Published online Dec 28, 2013. doi: 10.3748/wjg.v19.i48.9318
Revised: October 16, 2013
Accepted: November 1, 2013
Published online: December 28, 2013
Processing time: 139 Days and 4.1 Hours
AIM: To evaluate the potential effectiveness of hydroxynaphthoquinone mixture (HM) in rats with 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis.
METHODS: Colitis was induced by intracolonic administration of TNBS (80 mg/kg, dissolved in 50% ethanol). Rats were treated daily for 7 d with HM (2.5, 5, 10 mg/kg) and mesalazine 100 mg/kg 24 h after TNBS instillation. Disease progression was monitored daily by observation of clinical signs and body weight change. At the end of the experiment, macroscopic and histopathologic lesions of rats were scored, and myeloperoxidase (MPO) activity was determined. We also determined inflammatory cytokine tumor necrosis factor (TNF)-α level by ELISA, Western blotting and immunochemistry to explore the potential mechanisms of HM.
RESULTS: After intracolonic instillation of TNBS, animals developed colitis associated with soft stool, diarrhea and marked colonic destruction. Administration of HM significantly attenuated clinical and histopathologic severity of TNBS-induced colitis in a dose-dependent manner. It abrogated body weight loss, diarrhea and inflammation, decreased macroscopic damage score, and improved histological signs, with a significant reduction of inflammatory infiltration, ulcer size and the severity of goblet cell depletion (all P < 0.05 vs TNBS alone group). HM could reduce MPO activity. In addition, it also decreased serum TNF-α level and down-regulated TNF-α expression in colonic tissue. This reduction was statistically significant when the dose of HM was 10 mg/kg (P < 0.05 vs TNBS alone group), and the effect was comparable to that of mesalazine and showed no apparent adverse effect. The underlying mechanism may be associated with TNF-α inhibition.
CONCLUSION: These findings suggest that HM possesses favourable therapeutic action in TNBS-induced colitis, which provides direct pharmacological evidence for its clinical application.
Core tip: Current therapies for inflammatory bowel disease are limited by lack of effectiveness, drug refractoriness or severe adverse effects. Therefore, there is an urgent need for more effective and safe therapeutic approaches. Due to their favourable effect and less side effects, looking for novel agents from herbal and natural products has been a research focus for a long time. Previous studies demonstrate that hydroxynaphthoquinones exert therapeutic action on chronic inflammatory disease. In this study, hydroxynaphthoquinones showed beneficial effect on 2,4,6-trinitrobenzene sulfonic acid-induced colitis via tumor necrosis factor-α inhibition, and the effect was comparable to that of mesalazine. This provides pharmacological evidence for its clinical application.
-
Citation: Fan HY, Zhang ZL, Liu K, Yang MY, Lv WH, Che X, Xu H, Song WW. Effectiveness of a hydroxynaphthoquinone fraction from
Arnebia euchroma in rats with experimental colitis. World J Gastroenterol 2013; 19(48): 9318-9327 - URL: https://www.wjgnet.com/1007-9327/full/v19/i48/9318.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i48.9318
Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic, relapsing inflammatory intestinal disorder[1]. The incidence and prevalence of IBD are now increasing worldwide. The highest incidences have been reported in North American and Northern Europe. In recent years, the incidence rates appear to be increasing in developing countries in Europe and Asia with westernisation of lifestyle and industrialization, including China, South Korea and India[2]. The exact pathogenesis remains elusive, but thus far, IBD is thought to be the results of interaction between genetic alterations and environment factors that induce an aberrant mucosal immune response, in which inflammatory cytokines play a critical role in the induction of colonic tissue damage[3,4]. Available therapies for IBD include conventional anti-inflammatory agents (such as 5-aminosalicylates and corticosteroids), immune modulators and biological therapy. Biological therapy aims at antagonizing pro-inflammatory molecules. Thus, inflammatory cytokines are the most logical targets for IBD treatment. Among various cytokines, tumor necrosis factor (TNF)-α is the cytokine that has been widely studied. Currently, the use of TNF-α blockers is the only licensed biological therapy for IBD and several TNF-α blockers (infliximab, adalimumab and certolizumab) have been applied in clinical practice. Although these available agents have shown clinical benefits to some degree, they are not entirely effective and have multiple adverse effects. Furthermore, IBD management requires long-term treatment that often leads to drug refractoriness or intolerance[3]. Patients who are unresponsive to the current therapy still suffer from this common disease. Therefore, it is necessary to develop novel therapeutic approaches.
