Published online Dec 21, 2013. doi: 10.3748/wjg.v19.i47.9049
Revised: September 1, 2013
Accepted: September 15, 2013
Published online: December 21, 2013
Processing time: 198 Days and 10.9 Hours
AIM: To investigate serum insulin-like growth factor-binding protein 5 (IGFBP-5) levels and intestinal IGFBP-5 expression in patients with Crohn’s disease (CD).
METHODS: We analyzed the serum concentrations and intestinal expression of IGFBP-5 in 42 patients with CD, of whom 26 had endoscopically or radiologically proven stricture formation. Nine of the 42 patients had active disease, with a Crohn’s disease activity index > 150. Serum IGFBP-5 levels were analyzed in 20 healthy controls matched by sex and age to the CD patients. Serum IGFBP-5 was measured using an enzyme-linked immunosorbent assay. Intestinal tissue was obtained from patients through endoscopic biopsies. IGFBP-5 expression was detected using immunohistochemistry and was scored semiquantitatively.
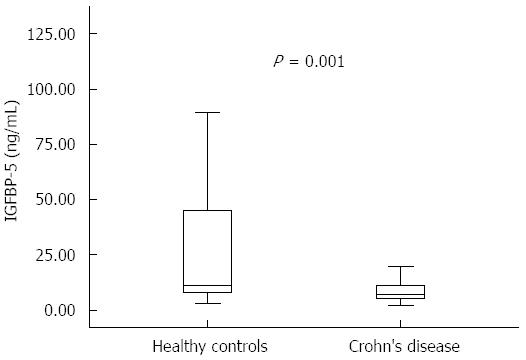
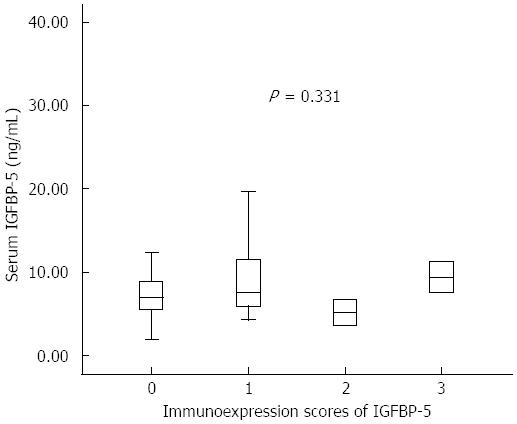
RESULTS: The median serum IGFBP-5 concentrations of CD patients were significantly lower compared with healthy controls [median 7.2 (IQR: 5.5-11.3) ng/mL vs 11.3 (8.0-44.6) ng/mL, P < 0.001]. There was no significant difference between median serum IGFBP-5 levels in CD patients with or without stricture formation [6.9 (5.5-11.3) ng/mL vs 7.8 (5.3-10.1) ng/mL, P = 0.815]. The serum IGFBP-5 levels were not significantly different between patients with active disease and inactive disease [7.2 (6.5-7.6) ng/mL vs 7.2 (5.5-11.3) ng/mL, P = 0.890]. However, a significant correlation was observed between serum IGFBP-5 levels and platelet count (PLT) (r = 0.319, P = 0.0395). No significant correlation was found between tissue IGFBP-5 immunohistochemical staining intensity scores and serum IGFBP-5 levels. No significant difference was found when comparing the serum IGFBP-5 levels among the patients with different tissue IGFBP-5 staining scores (absent/very weak, weak, moderate or strong). There was a significant correlation between tissue IGFBP-5 staining scores and white blood cell count (r = 0.391, P = 0.01) and PLT (r = 0.356, P = 0.021).
CONCLUSION: Our results indicate that serum IGFBP-5 concentrations were lower in CD patients compared to healthy controls regardless of disease activity or the presence of stricture formation.
Core tip: Previous studies have suggested that insulin-like growth factors are important for the growth and development of visceral smooth muscle. In particular, increased insulin-like growth factor-binding protein 5 expression has been described in inflamed and fibrotic intestinal tissue. In this study, we aim to investigate the possible role of insulin-like growth factor-binding protein 5 in Crohn’s disease with stricture involvement. Crohn’s disease patients had lower serum levels of IGFBP-5 compared to healthy controls. The results of the study suggest that additional research is necessary to explain the low circulating levels of IGFBP-5 in Crohn’s disease patients.
