BRAF and KRAS are common findings in SAC[26]. As mentioned above, these mutually exclusive mutations induce MAPK-ERK pathway that leads to the inhibition of apoptosis resulting in serrated appearance. Accumulation of CpG island methylation in the promoter regions and consequential silencing in key proapoptotic and tumor suppressor genes, such as p16 or IGFBP7 sets up a vicious circle. It is well established that methylation of MLH1 leading to MSI-H phenotype (closely linked with the sessile serrated pathway) and methylation of MGMT leading to MSI-L are the main inducing factors in the malignant progression of serrated adenomas (Figure 2).
It is generally thought that CpG island hypermethylation is confined to the serrated pathway (CIMP)[51,52], however a recent study showed that sporadic CRCs and precursors arising via the traditional adenoma-carcinoma pathway also have a characteristic DNA methylation pattern different from those evolving through the serrated pathway[53].
SAC predominantly locates to cecum (52%) and rectum (33%)[47]. It is estimated that 16% of proximal CRCs are SACs, whereas this proportion in the distal colon is only 6%[48]. It is hypothesized that proximal SACs (mostly MSI-H) arise from SSAs and distal SACs (MSI-L and MSS) originate from TSAs[38] (Figure 2). While serrated adenomas are more common in males, SACs are almost twice (1.9 : 1) as common in females, than in males[48]. The higher risk of malignant progression of serrated adenomas to SAC in (elderly) women was explained with postmenopausal estrogen deficiency and decreased folate level, however this needs to be further investigated[38].
The prognosis of SAC seems to be defined by its molecular profile (Figure 2). BRAF-mutated, microsatellite-stable cancers in the proximal colon confer a very poor survival[54] with adverse histological features such as lymphatic and perineural invasion and high tumor budding[55]. On the other hand, BRAF-mutated cancers with MSI-H phenotype (sporadic MSI-H CRCs) have a favorable prognosis[56].
Serrated polyposis, formerly called hyperplastic polyposis, is a rare form of intestinal polyposis, initially described in 1970 by Goldman et al[2]. Current diagnostic criteria, manifested in 2010[35], include (1) at least five serrated polyps proximal to the sigmoid colon with two or more of these being > 10 mm; (2) any number of serrated polyps proximal to the sigmoid colon in an individual who has a first-degree relative with serrated polyposis; and (3) > 20 serrated polyps of any size distributed throughout the colon (not all in the rectum).
Although serrated polyposis provided the first evidences for the malignant potential of serrated polyps, it is still one of the most under-recognized and poorly understood intestinal polyposis syndrome. This is probably due to its rarity (1 in 3000)[57], but also to the phenotypic plasticity[58] and overlapping clinical phenotypes within this disorder[59]. However, based on clinical observations including earlier onset of CRC, multiple cancers, increased individual and familial risk, accumulating evidence indicates that serrated polyposis is a genetic predisposition syndrome to CRC and probably confers also an increased risk for some extracolonic cancers[60].
It is imperative to detect and completely remove serrated lesions, as majority of these lesions tend to progress, and contribute to the development of interval cancers. Data on the natural history of serrated polyps is limited, only retrospective studies with small sample size[61] are currently available. High risk serrated polyps are frequently flat and associated with synchronous advanced colorectal neoplasms[62]. Magnifying chromoendoscopy can facilitate to differentiate between serrated polyps, but it is still difficult to distinguish between SSA and typical HP[63]. Both endoscopists and pathologists should know the most important features in order to detect and diagnose these lesions (Table 1).
To date, no consensus guidelines exist on the management of serrated polyps, but new guidelines including recommendations for management and follow-up of serrated polyps are expected to be available in the near future. With the exception of small and diminutive HPs in the rectosigmoid, that confer no malignant potential, all other serrated polyps should be endoscopically removed. If endoscopic resection cannot be implemented, then segmental colectomy is advised[51]. There is no consensus among experts on the optimal post-polypectomy surveillance intervals, however because of lack of data and presumed faster progression rate a more intensive surveillance is recommended (Table 2).