Zicao, the dried root of Arnebia euchroma (Royle) Johnst, is a traditional Chinese herbal medicine. It has been used in China for thousands of years for the treatment of various diseases[5,6]. Naphthoquinones (also termed as hydroxynaphthoquinones in Pharmacopoeia of China) have been identified as the main representative active ingredients of Zicao. Hydroxynaphthoquinones mainly consist of alkannin and shikonin as well as their derivatives. Modern pharmacological studies have demonstrated that hydroxynaphthoquinones possess multiple biological activities such as anti-inflammation, wound healing, antibacterial and antifungal[5-9]. In particular, shikonin exerts significant anti-arthritic and immunomodulatory effects. It could inhibit the expression and transcriptional activation of TNF-α and reduce the production of inflammatory mediators[10-12]. Furthermore, the ointment containing alkannin derivatives has been applied successfully to the treatment of traumatic ulcers and acute anal fissures[6]. Based on these characteristics, we hypothesized that hydroxynaphthoquinones may prevent and even cure IBD.
In previous research, our group isolated a mixture of hydroxynaphthoquinone alkannin derivatives and validated its protective effect against experimental arthritis and its analgesic effect, as well as its modulatory effect on the serum TNF-α level[13,14]. Chemical analysis identified seven constituents: alkannin, acetylalkannin, β-acetoxyisovalerylalkannin, deoxyalkannin, β,β’-dimethylacrylalkannin, α-methybutyrylalkannin, and isovalerylalkannin[14]. The objective of this study was to investigate the potential therapeutic action of this hydroxynaphthoquinone mixture (HM) in the murine model of 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis.
Male Sprague-Dawley (SD) rats (weight, 200-220 g) were purchased from the Animal Department of the College of Medicine, Beijing University [certificate No. SCXK (Jing) 2006-0008)]. All animals were allowed to acclimate for at least 1 wk at a temperature of 24 ± 1 °C and humidity of 55% ± 5%. All rats were housed in cages with food and tap water ad libitum. The experiment procedures were approved by Office of Experimental Animal Management Committee of Shandong Province, China.
HM was provided by Shandong Target Drug Research Co. Ltd. Mesalazine slow-release granule was the product of Ethypharm Industries (France). TNBS was supplied by Sigma Chemical Co. (United States). Polyclonal anti-TNF-α antibody was purchased from Santa Cruz Biotechnology (CA, United States). Anti-β-actin antibody was obtained from Beyotime Institute of Biotechnology (Jiangsu Province, China). Rabbit anti-goat IgG was purchased from Boster Biotechnology (Wuhan, Hubei Province, China). Myeloperoxidase (MPO) detection kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). TNF-α ELISA Kit was the product of R and D System (United States), which was obtained from Shanghai Chuanxiang Biotechnology Co. Ltd (Shanghai, China).
After a 24 h fasting, rats were anaesthetized with pentobarbital, and then TNBS (80 mg/kg, dissolved in 50% ethanol) was instilled into the colon[15]. Control rats received saline instead. Animals were orally administered with mesalazine 100 mg/kg and HM (2.5, 5, 10 mg/kg) 24 h after TNBS instillation, daily for 7 d. Body weight and diarrhea of rats were recorded daily. On day 8, rats were anaesthetized with pentobarbital. Blood was collected from the abdominal aorta of rats. The serum was prepared by centrifuging the blood at 3000 g for 15 min for TNF-α assay. The colon was removed and placed on an ice-cold plate, cleared of fat and mesentery, and blotted on filter paper. Then, the colon was longitudinally opened, washed gently with ice-cold saline and blotted dry with filter paper. Finally, colon tissue was weighted. Its length was measured and the extent of macroscopic damage was evaluated. Afterwards, the colon was divided into several segments. One segment of the colon (approximately 2 cm) was used for histological examination. The rest of tissue segments were snap-frozen in liquid nitrogen and stored at -80 °C for MPO activity measurement and Western blotting analysis.
Macroscopic colonic damage was assessed using a magnifying glass by an independent observer and was scored. The scale for macroscopic damage ranged from 0-10 and was based on the appearance of ulceration, thickening of the bowel wall, sites of ulceration and sites of inflammation[16].
The tissue was fixed in 10% neutral buffered formalin, embedded in paraffin and stained with hematoxylin and eosin (H and E). Colonic damage and inflammation were scored blindly according to the criteria described previously[17].
The assessment of MPO activity is a well established biochemical assay for quantifying intestinal inflammation[18]. The colonic samples (100 mg) were thawed and homogenized on ice in PBS buffer containing hexadecyl trimethyl ammonium bromide to prepare a 5% homogenate. The subsequent assay was performed according to the manufacturers’ instructions. The absorbance was read at 460 nm using visible spectrophotometer. MPO activity was determined using the O-dianisidine method and the final results were expressed as units per gram of wet tissue.