- Citation: Adali G, Yorulmaz E, Ozkanli S, Ulasoglu C, Bayraktar B, Orhun A, Colak Y, Tuncer I. Serum concentrations of insulin-like growth factor-binding protein 5 in Crohn’s disease. World J Gastroenterol 2013; 19(47): 9049-9056
- URL: https://www.wjgnet.com/1007-9327/full/v19/i47/9049.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i47.9049
Crohn’s disease (CD), a condition characterized by chronic inflammation of the alimentary tract, arises from a complex interaction between genetic, immunological, and microbial factors[1]. More than one-third of CD patients develop a distinct fibrostenosing phenotype with progressive intestinal strictures and potential intestinal obstruction[2]. Intestinal obstruction and fistulae are the main indications for surgery in patients with CD[3]. Stricture formation is caused by a combination of smooth muscle cell hyperplasia, smooth muscle cell hypertrophy, and excessive net extracellular matrix production by intestinal smooth muscle cells[4]. The factors underlying stricture development in CD are not completely understood. It is critical to develop markers that can be used to predict intestinal stricture formation in the early stages of CD.
The insulin-like growth factor (IGF) system has a critical role in regulating the growth and development of visceral and vascular smooth muscle[5]. Insulin-like growth factors (IGF-I and IGF-II) are transported in the serum by insulin-like growth factor-binding proteins (IGFBPs; IGFBP-1 to 6), which are produced in the liver. IGFBPs are also produced by non-liver tissues, in which they act in autocrine and paracrine manners to modulate responses to IGFs[6]. Despite their structural similarity, each IGFBP has unique characteristics and functions. Insulin-like growth factor-binding protein 5 (IGFBP-5) is the most conserved of the IGFBPs, has several regulatory functions, and is among the IGFBP subtypes that display IGF-independent effects. The most important in vivo regulator of IGFBP-5 expression is IGF-I. In normal adult human serum, IGFBP-5 levels are positively correlated with IGF-I concentrations[7]. In patients with CD, IGF-I expression is specifically upregulated in smooth muscle cells in regions of stricture compared to normal margins; this upregulation is accompanied by upregulated IGFBP-5 expression, which acts synergistically with IGF-I in these cells[8]. Increased IGFBP-5 expression has also been described in two human fibrotic disorders: systemic sclerosis and idiopathic pulmonary fibrosis[9,10].
Several studies have investigated the serum concentrations of IGF-1 and IGFBP-3 in active inflammatory bowel disease (IBD) patients and found significantly decreased serum levels[11-16]. There are currently no data available regarding serum IGFBP-5 levels in patients with CD. It is unknown whether circulating IGFBP-5 proteins influence local IGFBP-5 tissue expression or whether the protein levels are reflective of stricture formation in patients with CD. Therefore, we aimed to investigate the serum concentration of IGFBP-5 and intestinal IGFBP-5 expression in tissue taken from CD patients with and without stricture formation and to determine the correlation between serum IGFBP-5 levels and intestinal IGFBP-5 expression.
Forty-two patients with CD [20 female patients and 22 male patients; mean age (± SD) 38.79 ± 13.91 years; range 19-70 years, mean disease duration 4.74 ± 7.46 years] were enrolled from the inflammatory bowel disease outpatient clinic in Istanbul Medeniyet University, Goztepe Training and Research Hospital, Istanbul, Turkey, between March 2011 and September 2012. The study was conducted in accordance with the Declaration of Helsinki and according to the principles of Good Clinical Practice. The Goztepe Training and Research Hospital ethics committee approved the study (19/S-2012). All subjects gave informed consent. Twenty healthy controls who were matched to study patients by sex and age [10 female and 10 male, mean age (± SD) 38.4 ± 8.73 years; range 26-56 years] were also enrolled, and they provided written consent for the collection of blood samples.
CD was diagnosed based on the established criteria of clinical, endoscopic, and histological findings. All patients (n = 42) were evaluated endoscopically or radiologically for the presence of stricture formation. Twenty-six of the 42 patients (61.9%) had endoscopically or radiologically proven stricture formation. Eighteen of these 26 patients (69.2%) had a history of intestinal resection. The Crohn’s disease activity index (CDAI) was used in all patients to assess disease activity[17]. Nine of the 42 patients (21.4%) had active disease corresponding to a CDAI > 150. Only four of the 26 patients (15.4%) with stricture formation had active disease corresponding to a CDAI > 150.