| Serrated polyp | Intervention | Surveillance interval |
| Hyperplastic polyp in the rectosigmoid | No surveillance recommended | Screening colonoscopy at 10 yr |
| SSA without dysplasia | Endoscopic resection (EMR) | < 3 lesions, < 1 cm: 5 yr |
| ≥ 3 lesions, ≥ 1 cm: 3 yr | ||
| SSA with dysplasia | Endoscopic resection (EMR) | Complete: 2-6 mo |
| Incomplete: Segmental colectomy | ||
| TSA | Endoscopic resection (cold-snare) | Complete: 3 yr |
| Incomplete: Segmental colectomy | ||
| Serrated polyposis | Endoscopic resection | Follow-up colonoscopy at 6-12 mo |
| Children: screening at an age 10 yr younger than index case | ||
| Serrated polyposis in first-degree relative | Screening at an age 10 yr younger | Follow-up colonoscopy at 12 mo |
Due to their sessile nature, SSAs are difficult to detect and remove endoscopically. For the removal of flat SSAs endoscopic mucosal resection (EMR) is the method of choice[34]. It is recommended to use a chromoendoscopy contrast dye (either onto the surface or injected submucosally) to define the border of the lesion, lifting it, then snare removing in toto or in multiple sessions[28,34]. It is important to note that because of the thin wall of proximal colon (where SSAs typically locate[65]), this difficult technique is even more challenging, as one has take the complications of EMR (bleeding, perforation, incomplete resection) into account and it is advised to use argon plasma coagulation to reduce the risk of complications and recurrence[34,66].
It is getting generally accepted that CRC is a heterogeneous disease. Serrated pathway is serrated pathway is a rapidly evolving concept in colorectal carcinogenesis and it is postulated that 10%-20% of CRCs arise via this alternative pathway. In the past two decades since its original description our knowledge of morphologic and molecular alterations of serrated lesions has greatly expanded and these lesions are getting increasingly recognized. A major challenge is how to translate these new findings into clinical practice and how to determine appropriate surveillance intervals in order to avoid interval cancers. Further investigation is needed to better characterize natural history, optimize management and improve clinical outcomes.
We are deeply indebted to Orsolya Bakonyi for her assistance in the graphic design and Zsuzsa Kovács for her help in the preparation of the schematic figure.
P- Reviewers Hiyama T, Ho YH S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4464] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 2. | Goldman H, Ming S, Hickock DF. Nature and significance of hyperplastic polyps of the human colon. Arch Pathol. 1970;89:349-354. [PubMed] |
| 3. | Jass JR. Relation between metaplastic polyp and carcinoma of the colorectum. Lancet. 1983;1:28-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Young J, Jenkins M, Parry S, Young B, Nancarrow D, English D, Giles G, Jass J. Serrated pathway colorectal cancer in the population: genetic consideration. Gut. 2007;56:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 478] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 6. | Tateyama H, Li W, Takahashi E, Miura Y, Sugiura H, Eimoto T. Apoptosis index and apoptosis-related antigen expression in serrated adenoma of the colorectum: the saw-toothed structure may be related to inhibition of apoptosis. Am J Surg Pathol. 2002;26:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Higuchi T, Jass JR. My approach to serrated polyps of the colorectum. J Clin Pathol. 2004;57:682-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Carragher LA, Snell KR, Giblett SM, Aldridge VS, Patel B, Cook SJ, Winton DJ, Marais R, Pritchard CA. V600EBraf induces gastrointestinal crypt senescence and promotes tumour progression through enhanced CpG methylation of p16INK4a. EMBO Mol Med. 2010;2:458-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | O’Brien MJ. Hyperplastic and serrated polyps of the colorectum. Gastroenterol Clin North Am. 2007;36:947-968, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Chan AO, Broaddus RR, Houlihan PS, Issa JP, Hamilton SR, Rashid A. CpG island methylation in aberrant crypt foci of the colorectum. Am J Pathol. 2002;160:1823-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Suehiro Y, Hinoda Y. Genetic and epigenetic changes in aberrant crypt foci and serrated polyps. Cancer Sci. 2008;99:1071-1076. [PubMed] |
| 12. | Nucci MR, Robinson CR, Longo P, Campbell P, Hamilton SR. Phenotypic and genotypic characteristics of aberrant crypt foci in human colorectal mucosa. Hum Pathol. 1997;28:1396-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Takayama T, Ohi M, Hayashi T, Miyanishi K, Nobuoka A, Nakajima T, Satoh T, Takimoto R, Kato J, Sakamaki S. Analysis of K-ras, APC, and beta-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology. 2001;121:599-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Stevens RG, Swede H, Rosenberg DW. Epidemiology of colonic aberrant crypt foci: review and analysis of existing studies. Cancer Lett. 2007;252:171-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Rosenberg DW, Yang S, Pleau DC, Greenspan EJ, Stevens RG, Rajan TV, Heinen CD, Levine J, Zhou Y, O’Brien MJ. Mutations in BRAF and KRAS differentially distinguish serrated versus non-serrated hyperplastic aberrant crypt foci in humans. Cancer Res. 2007;67:3551-3554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1563] [Cited by in RCA: 1693] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 17. | Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Role for IGFBP7 in senescence induction by BRAF. Cell. 2010;141:746-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Aust DE, Baretton GB. Serrated polyps of the colon and rectum (hyperplastic polyps, sessile serrated adenomas, traditional serrated adenomas, and mixed polyps)-proposal for diagnostic criteria. Virchows Arch. 2010;457:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 431] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 20. | Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. 2002;11:1012-1018. [PubMed] |