Serum was obtained from all groups of rats on the final experimental day and then stored at -20 °C until analysis. The supernatant was used to measure the level of TNF-α with a rat TNF-α ELISA kit. The procedure was performed according to the manufacturer’s instructions.
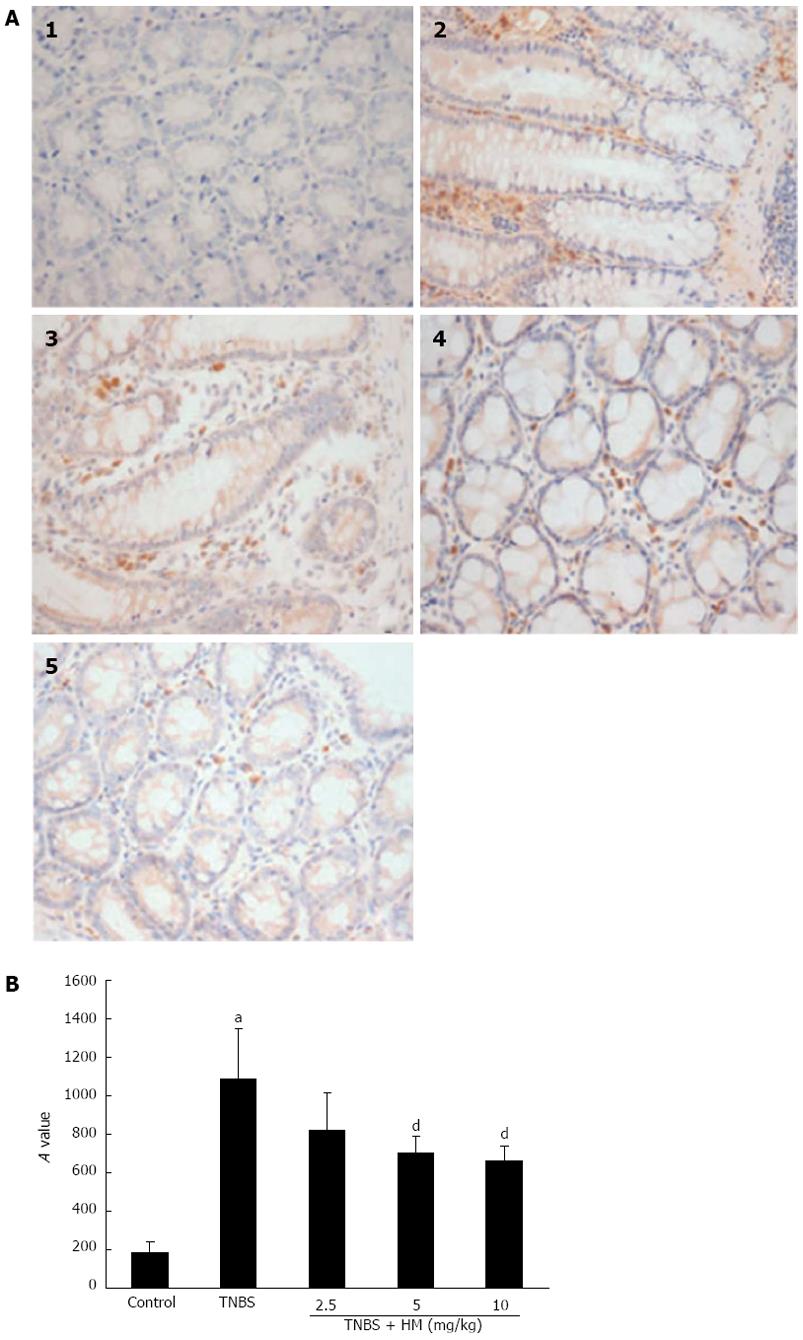
Paraffin-embedded colonic tissue sections (5 μmol/L) were fixed in 4% paraformaldehyde, deparaffinized and dehydrated through graded ethanol. The sections were washed three times with PBS for 5 min each, blotted drying, and then treated with 3% hydrogen peroxide for 30 min at room temperature to block the endogenous peroxidase activity. Afterwards, the sections were immersed in antigen retrieval solution (citrate buffer, pH 6.0) for 10 min. This was followed by rinsing with PBS. After blocking with normal goat serum for 30 min at 37 °C, sections were co-incubated with a primary anti-TNF-α antibody (1:150 dilution in PBS) overnight and then with a peroxidase-conjugated anti-rabbit IgG secondary antibody for 1 h at room temperature. Thereafter, the sections were incubated with 3,3-diaminobenzidine (DAB) regents for 10 min, counterstained with hematoxylin, dehydrated and mounted for microscopy analysis. The intensity of immunoreactivity was examined with a pathological image analyzer and IMAGE-PRO PLUS analyzing program. The results were represented as mean integrated optical density (A) value for each sample.
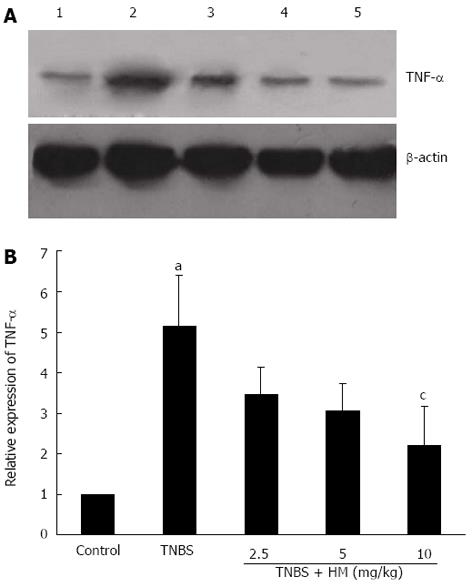
Frozen colonic tissues (60 mg) were thawed and later mechanically homogenized on ice. Homogenates were centrifuged at 10000 g for 20 min and total proteins were collected from the supernatant. The protein concentration was measured with a BCA protein assay kit. The protein was boiled in SDS sample loading buffer (Tris-HCl pH 6.8; 10% SDS; 0.5% bromophenol blue; 50% glycerine; 5% β-mercaptoethanol) for 5 min. Then equal amounts of protein (120 μg) were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis for 60 min, the protein was transferred onto polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 3% skim milk and saturated in Tris buffered saline with 1% Tween 20 for 1 h at room temperature. Subsequently, the membrane was incubated with the primary anti-TNF-α antibody (diluted 1:100 in TBST) overnight at 4 °C. After that, the membrane was washed three times for 5 min each and probed with a horseradish peroxidase-conjugated anti-rabbit IgG antibody (diluted 1:10000 in TBST). Protein was detected using an enhanced chemiluminescence (ECL) detection kit (Beyotime Institute of Biotechnology), and bands were visualized by exposure to photographic film. Densitometric analysis of protein bands was performed using Quantity One v4.4.0.36 analyzer software (BIO-RAD).
Statistical analysis was performed using SPSS software 11.5 for windows. All results are expressed as mean ± SD. Comparisons between groups of nonparametric data were made with the Kruskal-Wallis test followed by the Mann-Whitney U test. For evaluation of the body weight and TNF-α level, one-way ANOVA followed by Tukey’s post hoc test was used. P < 0.05 was considered significant.
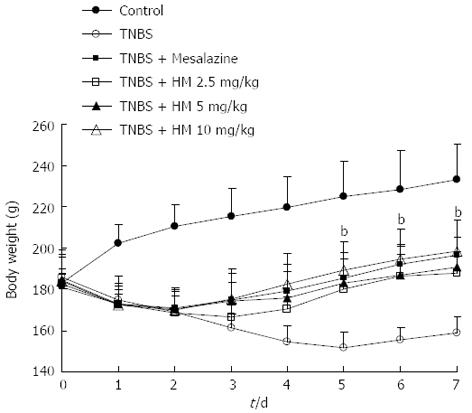
After intracolonic instillation of TNBS, animals developed colitis associated with soft stool and diarrhea. All TNBS-treated rats had profound weight loss compared with the weight gain seen in controls (all P < 0.01). Rats treated with HM and mesalazine gradually recovered the lost body weight beginning on day 3, accompanied by improving symptoms (Figure 1).
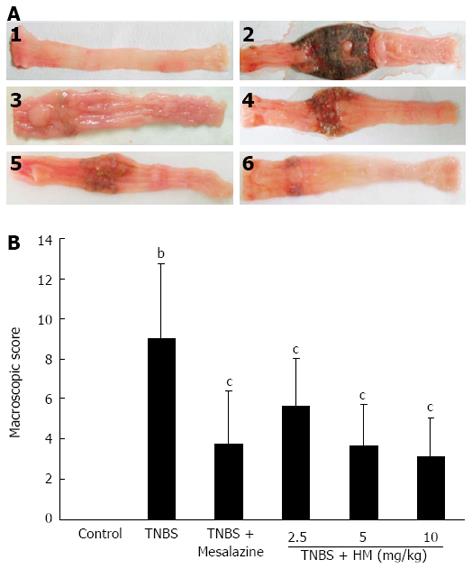
Control animals showed no colonic damage and the colonic damage score was zero. Rats in the TNBS group displayed hyperemia, thickening of the bowel, necrosis, inflammation, and a large area of ulceration. Moreover, moderate to severe adhesion of the colon to the surrounding organs was also observed. The macroscopic colon damage score was significantly increased. The severity of colonic destruction was markedly ameliorated after oral administration of HM and mesalazine, with reduced area of inflammation and ulcer (Figure 2).
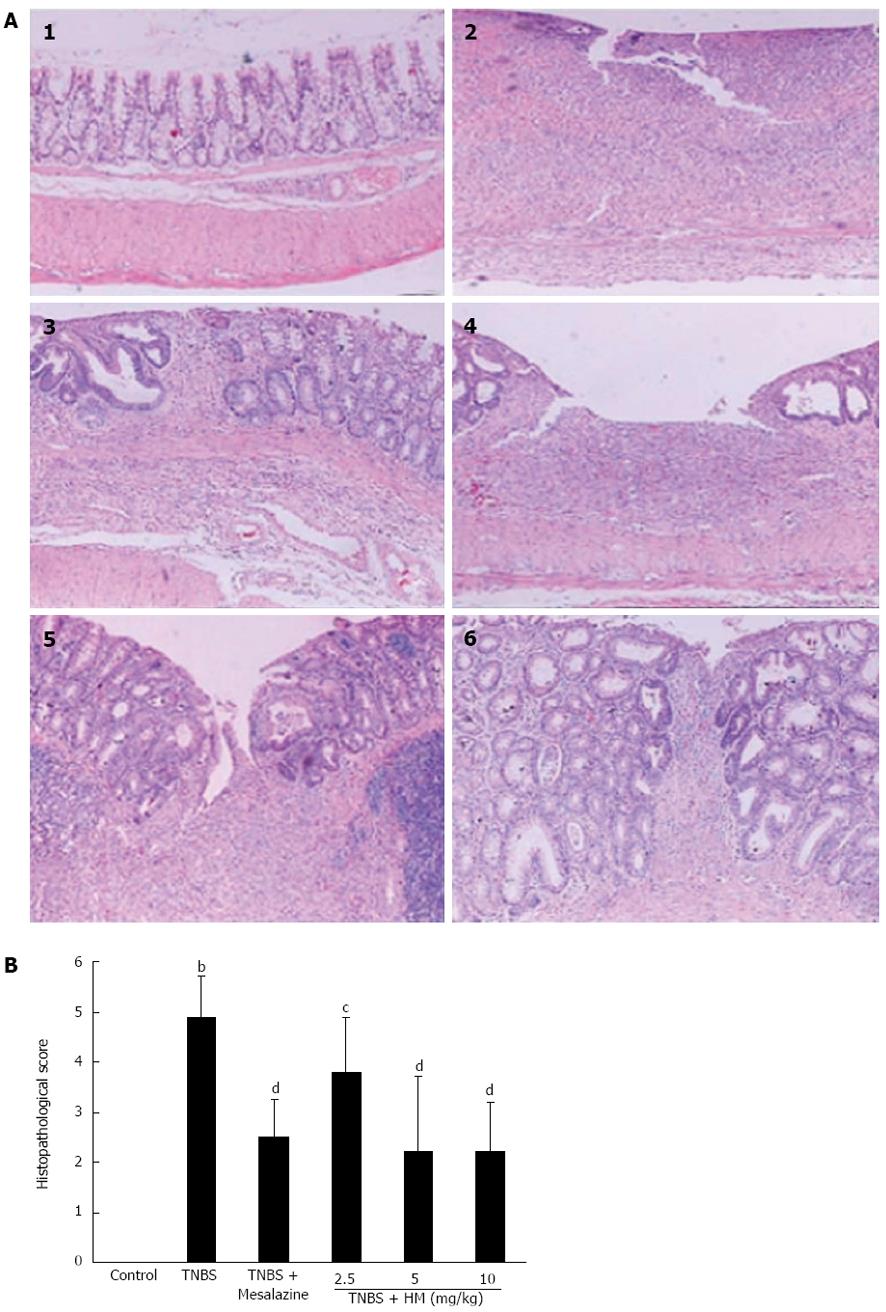
There was no histopathological change in the colons of control rats. Histologic evaluation of the colon of TNBS-treated rats showed transmural inflammation involving all layers of the bowel. The inflammatory process was associated with patchy ulceration, epithelial cell loss, pronounced depletion of goblet cells, distortion of the tubular glands, numerous inflammatory cell infiltrations, and dilated crypts. When rats were administered with HM, these histologic signs were much improved, with significant reduction of inflammatory infiltration and ulcer size. The transmural involvement of the lesions was reduced, and the goblet cell depletion was less severe (Figure 3).
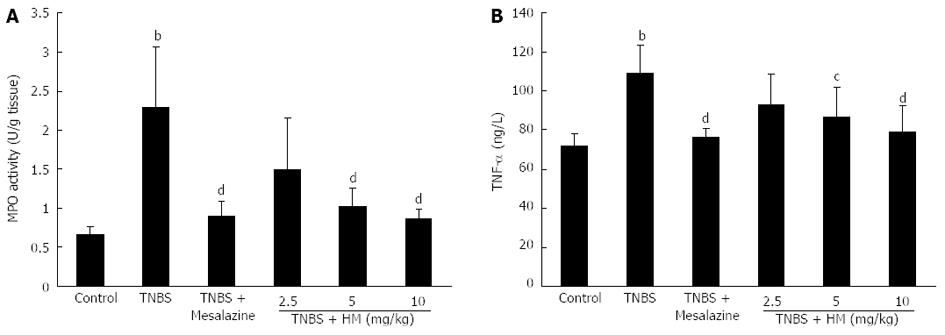
Myeloperoxidase in the intestine is a well-known enzyme that is directly correlated with the degree of neutrophil infiltration[18]. As shown in Figure 4A, MPO activity was markedly increased in colonic tissue following TNBS instillation compared to controls (P < 0.01, 2.29 ± 0.78 U/g vs 0.65 ± 0.11 U/g tissue). After treatment with HM, MPO activity were reduced to be 1.49 ± 0.66, 1.02 ± 0.24 and 0.86 ± 0.13 U/g tissue for HM 2.5, 5 and 10 mg/kg, respectively. The effect was significant at doses of 5 and 10 mg/kg. This indicated that treatment with HM attenuated the degree of inflammation response. The similar efficacy for mesalazine was also observed.
As depicted in Figure 4B, serum TNF-α level was obviously higher in TNBS-treated rats compared with that in controls. In contrast, pretreatment with HM and mesalazine prevented the increase in TNF-α level. The mean serum TNF-α levels were determined to be 71.90 ± 6.33 ng/L for the control group, 109.05 ± 14.30 ng/L for the TNBS group, 76.16 ± 4.64 ng/L for the mesalazine group, 93.00 ± 15.24 ng/L for the HM 2.5 mg/kg group, 86.96 ± 15.26 ng/L for the HM 5 mg/kg group and 78.72 ± 13.94 ng/L for the HM 10 mg/kg group.
Representative samples of each group were immunohistochemically stained for the expression of TNF-α. As shown in Figure 5A1, colonic section obtained from the control group showed negative staining. TNBS-treated rats showed strongly positive staining for TNF-α. Positively stained cells appeared in the mucosal and submucosal inflammatory cells (Figure 5A2 and B). Administration of HM attenuated the degree of TNF-α staining in the colon tissue. Significantly less positive cells were observed in tissues from HM 5 mg/kg- and 10 mg/kg-treated rats than in those from TNBS-treated rats (Figure 5A4, A5 and Figure 5B).
As shown in Figure 6, higher TNF-α expression was observed after TNBS instillation as compared with controls. HM treatment reduced the expression of TNF-α in a dose-dependent manner.
Many plant-derived extracts or chemicals have pharmacological effects and clinical benefits, which provide a great potential in improving the symptoms of IBD. The published literature has revealed that several natural products exhibit encouraging anti-IBD activity by inhibition of cytokine production, such as flavonoids or polyphenolic compounds[19]. TNBS-induced colitis is a Th1 cell-mediated inflammatory disease, associated with excessive secretion of cytokines as a consequence of exaggerated macrophage and neutrophil infiltration and activation, giving rise to transmurally inflamed intestinal mucosa, which displays clinical, biochemical, and pathological similarities to human CD. It is a commonly used experimental model system to test potential therapeutic agents[20]. Thus, the present study was performed to investigate the potential effect of HM on CD using TNBS-induced colitis.
The results obtained from the study demonstrate for the first time the efficacy of HM in intestinal inflammation, confirming the hypothesis mentioned in the introduction section that hydroxynaphthoquinones possess the ability to attenuate symptoms of IBD. Administration of HM at the onset of the disease ameliorated the clinical severity of the wasting disease, abrogating body weight loss, diarrhea, inflammation and the area of ulceration. This beneficial effect was further evidenced by histological evaluation, with a marked reduction in the extent and severity of inflamed tissue damage and infiltration of inflammatory cells. The beneficial effect was also established by a decrease in MPO activity. As mentioned above, MPO is a marker of neutrophil infiltration. It has been reported that MPO activity is increased in several experimental colitis models, including TNBS-induced colitis[18], and it is widely used to quantify intestinal inflammation and assess the degree of inflammation. Thus, a reduction of MPO activity can be interpreted as a manifestation of the anti-inflammatory effect of a given compound[18,21,22]. Inhibition of MPO activity by HM was consistent with the results observed in the histological examination, in which the level of inflammatory cell infiltration in colonic tissues was lower in HM-treated animals than in TNBS-treated rats.
Mesalazine was used as a reference in this study. Several reports suggest that mesalazine acts locally in the colon. After intragastric administration of mesalazine, it is absorbed from intestinal lumen and concentrates in the mucosa. The effectiveness of the drug directly depends on its mucosal concentration[23,24]. In the present experiment, oral administration with mesalazine 100 mg/kg resulted in a significant improvement in intestinal lesions, which was consistent with previous findings[24,25]. The effect of HM 10 mg/kg was comparable to that of mesalazine.
The anti-inflammatory effect and protection against tissue injury exerted by HM was further confirmed by the down-regulation of TNF-α in serum and colonic tissue. TNF-α is an important inflammatory mediator and plays a key role in the colonic damage. Among the various cytokines involved in the pathogenesis of TNBS colitis, TNF-α appears to be a key regulator since it has been reported that TNBS-induced colitis could not be induced in TNF-α-deficient mice and is far more severe in mice that over-express this inflammatory cytokine[26]. Different agents interfering with TNF-α signaling have been successful in the treatment of subsets of CD patients, and display favourable therapeutical effect, particularly TNF-α blockers[27]. Moreover, the majority of previous preclinical studies and current therapeutic approaches[27-29] have confirmed the idea that therapy that can address an essential element of a final common pathologic pathway participating in IBD could potentially treat this disease[30]. Therefore, we assessed the impact of HM on TNF-α signaling and explored the underlying mechanism. The results obtained from the present study showed that HM significantly lowered TNF-α level in serum and reduced TNF-α expression in colonic tissue. This indicates that the preventive effect of HM against TNBS-induced colonic injury is directly or indirectly related to its TNF-α inhibition. However, given that many other signaling pathways and transcription factors are involved in the regulation of TNF-α activity, additional research is needed to examine the exact mechanisms of action of HM.
In summary, the present study demonstrates that treatment with HM attenuates the clinical symptoms of TNBS-induced colitis, resulting in significant histological improvement, reduced MPO activity and TNF-α level in serum, and down-regulation of TNF-α expression in colonic tissue. The underlying mechanism of action of HM may be associated with TNF-α inhibition. These results provide supporting evidence for the clinical application of HM.
The incidence and prevalence of inflammatory bowel disease (IBD) are increasing at a disturbing rate worldwide, and now it has become a global disease. Current therapies for IBD are limited by lack of effectiveness, drug refractoriness or severe adverse effects. Therefore, there is an urgent need for more effective and safe therapeutic approaches.
Looking for novel agents from herbal or natural products has been a research focus for a long time. Many plant-derived extracts or chemicals have pharmacological effects and clinical benefits. The published literature has revealed that several natural products exhibit encouraging anti-IBD activity by inhibition of cytokine production, such as flavonoids or polyphenolic compounds. Hydroxynaphthoquinones have been identified as the main active ingredients of Zicao, a traditional Chinese herbal medicine, and possess potent anti-inflammatory, wound healing and antibacterial activities. Yet no studies have evaluated the therapeutical effect of hydroxynaphthoquinones on treating inflammatory intestinal diseases so far. In this study, the authors isolate a hydroxynaphthoquinone mixture (HM) from Zicao, which mainly contains seven alkannin derivatives, and demonstrate that HM showed beneficial effect on TNBS-induced colitis.
This is the first study to report that hydroxynaphthoquinones exert therapeutical effect on mucosal inflammation by modulating tumor necrosis factor-α, and the effect of hydroxynaphthoquinones is comparable to that of mesalazine, a classical anti-IBD drug.
Elucidation of the effect and mechanism of action of hydroxynaphthoquinone mixture (HM) in the treatment of inflammatory intestinal disease provides pharmacological evidence for its further development as a candidate for treating IBD, and for its clinical application.
This study describes the beneficial effects of HM from Arnebia euchroma in an experimental model of colitis in rats. It revealed that HM significantly attenuated the severity of mucosal lesions. The authors have made a complex and interesting study.
P- Reviewers: Boros M, Fitzpatrick LR S- Editor: Cui XM L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12 Suppl 1:S3-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 636] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 2. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1561] [Article Influence: 111.5] [Reference Citation Analysis (1)] |
| 3. | Biasi F, Leonarduzzi G, Oteiza PI, Poli G. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid Redox Signal. 2013;19:1711-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 4. | Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 887] [Cited by in RCA: 852] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 5. | Huang ZS, Zhang M, Ma L, Gu LQ. A survery of chemical and pharmacologic studies on Zicao. Tianranchanwu Yanjiu Yu Kaifa. 1999;12:73-82. |
| 6. | Papageorgiou VP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew Chem Int Ed. 1999;38:270-300. |
| 7. | Singh B, Sahu PM, Jain SC, Singh S. Estimation of naphthaquinones from Arnebia hispidissima (Lehm.) DC. In vivo and in vitro. I. Anti-inflammatory screening. Phytother Res. 2004;18:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Seto Y, Motoyoshi S, Nakamura H, Imuta J, Ishitoku T, Isayama S. [Effect of shikonin and its derivatives, pentaacetylated shikonin (MDS-004) on granuloma formation and delayed-type allergy in experimental animals]. Yakugaku Zasshi. 1992;112:259-271. [PubMed] |
| 9. | Shen CC, Syu WJ, Li SY, Lin CH, Lee GH, Sun CM. Antimicrobial activities of naphthazarins from Arnebia euchroma. J Nat Prod. 2002;65:1857-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Dai Q, Fang J, Zhang FS. Dual role of shikonin in early and late stages of collagen type II arthritis. Mol Biol Rep. 2009;36:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Staniforth V, Wang SY, Shyur LF, Yang NS. Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor alpha promoter in vivo. J Biol Chem. 2004;279:5877-5885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Su PF, Staniforth V, Li CJ, Wang CY, Chiao MT, Wang SY, Shyur LF, Yang NS. Immunomodulatory effects of phytocompounds characterized by in vivo transgenic human GM-CSF promoter activity in skin tissues. J Biomed Sci. 2008;15:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Zhao HQ, Liu JF, Liu K. Hydroxynaphthoquinone compounds from Arnebia euchroma and their inhibitory activities of phosphodiesterase 4. Zhongguo Shiyanfangjixue Zazhi. 2010;16:96-99. |
| 14. | Fan H, Yang M, Che X, Zhang Z, Xu H, Liu K, Meng Q. Activity study of a hydroxynaphthoquinone fraction from Arnebia euchroma in experimental arthritis. Fitoterapia. 2012;83:1226-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [PubMed] |
| 16. | Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29-36. [PubMed] |
| 17. | Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, Ito E, Suzuki I, Kulkarni AD, Kawajiri A. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 196] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344-1350. [PubMed] |
| 19. | Hur SJ, Kang SH, Jung HS, Kim SC, Jeon HS, Kim IH, Lee JD. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr Res. 2012;32:801-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1024] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 21. | Camuesco D, Peran L, Comalada M, Nieto A, Di Stasi LC, Rodriguez-Cabezas ME, Concha A, Zarzuelo A, Galvez J. Preventative effects of lactulose in the trinitrobenzenesulphonic acid model of rat colitis. Inflamm Bowel Dis. 2005;11:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Frieri G, Giacomelli R, Pimpo M, Palumbo G, Passacantando A, Pantaleoni G, Caprilli R. Mucosal 5-aminosalicylic acid concentration inversely correlates with severity of colonic inflammation in patients with ulcerative colitis. Gut. 2000;47:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Rousseaux C, Lefebvre B, Dubuquoy L, Lefebvre P, Romano O, Auwerx J, Metzger D, Wahli W, Desvergne B, Naccari GC. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med. 2005;201:1205-1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 402] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 25. | Rachmilewitz D, Karmeli F, Schwartz LW, Simon PL. Effect of aminophenols (5-ASA and 4-ASA) on colonic interleukin-1 generation. Gut. 1992;33:929-932. [PubMed] |
| 26. | Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Büschenfelde KH, Strober W, Kollias G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immunol. 1997;27:1743-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 309] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Löwenberg M, D’Haens G. Novel targets for inflammatory bowel disease therapeutics. Curr Gastroenterol Rep. 2013;15:311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Ten Hove T, Corbaz A, Amitai H, Aloni S, Belzer I, Graber P, Drillenburg P, van Deventer SJ, Chvatchko Y, Te Velde AA. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-alpha production in mice. Gastroenterology. 2001;121:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Kretzmann NA, Fillmann H, Mauriz JL, Marroni CA, Marroni N, González-Gallego J, Tuñón MJ. Effects of glutamine on proinflammatory gene expression and activation of nuclear factor kappa B and signal transducers and activators of transcription in TNBS-induced colitis. Inflamm Bowel Dis. 2008;14:1504-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1003] [Article Influence: 55.7] [Reference Citation Analysis (0)] |