All patients were subdivided into disease phenotypes according to the Montreal Classification[18]: ileal disease only (L1) (n = 9, 21.4%), colonic disease only (L2) (n = 2, 4.8%), and ileocolonic disease (L3) (n = 31, 73.8%). Clinical data regarding each patient’s duration and localization of the disease, history of bowel resection, and current medications were obtained and recorded. Exclusion criteria included the presence of liver fibrosis or cirrhosis, systemic sclerosis, idiopathic pulmonary fibrosis, or a history of cancer.
The recruited patients were scheduled to undergo an ileocolonoscopy. The reasons for the scheduled endoscopy were as follows: to assess the disease extent and activity (n = 24, 57.1%), to monitor response to therapy (n = 9, 21.4%), and to perform stricture dilation (n = 9, 21.4%). Endoscopy was performed by experienced gastroenterologists, who collected biopsies with standard-sized flexible endoscopic forceps from areas that were endoscopically strictured and/or ulcerated (ileal and/or colonic) in patients with stricture formation. The biopsies were taken from inflamed or ulcerated areas (ileal and/or colonic) in patients without stricture formation. At least 2 biopsy specimens were collected from each area. One specimen was stained with immunohistochemistry to determine IGFBP-5 expression, and the other was stained with hematoxylin and eosin (HE). Routine histological examination of the biopsy specimens was performed by an experienced pathologist. Blood samples were collected on the same day, and sera were frozen at -80 °C until testing was performed.
Human serum IGFBP-5 levels were determined using an ELISA kit (RayBiotech, Norcross, GA) according to the manufacturer’s instructions. The sensitivity of the assay was less than 2 ng/mL. The intra- and inter-assay coefficients of variation (CVs) were < 10% and < 12%, respectively.
Immunohistochemical staining was performed using polyclonal antibodies against IGFBP-5 (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Tissue samples from the diagnostic ileocolonoscopy (ileal and/or colonic) were stained with immunohistochemistry to determine IGFBP-5 expression. HE staining was performed on parallel sections of the tissue samples. Tissue samples were fixed in 4% paraformaldehyde and embedded in paraffin. Sections (3 μm) were deparaffinized with xylene for 10 min. After deparaffinization, the sections were incubated in a 3% hydrogen peroxide block for 10 min to reduce nonspecific background staining due to endogenous peroxidase. After a wash in phosphate-buffered saline plus Tween 20 (20 ×) (PBS; ScyTek Laboratories, Logan, Utah, United States), the sections were incubated in an ultra V block (ScyTek Laboratories, Logan, Utah, United States) for 5 min at room temperature (RT) to block nonspecific binding. A primary antibody against IGFBP-5 (dilution 1:50) was added to the tissue sections, and the sections were incubated at RT for 90 min, followed by incubation with a secondary antibody (dilution 1:200, Ultra Tek antipolyvalent biotinylated antibody, ScyTek Laboratories Logan, Utah, United States) at RT for 15 min. After rehydration with PBS, Ultra Tek HRP (ScyTek Laboratories, Logan, Utah, United States) was added to the specimens. The DAB chromogen system (DAB substrate kit, ScyTek Laboratories, Logan, Utah, United States) was added to the specimens after rehydration with PBS. Mayer’s hematoxylin stain was used as a counterstain.
IGFBP-5 immunohistochemical staining was scored semiquantitatively by an independent pathologist who was blinded to clinical information. Positive staining for IGFBP-5 was observed as diffuse brown staining. The intensity of staining was scored as follows: 0 = absent or very weak staining, 1 = weak staining, 2 = moderate staining and 3 = strong staining.
The SPSS statistical software package (SPSS version 19.0, SPSS, Chicago, IL, United States) was used for data management and analyses. CD patients were matched by age and sex with healthy controls to minimize confounding factors; matched controls were included because the numbers of patients in the study was not large enough to carry out the modeling necessary to adjust for possible effects of age and gender. For continuous normally distributed variables, the mean and standard deviation were reported. Median and interquartile range were reported for non-normally distributed continuous variables. Frequencies and percentages were given for categorical variables. The Mann-Whitney test was used to evaluate the median difference between groups, and a t test was used to compare differences in mean scores. Fisher’s exact test was used instead of the typical χ2 test to compare the frequencies or categorical variables because there were few subjects in each category (n < 10 subjects). Spearman’s correlation coefficient was used to assess the association between continuous variables in the CD group. The Kruskal-Wallis test was used to assess differences in serum concentrations of IGFBP-5 and expression in tissue specimens (i.e., scores of 0, 1, 2, and 3). Statistical significance was set at a 95%CI level using a 2-sided P value.
The baseline characteristics of the CD patients and healthy controls are summarized in Table 1. The median duration of CD diagnosis was 1.2 years (IQR: 0.16-7). Of the 42 patients with CD, 31(73%) had ileocolonic disease, and 33 (78.6%) were treated with azathioprine.
| Crohn’s disease | Healthy controls | |
| (n = 42) | (n = 20) | |
| Age, mean ± SD (yr) | 38.7 ± 13.9 | 38.4 ± 8.7 |
| Gender | ||
| Female | 20 (47.6) | 10 (50) |
| Male | 22 (52.4) | 10 (50) |
| Disease localization | - | |
| Ileum | 9 (21.4) | |
| Colon | 2 (4.8) | |
| Ileum + colon | 31 (73.8) | |
| Medical treatment | - | |
| None | 9 (21.4) | |
| Corticosteroids | 1 (2.4) | |
| 5-aminosalicylate | 8 (19) | |
| Azathioprine | 33 (78.6) | |
| Sulfasalazine | 1 (2.4) | |
| More than one drug | 23 (54.8) | |
| Median disease duration (yr) (IQR) | 1.2 (0.17) | - |
| Prior intestinal resection | 18 (42.9) | - |
The main clinical and biochemical characteristics of CD patients and healthy controls are presented in Table 2. The median values for erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), white blood cell (WBC) and platelet count (PLT) were significantly higher in CD patients compared to controls (for ESR and CRP, P < 0.001; for PLT, P = 0.0001; and for WBC count, P = 0.036). However, the median levels of albumin (Alb) and hemoglobin (Hb) were significantly lower in the CD group compared to healthy controls, with P values of < 0.001 and 0.0063, respectively. Serum IGFBP-5 levels were significantly reduced in patients with CD [7.2 (5.5 -11.3) ng/mL] compared to healthy controls [11.3 (8.0- 44.6) ng/mL, P = 0.001] (Figure 1 and Table 2).
| Crohn’s disease (n = 42) | Healthy controls (n = 20) | P value1 | |
| CDAI | 87.0 (52-138) | - | |
| ESR (mm/h) | 35.5 (17.0-48) | 12.0 (10.5-14.5) | < 0.001 |
| CRP (mg/dL) | 0.6 (0.3-1.5) | 0.2 (0.1-0.3) | < 0.001 |
| Hb (g/dL) | 13.0 (11.8-13.9) | 14.0 (12.9-15) | 0.0063 |
| WBC (× 103/mm3) | 7.7 (5.9-9.2) | 6.2 (5.3-7.9) | 0.0356 |
| PLT (× 103/mm3) | 309.5 (245-359) | 240.0 (229.0-243.5) | 0.0001 |
| Alb (g/L) | 3.9 (3.7-4.4) | 4.7 (4-5) | < 0.001 |
| IGFBP-5 (ng/mL) | 7.2 (5.5-11.3) | 11.3 (8.0-44.6) | 0.0019 |
Table 3 shows the demographic and biochemical characteristics of CD patients with and without stricture formation. There were no significant differences between the CD patients with stricture formation and those without stricture formation with regards to age, gender, disease activity, disease localization or disease duration. Additionally, there was no significant difference in median values for biochemical parameters in CD patients with or without stricture formation. There was also no significant difference between serum IGFBP-5 levels in CD patients with and without stricture formation [6.9 (5.5-11.3) ng/mL and 7.8 (5.3-10.1) ng/mL (P = 0.815), respectively].
| CD patients with stricture formation (n = 26) | CD patients without stricture formation (n = 16) | P value1 | |
| Age mean ± SD (yr) | 41.4 (15.0) | 34.6 (11.0) | 0.124 |
| Gender (F/M) | 11 (42.3)/15 (57.7) | 9 (56.3)/7 (43.8) | 0.527 |
| Disease localization | 0.083 | ||
| Ileum | 3 (11.5) | 6 (37.5) | |
| Colon | 1 (3.9) | 1 (6.3) | |
| Ileum + colon | 22 (84.6) | 9 (56.3) | |
| Disease duration (yr) | 1.25 (0.25-12) | 1.2 (0-3.1) | 0.254 |
| CDAI | 78.5 (50.0-122.0) | 112.0 (74.5-165.5) | 0.090 |
| ESR (mm/h) | 36.5 (17.0-54.0) | 35.0 (21.0-39.5) | 0.660 |
| CRP (mg/dL) | 0.4 (0.3-1.2) | 1.0 (0.4-3.0) | 0.239 |
| Hb (g/dL) | 13.0 (12.4-14.1) | 12.4 (11.5-13.7) | 0.468 |
| WBC (× 103/mm3) | 7.25 (5.5-9.6) | 8.8 (6.15-9.15) | 0.509 |
| PLT (× 103/mm3) | 309.5 (262-383) | 288.5 (234.0-334.0) | 0.399 |
| Alb (g/L) | 3.9 (3.6-4.4) | 4.1 (3.8-4.5) | 0.233 |
| IGFBP-5 (ng/mL) | 6.9 (5.5-11.3) | 7.8 (5.3-10.1) | 0.815 |
The serum median IGFBP-5 levels were not significantly different in patients with active CD [7.2 (6.3-7.6) ng/mL] compared to patients with inactive CD [7.2 (5.5-11.3) ng/mL; P = 0.8901]. However, patients with active disease had lower 75 percentile values compared to patients with inactive disease (7.6 vs 11.3) (data not shown).
The serum IGFBP-5 levels were not correlated with clinical baseline parameters reflecting disease activity (CDAI, Alb, and Hb) or the presence of stricture formation. However, there was a significant correlation between serum IGFBP-5 levels and PLT (r = 0.319, P = 0.0395).
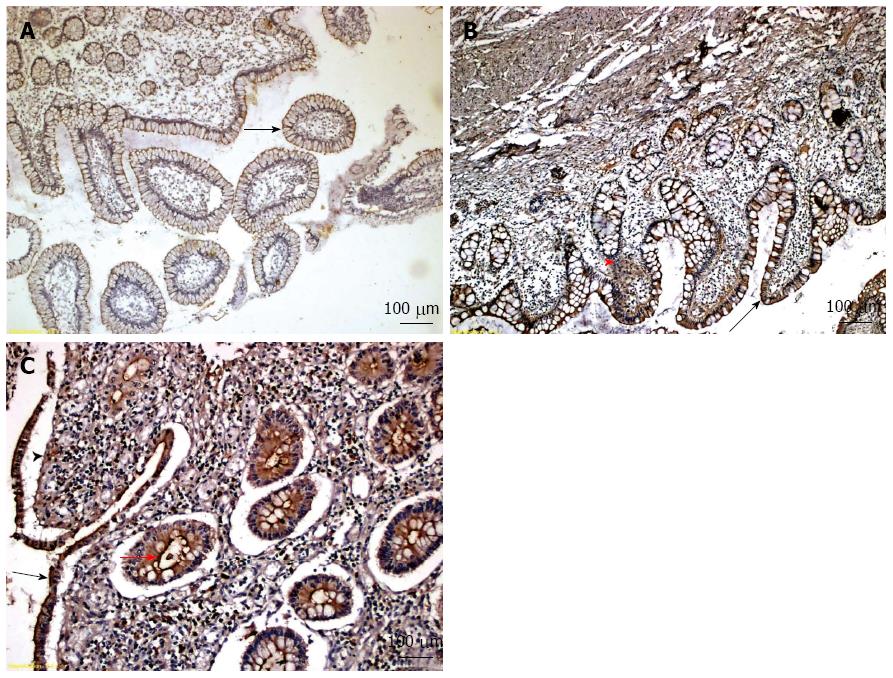
We evaluated IGFBP-5 expression in ileal biopsies using immunohistochemistry in all CD patients. Figure 2 presents representative examples of different immunohistochemical staining intensity scores (scores 1, 2 and 3) for IGFBP-5 expression using tissue samples from 3 different CD patients. Among all 42 CD patients, 24 (57.1%) had tissue samples stain positive for IGFBP-5 expression. The median level of IGFBP-5 intensity score was 1.0. The frequency of IGFBP-5 positive samples and the median intensity score did not differ significantly between CD patients with stricture formation and those without stricture formation (Table 4) or between CD patients with active disease and those with inactive disease (data not shown). The IGFBP-5 intensity scores were positively correlated with WBC count (r = 0.391; P = 0.01) and PLT (r = 0.356; P = 0.021).
| CD patients with stricture formation (n = 26) | CD patients without stricture formation (n = 16) | P value1 | |
| Number of IGFBP-5 positive samples | 16 (61.5) | 8 (50) | 0.463 |
| Median intensity score of IGFBP-5 immunostaining | 1 | 0.5 | 0.405 |
No significant correlation was found between ileal IGFBP-5 immunohistochemical expression and serum IGFBP-5 levels. The serum IGFBP-5 concentrations were not significantly different for individuals with biopsies with absent/very weak (0), weak (1), moderate (2), or strong (3) IGFBP-5 staining scores (Figure 3).
This study revealed that circulating levels of IGFBP-5 were significantly reduced in patients with CD compared to healthy controls [7.2 (5.5-11.3) ng/mL vs 11.3 (8.0-44.6) ng/mL (P = 0.002), respectively]. To our knowledge, this is the first study that demonstrates low serum IGFBP-5 levels in patients with CD compared to healthy controls. Despite the low serum IGFBP-5 levels described in CD patients, the authors did not observe any significant differences between the median serum IGFBP-5 levels for patients with and without strictures [6.9 (5.5-11.3) ng/mL vs 7.8 (5.3-10.1) ng/mL (P = 0.815), respectively]. Previous studies have mainly evaluated serum total and free IGF-I , IGFBP-1, IGFBP-2 and IGFBP-3 levels in the context of disease activity. Our results were similar to these studies, demonstrating low levels of IGF system proteins in IBD patients[11-16]. Katsanos et al[11] reported that circulating levels of IGF-I and IGFBP-3 were reduced in patients with active IBD[9]. Grønbek et al[12] demonstrated reduced serum total and free IGF-I and IGFBP-3 levels in patients with active IBD without complete normalization during high-dose prednisolone treatment and tapering[10]. Vespasiani Gentilucci et al[14] observed low IGF-1 and IGFBP-3 levels in active IBD patients before infliximab therapy, and a repeated drop in levels after normalization and clinical remission. However, we did not observe any significant difference between serum IGFBP-5 levels in patients with active and inactive disease [7.2 (5.5-11.3) ng/mL vs 7.2 (6.3-7.6) ng/mL (P = 0.8901), respectively]. Moreover, serum IGFBP-5 levels were moderately correlated with PLT, but not with hemoglobin, albumin levels, or CDAI. This finding may be explained by the low number of active CD patients (n = 9, 21.4%) in our study or by the presence of low-grade subclinical transmural inflammation, which is not detectable with clinical or biochemical markers. The most important in vivo regulator of IGFBP-5 expression is IGF-I[7]. In normal adult human serum, IGFBP-5 levels are positively correlated with IGF-I concentrations[19]. Although we did not evaluate serum IGF-I levels, the mechanism underlying low IGFBP-5 levels in CD patients may be similar to the mechanism for low IGF-I levels, as IGFBP-5 expression is mainly regulated by IGF-I . Previous studies have suggested that low IGF-1 levels in active IBD patients may be due to the direct adverse effects of circulating inflammatory cytokines[19,20]. Katsanos et al[11] also showed that serum IL-6 levels were increased in IBD patients with active disease compared to healthy controls. IGFBP-5 expression in vitro can be regulated by hormones and cytokines[7]. However, as inflammatory cytokines were not evaluated in our study, further investigations are needed to examine the effects of cytokines on circulating IGFBP-5 levels. Previous studies demonstrated partially normalized or unchanged low IGF levels during corticosteroid and infliximab treatments[12,14-16], and the low serum IGFBP-5 levels in our study may be explained by the previously described poor correlation between clinical activity, endoscopic severity and biological parameters in CD patients[21].
To our knowledge, we are the first researchers to investigate the relationship between circulating IGFBP-5 concentrations and intestinal IGFBP-5 expression in CD patients. No correlation was found between circulating IGFBP-5 concentrations and intestinal IGFBP-5 expression. The serum IGFBP-5 concentrations were not significantly different between individuals with biopsies with absent/very weak, weak, moderate, or strong IGFBP-5 staining. Previous studies demonstrated that serum levels of hepatic-derived IGF-I and IGFBP-3 were lower in patients with active CD than in normal subjects, whereas the expression of IGF-I, IGFBP-5, and IGFBP-3 in smooth muscle cells in strictured intestines was increased compared to adjacent nonstrictured intestinal muscle from the margin of resected tissue[22-24]; however, the reasons for the discrepancy in the levels of these mediators in the serum compared to the expression in the muscle layer of the intestine remain unclear. It has been suggested that serum levels and intestinal expression of the IGF system are differentially regulated.
Three pathophysiologic events occurring within smooth muscle cells of the muscularis propria contribute to stricture formation in CD: increased smooth muscle cell hyperplasia, increased smooth muscle cell hypertrophy, and excess net extracellular matrix proteins, including collagen. IGF-I up-regulation is accompanied by IGFBP-5 up-regulation and collagen I, III, and V up-regulation[4,5,25]. Zimmermann et al[5] showed that IGF-I and IGFBP-5 mRNA was increased in inflamed/fibrotic intestines compared with normal-appearing intestines. However, we could not demonstrate increased intestinal IGFBP-5 expression in CD patients with stricture formation compared to those without stricture formation. Unfortunately, we were not able to analyze and compare intestinal IGFBP-5 expression in both fibrotic and normal-appearing tissue in CD patients with stricture formation. Moreover, the intestinal tissue was obtained with standard endoscopic biopsies, and the majority of our patients had inactive disease (n = 33, 78.6%), whereas previous studies analyzed active inflamed intestinal tissues from resection samples. Although we did not observe any significant difference between active and inactive patient groups regarding intestinal IGFBP-5 expression, intestinal IGFBP-5 expression was positively correlated with WBC count and PLT. This finding may be due to a poor correlation between clinical activity indices and actual endoscopic disease activity. One limitation of our study is the small sample size. Additionally, we were unable to obtain biopsies from normal-appearing intestinal mucosa of CD patients to compare with intestinal IGFBP-5 expression in inflamed/strictured mucosa. Moreover, intestinal IGFBP-5 expression could be affected by the area of the biopsy samples.
In conclusion, our results indicate that serum IGFBP-5 concentrations are lower in CD patients compared to healthy controls regardless of disease activity or the presence of stricture formation. Serum IGFBP-5 concentrations were not associated with intestinal IGFBP-5 tissue expression. Therefore, our results do not answer the question of whether IGFBP-5 is involved in the stricture formation of CD, and thus, more research is necessary. Directions for future research include examination of other serum IGF system components, use of a larger patient population with active and inactive disease, endoscopic determination of disease activity and collection of biopsy tissue from both normal and inflamed/strictured areas.
Crohn’s disease (CD) is a multifactorial disorder and its behavior may change throughout the course of the disease. Approximately 30 % of the CD patients will develop strictures and experience complications related to the stricture formation. It is crucial to understand the pathophysiology of bowel-wall stricturing in CD. Members of the insulin-like growth factor (IGF) system have been implicated as central players in stricture formation.
It has been shown that both insulin-like growth factor 1 (IGF-I) and insulin-like growth factor-binding protein 5 (IGFBP-5) expressions are increased in inflamed/fibrotic intestine. Many studies showed that circulating levels of IGF-I and its binding protein proteins (IGFBPs) are decreased in inflammatory bowel disease. In the present study serum IGFBP-5 levels and intestinal IGFBP-5 expression was investigated in CD patients with and without stricture formation.
The serum levels of IGFBP-5 and intestinal expression of IGFBP-5 with immunohistochemistry in CD patients has not been studied previously. This study, for first time, reports that serum IGFBP-5 levels are decreased in CD patients regardless the presence of stricture formation or disease activity.
By understanding the circulating IGFBP-5 profile in CD patients, this study may represent a future strategy for prospective studies to understand the interaction between IGF system and CD pathophysiology, which may in turn aid in finding specific biomolecular targets for treatment of CD.
IGFBP-5 is a member of six IGFBPs. It is binding to IGFs with high affinity and has several regulatory functions. IGFBP-5 stimulates muscle hyperplasia and collagen secretion in human intestinal smooth muscle. It has been suggested that IGFBP-5, may be important in the pathogenesis of intestinal fibrosis in inflammatory bowel disease.
The authors examined the serum levels of IGFBP-5 and its intestinal expression in CD. It revealed that circulating IGFBP-5 levels are lower in CD patients compared to healthy controls. The results are interesting and may address the potential role of circulating IGFBP-5 in the pathophysiology of CD.
P- Reviewers: Munoz M, Zhou M S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
| 1. | Shanahan F. Crohn’s disease. Lancet. 2002;359:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 353] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 2. | Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis. 2004;10:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Longo WE, Virgo KS, Bahadursingh AN, Johnson FE. Patterns of disease and surgical treatment among United States veterans more than 50 years of age with ulcerative colitis. Am J Surg. 2003;186:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Graham MF, Diegelmann RF, Elson CO, Lindblad WJ, Gotschalk N, Gay S, Gay R. Collagen content and types in the intestinal strictures of Crohn’s disease. Gastroenterology. 1988;94:257-265. [PubMed] |
| 5. | Zimmermann EM, Li L, Hou YT, Mohapatra NK, Pucilowska JB. Insulin-like growth factor I and insulin-like growth factor binding protein 5 in Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1022-G1029. [PubMed] |
| 6. | Zimmermann EM, Li L, Hou YT, Cannon M, Christman GM, Bitar KN. IGF-I induces collagen and IGFBP-5 mRNA in rat intestinal smooth muscle. Am J Physiol. 1997;273:G875-G882. [PubMed] |
| 7. | Schneider MR, Wolf E, Hoeflich A, Lahm H. IGF-binding protein-5: flexible player in the IGF system and effector on its own. J Endocrinol. 2002;172:423-440. [PubMed] |
| 8. | Kuemmerle JF. Occupation of alphavbeta3-integrin by endogenous ligands modulates IGF-I receptor activation and proliferation of human intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1194-G1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Yeh CC, Chang SF, Huang TY, Chang HI, Kuo HC, Wu YC, Hsieh CH, Shi CS, Chen CN. Shear stress modulates macrophage-induced urokinase plasminogen activator expression in human chondrocytes. Arthritis Res Ther. 2013;15:R53. [PubMed] |
| 10. | Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol. 2005;166:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Katsanos KH, Tsatsoulis A, Christodoulou D, Challa A, Katsaraki A, Tsianos EV. Reduced serum insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 levels in adults with inflammatory bowel disease. Growth Horm IGF Res. 2001;11:364-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Grønbek H, Thøgersen T, Frystyk J, Vilstrup H, Flyvbjerg A, Dahlerup JF. Low free and total insulinlike growth factor I (IGF-I) and IGF binding protein-3 levels in chronic inflammatory bowel disease: partial normalization during prednisolone treatment. Am J Gastroenterol. 2002;97:673-678. [PubMed] |
| 13. | Kirman I, Whelan RL, Jain S, Nielsen SE, Seidelin JB, Nielsen OH. Insulin-like growth factor binding protein 3 in inflammatory bowel disease. Dig Dis Sci. 2005;50:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Vespasiani Gentilucci U, Caviglia R, Picardi A, Carotti S, Ribolsi M, Galati G, Petitti T, Afeltra A, Cicala M. Infliximab reverses growth hormone resistance associated with inflammatory bowel disease. Aliment Pharmacol Ther. 2005;21:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Eivindson M, Grønbaek H, Skogstrand K, Thorsen P, Frystyk J, Flyvbjerg A, Dahlerup JF. The insulin-like growth factor (IGF) system and its relation to infliximab treatment in adult patients with Crohn’s disease. Scand J Gastroenterol. 2007;42:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Eivindson M, Grønbaek H, Flyvbjerg A, Frystyk J, Zimmermann-Nielsen E, Dahlerup JF. The insulin-like growth factor (IGF)-system in active ulcerative colitis and Crohn’s disease: relations to disease activity and corticosteroid treatment. Growth Horm IGF Res. 2007;17:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 18. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2350] [Article Influence: 123.7] [Reference Citation Analysis (2)] |
| 19. | Mohan S, Baylink DJ, Pettis JL. Insulin-like growth factor (IGF)-binding proteins in serum--do they have additional roles besides modulating the endocrine IGF actions? J Clin Endocrinol Metab. 1996;81:3817-3820. [PubMed] |
| 20. | Fan J, Char D, Bagby GJ, Gelato MC, Lang CH. Regulation of insulin-like growth factor-I (IGF-I) and IGF-binding proteins by tumor necrosis factor. Am J Physiol. 1995;269:R1204-R1212. [PubMed] |
| 21. | Cellier C, Sahmoud T, Froguel E, Adenis A, Belaiche J, Bretagne JF, Florent C, Bouvry M, Mary JY, Modigliani R. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231-235. [PubMed] |
| 22. | Flynn RS, Murthy KS, Grider JR, Kellum JM, Kuemmerle JF. Endogenous IGF-I and alphaVbeta3 integrin ligands regulate increased smooth muscle hyperplasia in stricturing Crohn’s disease. Gastroenterology. 2010;138:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Zimmermann EM, Sartor RB, McCall RD, Pardo M, Bender D, Lund PK. Insulinlike growth factor I and interleukin 1 beta messenger RNA in a rat model of granulomatous enterocolitis and hepatitis. Gastroenterology. 1993;105:399-409. [PubMed] |
| 24. | Flynn RS, Mahavadi S, Murthy KS, Grider JR, Kellum JM, Akbari H, Kuemmerle JF. Endogenous IGFBP-3 regulates excess collagen expression in intestinal smooth muscle cells of Crohn’s disease strictures. Inflamm Bowel Dis. 2011;17:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Kuemmerle JF. Endogenous IGF-I regulates IGF binding protein production in human intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G710-G717. [PubMed] |