| 21. | Kearney J, Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Bleday R, Willett WC. Diet, alcohol, and smoking and the occurrence of hyperplastic polyps of the colon and rectum (United States). Cancer Causes Control. 1995;6:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Samowitz WS, Albertsen H, Sweeney C, Herrick J, Caan BJ, Anderson KE, Wolff RK, Slattery ML. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98:1731-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Limsui D, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW, Lynch CF, Anderson KE, French AJ, Haile RW. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102:1012-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 24. | O’Brien MJ, Yang S, Mack C, Xu H, Huang CS, Mulcahy E, Amorosino M, Farraye FA. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 381] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 25. | O’Brien MJ, Yang S, Clebanoff JL, Mulcahy E, Farraye FA, Amorosino M, Swan N. Hyperplastic (serrated) polyps of the colorectum: relationship of CpG island methylator phenotype and K-ras mutation to location and histologic subtype. Am J Surg Pathol. 2004;28:423-434. [PubMed] |
| 26. | Yang S, Farraye FA, Mack C, Posnik O, O’Brien MJ. BRAF and KRAS Mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation status. Am J Surg Pathol. 2004;28:1452-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, Simms LA, Young J, James M, Montgomery GW. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 410] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 28. | Huang CS, Farraye FA, Yang S, O’Brien MJ. The clinical significance of serrated polyps. Am J Gastroenterol. 2011;106:229-240; quiz 241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 29. | Arnold CA, Montgomery E, Iacobuzio-Donahue M. The serrated pathway of neoplasia: new insights into an evolving concept. Diagn Histopathol. 2011;17:367-375. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 418] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 31. | Goldstein NS, Bhanot P, Odish E, Hunter S. Hyperplastic-like colon polyps that preceded microsatellite-unstable adenocarcinomas. Am J Clin Pathol. 2003;119:778-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology. 2005;47:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Tannapfel A, Neid M, Aust D, Baretton G. The origins of colorectal carcinoma: specific nomenclature for different pathways and precursor lesions. Dtsch Arztebl Int. 2010;107:760-766. [PubMed] |
| 34. | East JE, Saunders BP, Jass JR. Sporadic and syndromic hyperplastic polyps and serrated adenomas of the colon: classification, molecular genetics, natural history, and clinical management. Gastroenterol Clin North Am. 2008;37:25-46, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Snover D, Ahnen D, Burt R, Odze RD. Serrated Polyps of the Colon and Rectum and Serrated Polyposis. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: IARC 2010; . |
| 36. | Fidalgo CA, Santos L, Rosa I, Fonseca R, Lage P, Claro I, Chaves P, Dias Pereira A. Hyperplastic polyp? The impact of the new classification for serrated polyps. Gut. 2011;60 Suppl 3:A306. |
| 37. | Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 240] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Mäkinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50:131-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 39. | Kimura T, Yamamoto E, Yamano HO, Suzuki H, Kamimae S, Nojima M, Sawada T, Ashida M, Yoshikawa K, Takagi R. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am J Gastroenterol. 2012;107:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Sheridan TB, Fenton H, Lewin MR, Burkart AL, Iacobuzio-Donahue CA, Frankel WL, Montgomery E. Sessile serrated adenomas with low- and high-grade dysplasia and early carcinomas: an immunohistochemical study of serrated lesions “caught in the act”. Am J Clin Pathol. 2006;126:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Oono Y, Fu K, Nakamura H, Iriguchi Y, Yamamura A, Tomino Y, Oda J, Mizutani M, Takayanagi S, Kishi D. Progression of a sessile serrated adenoma to an early invasive cancer within 8 months. Dig Dis Sci. 2009;54:906-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Torlakovic EE, Gomez JD, Driman DK, Parfitt JR, Wang C, Benerjee T, Snover DC. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA). Am J Surg Pathol. 2008;32:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 43. | Yantiss RK, Oh KY, Chen YT, Redston M, Odze RD. Filiform serrated adenomas: a clinicopathologic and immunophenotypic study of 18 cases. Am J Surg Pathol. 2007;31:1238-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Ha SY, Lee SM, Lee EJ, Kang SY, Jang KT, Park CK, Kim JY, Kim YH, Chang DK, Kim KM. Filiform serrated adenoma is an unusual, less aggressive variant of traditional serrated adenoma. Pathology. 2012;44:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Jass JR, Whitehall VL, Young J, Leggett BA. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002;123:862-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 323] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 46. | Jass JR, Smith M. Sialic acid and epithelial differentiation in colorectal polyps and cancer--a morphological, mucin and lectin histochemical study. Pathology. 1992;24:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Mäkinen MJ, George SM, Jernvall P, Mäkelä J, Vihko P, Karttunen TJ. Colorectal carcinoma associated with serrated adenoma--prevalence, histological features, and prognosis. J Pathol. 2001;193:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Tuppurainen K, Mäkinen JM, Junttila O, Liakka A, Kyllönen AP, Tuominen H, Karttunen TJ, Mäkinen MJ. Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J Pathol. 2005;207:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Laiho P, Kokko A, Vanharanta S, Salovaara R, Sammalkorpi H, Järvinen H, Mecklin JP, Karttunen TJ, Tuppurainen K, Davalos V. Serrated carcinomas form a subclass of colorectal cancer with distinct molecular basis. Oncogene. 2007;26:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 50. | García-Solano J, Pérez-Guillermo M, Conesa-Zamora P, Acosta-Ortega J, Trujillo-Santos J, Cerezuela-Fuentes P, Mäkinen MJ. Clinicopathologic study of 85 colorectal serrated adenocarcinomas: further insights into the full recognition of a new subset of colorectal carcinoma. Hum Pathol. 2010;41:1359-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Leonard DF, Dozois EJ, Smyrk TC, Suwanthanma W, Baron TH, Cima RR, Larson DW. Endoscopic and surgical management of serrated colonic polyps. Br J Surg. 2011;98:1685-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Patai AV, Molnár B, Kalmár A, Schöller A, Tóth K, Tulassay Z. Role of DNA methylation in colorectal carcinogenesis. Dig Dis. 2012;30:310-315. [PubMed] [DOI] [Full Text] |
| 53. | Patai AV, Galamb O, Kalmár A, Wichmann B, Valcz G, Patai A, Leiszter K, Tóth K, Tulassay Z, Molnár B. Non-sequential DNA methylation alterations in the adenoma-dysplasia-carcinoma sequence in the distal colon. Gut. 2011;60 Suppl 3:A76. |
| 54. | Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, Wolff RK, Slattery ML. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063-6069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 598] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 55. | Pai RK, Jayachandran P, Koong AC, Chang DT, Kwok S, Ma L, Arber DA, Balise RR, Tubbs RR, Shadrach B. BRAF-mutated, microsatellite-stable adenocarcinoma of the proximal colon: an aggressive adenocarcinoma with poor survival, mucinous differentiation, and adverse morphologic features. Am J Surg Pathol. 2012;36:744-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 56. | Samowitz WS, Curtin K, Ma KN, Schaffer D, Coleman LW, Leppert M, Slattery ML. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10:917-923. [PubMed] |
| 57. | Lockett MJ, Atkin WS. Hyperplastic polyposis: prevalence and cancer risk. Gut. 2001;48 Suppl 1:A4. |
| 58. | Roberts A, Nancarrow D, Clendenning M, Buchanan DD, Jenkins MA, Duggan D, Taverna D, McKeone D, Walters R, Walsh MD. Linkage to chromosome 2q32.2-q33.3 in familial serrated neoplasia (Jass syndrome). Fam Cancer. 2011;10:245-254. [PubMed] |
| 59. | Kalady MF, Jarrar A, Leach B, LaGuardia L, O’Malley M, Eng C, Church JM. Defining phenotypes and cancer risk in hyperplastic polyposis syndrome. Dis Colon Rectum. 2011;54:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 60. | Jarrar AM, Church JM, Fay S, Kalady MF. Is the phenotype mixed or mistaken? Hereditary nonpolyposis colorectal cancer and hyperplastic polyposis syndrome. Dis Colon Rectum. 2009;52:1949-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Teriaky A, Driman DK, Chande N. Outcomes of a 5-year follow-up of patients with sessile serrated adenomas. Scand J Gastroenterol. 2012;47:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Rondagh EJ, Masclee AA, Bouwens MW, Winkens B, Riedl RG, de Bruïne AP, de Ridder R, Kaltenbach T, Soetikno RM, Sanduleanu S. Endoscopic red flags for the detection of high-risk serrated polyps: an observational study. Endoscopy. 2011;43:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Kashida H, Ikehara N, Hamatani S, Kudo SE, Kudo M. Endoscopic characteristics of colorectal serrated lesions. Hepatogastroenterology. 2011;58:1163-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Terdiman JP, McQuaid KR. Surveillance guidelines should be updated to recognize the importance of serrated polyps. Gastroenterology. 2010;139:1444-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Gurudu SR, Heigh RI, De Petris G, Heigh EG, Leighton JA, Pasha SF, Malagon IB, Das A. Sessile serrated adenomas: demographic, endoscopic and pathological characteristics. World J Gastroenterol. 2010;16:3402-3405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 66. | Brooker JC, Saunders BP, Shah SG, Thapar CJ, Suzuki N, Williams CB. Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: a randomized trial and recommendations. Gastrointest Endosc. 2002;55:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |