Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8652
Revised: October 7, 2013
Accepted: October 13, 2013
Published online: December 14, 2013
Processing time: 231 Days and 6.6 Hours
AIM: To investigate a classification of endocytoscopy (ECS) images in Barrett’s esophagus (BE) and evaluate its diagnostic performance and interobserver variability.
METHODS: ECS was applied to surveillance endoscopic mucosal resection (EMR) specimens of BE ex-vivo. The mucosal surface of specimen was stained with 1% methylene blue and surveyed with a catheter-type endocytoscope. We selected still images that were most representative of the endoscopically suspect lesion and matched with the final histopathological diagnosis to accomplish accurate correlation. The diagnostic performance and inter-observer variability of the new classification scheme were assessed in a blinded fashion by physicians with expertise in both BE and ECS and inexperienced physicians with no prior exposure to ECS.
RESULTS: Three staff physicians and 22 gastroenterology fellows classified eight randomly assigned unknown still ECS pictures (two images per each classification) into one of four histopathologic categories as follows: (1) BEC1-squamous epithelium; (2) BEC2-BE without dysplasia; (3) BEC3-BE with dysplasia; and (4) BEC4-esophageal adenocarcinoma (EAC) in BE. Accuracy of diagnosis in staff physicians and clinical fellows were, respectively, 100% and 99.4% for BEC1, 95.8% and 83.0% for BEC2, 91.7% and 83.0% for BEC3, and 95.8% and 98.3% for BEC4. Interobserver agreement of the faculty physicians and fellows in classifying each category were 0.932 and 0.897, respectively.
CONCLUSION: This is the first study to investigate classification system of ECS in BE. This ex-vivo pilot study demonstrated acceptable diagnostic accuracy and excellent interobserver agreement.
Core tip: The current gold standard for surveillance of esophageal adenocarcioma in Barretts’s esophagus (BE) is endoscopic random biopsy and pathological diagnosis. Endocytoscopy (ECS) has the potential to provide a virtual histological diagnosis in vivo and in real-time. However, a major issue relates to that interpretation of cellular and nuclear images may be subject to similar interobserver variability associated with conventional histopathological diagnosis, and there have been no reliable classification systems for the endocytoscopic diagnosis. We presented the first study to investigate classification system of ECS in BE. This ex-vivo pilot study demonstrated acceptable diagnostic accuracy and excellent interobserver agreement.
-
Citation: Tomizawa Y, Iyer PG, Wongkeesong LM, Buttar NS, Lutzke LS, Wu TT, Wang KK. Assessment of the diagnostic performance and interobserver variability of endocytoscopy in Barrett’s esophagus: A pilot
ex-vivo study. World J Gastroenterol 2013; 19(46): 8652-8658 - URL: https://www.wjgnet.com/1007-9327/full/v19/i46/8652.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i46.8652
Recent advances in endoscopic imaging may lead to improved detection and facilitate therapy of dysplasia and esophageal adenocarcinoma (EAC) in Barrett’s esophagus (BE)[1-5]. Histology is regarding as the gold standard for diagnosis of dysplasia and EAC[6,7], but sampling error and interobserver variability among gastrointestinal pathologists have been well described[8-11].
Endocytoscopy (ECS) is a probe-based technique which captures ultra-high magnified images of the epithelial surface, with the capability to discriminate cellular and subcellular features. ECS, thus, has the potential to provide a virtual histological diagnosis in vivo and in real-time. ECS has been investigated throughout the gastrointestinal tract for the identification of lesions in the esophagus[12-16], small intestine[17], and colon[18-21]. However, a major issue relates to the fact that interpretation of cellular and nuclear images may be subject to similar interobserver variability associated with conventional histopathological diagnosis[22,23]. To date, there have been no reliable classification systems for the endocytoscopic diagnosis of BE and Barrett’s EAC. Accurate diagnosis based on a simple and reproducible classification system is warranted before ECS can be implemented into clinical practice.
Our aim was to develop simplified scheme for the classification of endocytoscopic images in BE and to evaluate its diagnostic performance and interobserver variability among experienced and inexperienced users of ECS in an ex-vivo setting.
ECS was performed ex-vivo on endoscopic mucosal resection (EMR) specimens obtained from patients undergoing endoscopic surveillance of BE at our institution. All EMR procedures were performed by a single endoscopist using the cap technique (EMR Kit; Olympus America, Center Valley, PA). Lesions targeted for EMR were endoscopically suspect areas, such as nodules or polyps, or dysplastic/neoplastic-appearing mucosa, such as irregular, friable, ulcerated, or villous-appearing mucosa, as seen under high-definition white-light imaging and narrow band imaging[24]. The study was approved by the Institutional Review Board and a written informed consent was obtained from all patients.
As soon as retrieved from the patient, the mucosal surface of each EMR specimen was immediately rinsed with 3-5 mL of 20% N-acetylcysteine to remove excess mucus, followed by the application of 1-1.5 mL of 1% methylene blue solution as contrast agent. Ex-vivo ECS imaging was performed using a flexible, catheter-type endocytoscope (XEC120, Olympus Medical Systems Co., Tokyo, Japan) which provides 1100 × magnification at a 120 μm × 120 μm field of view. The stained surface of each specimen was surveyed with the endocytoscope, and the area most representative of the endoscopically suspect lesion was identified. ECS imaging of the lesion was videotaped for approximately one minute.
Histopathological assessment of the EMR specimens was performed according to the protocol in our BE unit, as previously published[25]. Patients had their pathological diagnosis of EMR specimens confirmed by at least two experienced gastrointestinal pathologists with expertise in Barrett-associated neoplasia.
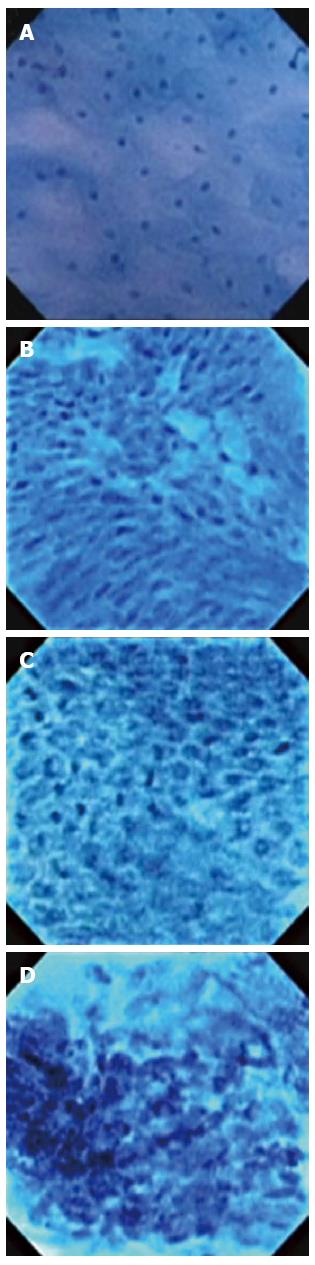
Following histopathological diagnoses of EMR specimens, the corresponding ECS videos and images were reviewed by investigators uninvolved in the subsequent blinded image assessment. To accomplish accurate correlation of endocytoscopic images with histological findings, we selected snapshots that were most representative of the histopathological findings for each specimen and matched final histopathological findings with their respective ECS images. With consideration to the previously proposed esophageal endocytoscopic atypia classification by Inoue et al[26], we classified the endocytoscopic images as follows (BEC; Barrett’s EndoCytoscopy): (1) BEC1 - squamous epithelium; (2) BEC2-BE without dysplasia; (3) BEC3 - BE with dysplasia; and (4) BEC4-BE with EAC.
This classification scheme is based on the interpretation of ECS features (Figure 1): BEC1 images consist of cytoplasm-rich, rhomboid cells in a regular pattern; BEC2 images consist of increased cell numbers and different-sized nuclei/cells; BEC3 images consist of increased nucleus-cytoplasm ratio with dense chromatin and prominent nuclear fission; BEC4 images consist of cells of various sizes that are irregularly arranged, with blurred and enlarged nuclei. Two representative endocytoscopic images for each classification were selected for analysis of diagnostic performance and interobserver variability.
The diagnostic performance and inter-observer variability of the new classification scheme were assessed in a blinded fashion. Experienced physicians with expertise in both BE and ECS were provided with a brief 5-min presentation on the new classification, as shown in Figure 1. Inexperienced physicians consisted of clinical fellows (n = 22) in the Division of Gastroenterology and Hepatology with no prior exposure to ECS. They were provided with a 30 min presentation on ECS imaging and the new classification scheme. During the training session, fellows were presented with two non-study sets of pictures representative of each BEC classification for learning purposes. They were given the opportunity to ask questions and review the criteria. The training session and image classification by experienced and inexperienced physicians were conducted separately.
Immediately following the training session, participants were shown the randomly assigned unknown ECS pictures and asked to classify each image as: (1) BEC1; (2) BEC2; (3) BEC3; and (4) BEC4. The participants were blinded to patient history, endoscopic findings, and histopathological diagnoses. During the image classification session, the participants were not allowed to review previously seen images or to change their answers.
Data analysis was performed using the SPSS (Chicago, Illinois, United States) statistical software program. Classification accuracy, sensitivity, specificity, and positive and negative predictive values were calculated to assess diagnostic performance. Interobserver agreement was determined using intraclass correlation coefficient (ICC), which assesses agreement beyond chance among investigators. ICC was derived from a 2-way random effects model because both people effects and measures effects were random. An ICC of 0.4-0.75 indicates fair to good reliability, whereas an ICC greater than 0.75 shows excellent reliability.
A total of 20 patients were included in this study: squamous epithelium (n = 2), BE without dysplasia (n = 6), BE with dysplasia (n = 6), and BE with EAC (n = 6). A total of eight representative endocytoscopic images (two images per each classification) from different patients were utilized for this study. The overall classification accuracy for each category among experienced (n = 3) and inexperienced (n = 22) physicians were 100% and 99.4% for BEC1, 95.8% and 83.0% for BEC2, 91.7% and 83.0% for BEC3, and 95.8% and 98.3% for BEC4, respectively. If we combined BEC2 and BEC3 as diagnosis of BE, the classification accuracy would be 95.8%, even in ECS naive observers. The sensitivities, specificities, positive predictive values and negative predictive values for each category are shown in Table 1.
| Classification | Sensitivity | Specificity | PPV | NPV | |
| Experienced physicians (staff physician) | BEC 1 | 1.000% | 1.000% | 1.000% | 1.000% |
| BEC 2 | 1.000% | 0.944% | 0.857% | 1.000% | |
| BEC 3 | 0.833% | 0.944% | 0.833% | 0.944% | |
| BEC 4 | 0.833% | 1.000% | 1.000% | 0.947% | |
| Inexperienced physicians (clinical fellow) | BEC 1 | 0.977% | 1.000% | 1.000% | 0.992% |
| BEC 2 | 0.636% | 0.894% | 0.667% | 0.881% | |
| BEC 3 | 0.705% | 0.871% | 0.646% | 0.898% | |
| BEC 4 | 0.955% | 0.992% | 0.977% | 0.985% | |
| Both experienced and inexperienced (physicians staff physician and clinical fellow) | BEC 1 | 0.980% | 1.000% | 1.000% | 0.993% |
| BEC 2 | 0.680% | 0.900% | 0.694% | 0.894% | |
| BEC 3 | 0.720% | 0.880% | 0.667% | 0.904% | |
| BEC 4 | 0.940% | 0.993% | 0.979% | 0.980% |
The interobserver agreements for the experienced and inexperienced physicians in classifying each category were 0.932 and 0.897, respectively. When a dichotomized category (BEC 1 and 2 vs BEC 3 and 4) was used, interobserver agreements for the experienced and inexperienced physicians in classifying into this category were 0.851 and 0.581, respectively (Table 2).
| Classification | Staff physician (95%CI) | GI fellow (95%CI) |
| BEC 1-4 | 0.932 (0.794-0.985) | 0.897 (0.784-0.973) |
| BEC 1 and 2 vs 3 and 4 | 0.851 (0.593-0.965) | 0.581 (0.358-0.856) |
Barrett’s esophagus (BE) is a well-established precursor of esophageal adenocarcinoma (EAC) whose incidence is rising in Western countries. Patients with BE are therefore advised to undergo periodic surveillance to detect dysplastic mucosa and pre-cancerous lesions at an early stage at a time where intervention can be curative. The current gold standard for surveillance is periodic endoscopic random biopsy within the BE segment and pathological diagnosis. Due to inherent limitations of the gold standard, there have been considerable interests in advanced endoscopic imaging techniques to enhance dysplasia and EAC detection. Dysplasia can be patchy in distribution within the BE segment and sampling error can occur with random biopsy techniques. Novel imaging technologies that can reliably detect dysplasia or EAC in real-time would facilitate targeted biopsy and, hence, a reduction in sampling error.
Endocytoscopy (ECS) can provide real-time virtual histological images during endoscopic observation and potentially identify areas that harbor dysplastic or cancerous cells. However, interpretation of ECS images may be subject to similar interobserver variability associated with conventional histopathological diagnosis. A first step is to standardize ECS image criteria for accurate tissue diagnosis. To date, no studies have been conducted on the development and use of a classification system for endocytoscopic images in BE. In this study, we proposed a classification scheme based on ECS cellular and architectural features for categorizing Barrett’s tissue, with the aim that the classification remains simple and easy to learn and adopt.
Overall, we had an acceptable classification accuracy for each Barrett tissue category when using our classification system and high accuracy was obtained for the differentiation of BE without dysplasia from dysplastic tissue among experienced observers. Although the number of percentage of accuracy among staff physicians appears low in BE with dysplasia, it is obvious that the small number of denominator is the reason. Our group of inexperienced observers (clinical fellows) classified squamous epithelium and EAC with high accuracy of 99.4% and 98.3%, respectively, and BE with and without dysplasia with acceptable accuracy of 83.0%. These results suggest our classification scheme is reliable and easy to learn. Interobserver agreement regarding both experienced and inexperienced groups was interpreted as excellent (ICC = 0.932 and 0.897, respectively). In classifying into two dichotomized category (BEC 1 and 2 vs BEC 3 and 4), interobserver agreement for the experienced physicians was still interpreted as excellent (ICC = 0.851) and interobserver agreement for the fellows showed good reliability (ICC = 0.581).
In this study, all the misdiagnoses of BE with dysplasia (BEC 3) were answered as non-dysplasia (BEC 2). It may imply that our criteria are similar to those of histological diagnosis. Misdiagnosis for non-dysplastic BE occurred in the inexperienced group, and all the misdiagnoses were answered as dysplasia. These facts probably reflect some of the dilemma that exists with pathological interpretation of non-dysplastic and dysplastic BE.
The reported diagnostic accuracy and interobserver agreement of ECS images should be interpreted with caution. In this structured pilot ex-vivo study, we intended to show a “classic” unambiguous image for each category and, thus, selected representative images. In BE, the microscopic epithelial changes that represent transition from metaplasia to dysplasia to cancer occur on a continuum. We did not assess how our classification performs near the margins of the transitions. The use of the representative images may maximize diagnostic accuracy and interobserver agreement and minimize the correlation of the study findings with what will be observed during real-time use in vivo. We did not evaluate using “real-time” images correlated with biopsy results using our classification. Further study is warranted in real scanning to validate our classification. An additional study limitation is related to the ex-vivo nature of this preliminary study. The performance of in-vivo catheter-type ECS is clearly operator dependent. Challenges that lie ahead are the difficulty of maintaining a long, thin, flexible catheter onto the esophageal surface in stable position and in focus. During in-vivo observations, gastrointestinal motility may hinder collection of interpretable images. In one in-vivo study of ECS in BE[27], 76% of ECS images were recognized as poor quality. The study also had a high false-positive rate of 43%, resulting in both a sensitivity and positive predictive value of 42% in all image sequences. Conversely, an in-vivo feasibility ECS study for esophageal cancer conducted in Japan reported that clear and interpretable images were obtained in all cases, with the positive predictive value and the false-positive rate for esophageal malignancy being 94% and 6.3%, respectively[26]. Another in-vivo feasibility study also presented high quality images for interpretation[12]. Expertise in handling the ECS device could explain the difference in image quality obtained, and we believe the technical aspects can be overcome as already reported in the previous two in-vivo studies. A new endoscope-type ECS (XGIF-Q260EC1 and XCF-Q260EC1; Olympus) has recently been introduced and been reported to obtain more sensitive, ultra-magnified images[28-30]. The new ECS enables easy switch from a conventional endoscopic view to ultra-magnification endocytoscopic view by the press of a button at the top of the endoscope. The new device could reduce the technical burden of maintaining an ECS probe on a moving surface.
Our classification system does not differentiate between low grade (LGD) and high grade (HGD) dysplasia. It is well known the reproducibility of histopathological interpretation of LGD and HGD even among skilled pathologists is a challenge[10]. We still do not have definitive consensus about the management of LGD in BE, and management is individualized. It is clear that surveillance of any dysplastic lesions in BE segment is of importance given the established dysplasia-carcinoma sequence in EAC. In this study, we aimed to assess the potential of ECS in enhancing surveillance of dysplastic lesions in BE. We therefore proposed the two distinct criteria of dysplastic BE vs non-dysplastic BE instead of LGD and HGD.
In conclusion, we proposed a simple diagnostic classification system for ECS in BE. In this pilot ex-vivo study, acceptable accuracies regarding the diagnosis of squamous epithelium, non-dysplastic BE, dysplastic BE, and EAC were demonstrated. Interobserver agreement in classifying each category was interpreted as excellent, even among observers inexperienced in ECS. The applicability of the proposed classification scheme in the in-vivo setting remains to be seen.
We thank Olympus America for providing the catheter-type endocytoscope for this pilot study.
Barrett’s esophagus is a well-established precursor of esophageal adenocarcinoma, therefore it is very important to detect dysplastic pre-cancerous lesions at an early stage at a time where intervention can be curative. The current gold standard of endoscopic random biopsy has inherent limitations. There have been considerable interests in advanced endoscopic imaging techniques to enhance dysplasia detection.
Novel imaging technologies that can reliably detect dysplasia or early esophageal cancer would facilitate targeted biopsy and, hence, a reduction in sampling error. Endocytoscopy can provide real-time virtual histological images during endoscopic observation and potentially identify areas that harbor dysplastic or cancerous cells and facilitate targeted biopsy.
A major issue relates to the fact that interpretation of cellular and nuclear images by endocytoscopy may be subject to similar interobserver variability associated with conventional histopathological diagnosis. To date, there have been no reliable classification systems for the endocytoscopic diagnosis of Barrett’s esophagus and esophageal adenocarcinoma. In this study, the authors proposed a classification scheme based on endocytoscopy cellular and architectural features for categorizing Barrett’s tissue, with the aim that the classification remains simple and easy to adopt. This is the first study to investigate classification system of endocytoscopy in Barrett’s esophagus. In this study, the diagnostic performance and inter-observer variability of the new classification scheme were assessed in a blinded fashion by physicians with expertise in both Barrett’s esophagus and endocytoscopy and inexperienced physicians with no prior exposure to endocytosocpy. Overall, the authors had an acceptable classification accuracy for each Barrett tissue category when using our classification system, and high accuracy was obtained for the differentiation of Barrett’s esophagus without dysplasia from dysplastic tissue among experienced observers. Interobserver agreement in classifying each category was interpreted as excellent among both experienced and inexperienced observers.
The results of this structured pilot ex-vivo study suggest that our classification scheme is reliable and easy to learn.
The study to investigate classification system of endocytoscopy in Barrett’s esophagus is very interesting. This ex-vivo pilot study demonstrated acceptable diagnostic accuracy and excellent interobserver agreement.
P- Reviewer: Siriwardana HPP S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Sturm MB, Piraka C, Elmunzer BJ, Kwon RS, Joshi BP, Appelman HD, Turgeon DK, Wang TD. In vivo molecular imaging of Barrett’s esophagus with confocal laser endomicroscopy. Gastroenterology. 2013;145:56-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Gorospe EC, Leggett CL, Sun G, Anderson MA, Gupta M, Penfield JD, Lutzke L, Lewis JT, Wong Kee Song LM, Wang KK. Diagnostic performance of two confocal endomicroscopy systems in detecting Barrett’s dysplasia: a pilot study using a novel bioprobe in ex vivo tissue. Gastrointest Endosc. 2012;76:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Wallace MB, Crook JE, Saunders M, Lovat L, Coron E, Waxman I, Sharma P, Hwang JH, Banks M, DePreville M. Multicenter, randomized, controlled trial of confocal laser endomicroscopy assessment of residual metaplasia after mucosal ablation or resection of GI neoplasia in Barrett’s esophagus. Gastrointest Endosc. 2012;76:539-47.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Wolfsen HC, Crook JE, Krishna M, Achem SR, Devault KR, Bouras EP, Loeb DS, Stark ME, Woodward TA, Hemminger LL. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s Esophagus. Gastroenterology. 2008;135:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Sharma P, Hawes RH, Bansal A, Gupta N, Curvers W, Rastogi A, Singh M, Hall M, Mathur SC, Wani SB. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: a prospective, international, randomised controlled trial. Gut. 2013;62:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 6. | Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788-797. [PubMed] [DOI] [Full Text] |
| 7. | Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140:e18-52; quiz e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 802] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 8. | Peters FP, Curvers WL, Rosmolen WD, de Vries CE, Ten Kate FJ, Krishnadath KK, Fockens P, Bergman JJ. Surveillance history of endoscopically treated patients with early Barrett’s neoplasia: nonadherence to the Seattle biopsy protocol leads to sampling error. Dis Esophagus. 2008;21:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Eloubeidi MA, Provenzale D. Does this patient have Barrett’s esophagus? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol. 1999;94:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, Lamps LW, Lauwers GY, Lazenby AJ, Lewin DN. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 566] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 11. | Downs-Kelly E, Mendelin JE, Bennett AE, Castilla E, Henricks WH, Schoenfield L, Skacel M, Yerian L, Rice TW, Rybicki LA. Poor interobserver agreement in the distinction of high-grade dysplasia and adenocarcinoma in pretreatment Barrett’s esophagus biopsies. Am J Gastroenterol. 2008;103:2333-2340; quiz 2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Kumagai Y, Monma K, Kawada K. Magnifying chromoendoscopy of the esophagus: in-vivo pathological diagnosis using an endocytoscopy system. Endoscopy. 2004;36:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Fujishiro M, Kodashima S, Takubo K, Kakushima N, Omata M. Detailed comparison between endocytoscopy and horizontal histology of an esophageal intraepithelial squamous cell carcinoma. Dis Esophagus. 2008;21:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Eleftheriadis N, Inoue H, Ikeda H, Onimaru M, Yoshida A, Hosoya T, Maselli R, Kudo SE. Endocytoscopic visualization of squamous cell islands within Barrett’s epithelium. World J Gastrointest Endosc. 2013;5:174-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Kumagai Y, Kawada K, Yamazaki S, Iida M, Momma K, Odajima H, Kawachi H, Nemoto T, Kawano T, Takubo K. Endocytoscopic observation for esophageal squamous cell carcinoma: can biopsy histology be omitted? Dis Esophagus. 2009;22:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Fujishiro M, Takubo K, Sato Y, Kaise M, Niwa Y, Kato M, Muto M. Potential and present limitation of endocytoscopy in the diagnosis of esophageal squamous-cell carcinoma: a multicenter ex vivo pilot study. Gastrointest Endosc. 2007;66:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Pohl H, Rösch T, Tanczos BT, Rudolph B, Schlüns K, Baumgart DC. Endocytoscopy for the detection of microstructural features in adult patients with celiac sprue: a prospective, blinded endocytoscopy-conventional histology correlation study. Gastrointest Endosc. 2009;70:933-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Rotondano G, Bianco MA, Salerno R, Meucci C, Prisco A, Garofano ML, Sansone S, Cipolletta L. Endocytoscopic classification of preneoplastic lesions in the colorectum. Int J Colorectal Dis. 2010;25:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Kudo SE, Wakamura K, Ikehara N, Mori Y, Inoue H, Hamatani S. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy. 2011;43:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Mori Y, Kudo S, Ikehara N, Wakamura K, Wada Y, Kutsukawa M, Misawa M, Kudo T, Kobayashi Y, Miyachi H. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: a prospective randomized noninferiority trial. Endoscopy. 2013;45:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Ichimasa K, Kudo SE, Mori Y, Wakamura K, Ikehara N, Kutsukawa M, Takeda K, Misawa M, Kudo T, Miyachi H. Double staining with crystal violet and methylene blue is appropriate for colonic endocytoscopy: An in vivo prospective pilot study. Dig Endosc. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Giacchino M, Bansal A, Kim RE, Singh V, Hall SB, Singh M, Rastogi A, Moloney B, Wani SB, Gaddam S. Clinical utility and interobserver agreement of autofluorescence imaging and magnification narrow-band imaging for the evaluation of Barrett’s esophagus: a prospective tandem study. Gastrointest Endosc. 2013;77:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Baldaque-Silva F, Marques M, Lunet N, Themudo G, Goda K, Toth E, Soares J, Bastos P, Ramalho R, Pereira P. Endoscopic assessment and grading of Barrett’s esophagus using magnification endoscopy and narrow band imaging: impact of structured learning and experience on the accuracy of the Amsterdam classification system. Scand J Gastroenterol. 2013;48:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett’s esophagus. Gastrointest Endosc. 2000;52:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Prasad GA, Wu TT, Wigle DA, Buttar NS, Wongkeesong LM, Dunagan KT, Lutzke LS, Borkenhagen LS, Wang KK. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology. 2009;137:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 26. | Inoue H, Sasajima K, Kaga M, Sugaya S, Sato Y, Wada Y, Inui M, Satodate H, Kudo SE, Kimura S. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy. 2006;38:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Pohl H, Koch M, Khalifa A, Papanikolaou IS, Scheiner K, Wiedenmann B, Rösch T. Evaluation of endocytoscopy in the surveillance of patients with Barrett’s esophagus. Endoscopy. 2007;39:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Arya AV, Yan BM. Ultra high magnification endoscopy: Is seeing really believing? World J Gastrointest Endosc. 2012;4:462-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Kumagai Y, Kawada K, Yamazaki S, Iida M, Odajima H, Ochiai T, Kawano T, Takubo K. Current status and limitations of the newly developed endocytoscope GIF-Y0002 with reference to its diagnostic performance for common esophageal lesions. J Dig Dis. 2012;13:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Hosoe N, Kobayashi T, Kanai T, Bessho R, Takayama T, Inoue N, Imaeda H, Iwao Y, Kobayashi S, Mukai M. In vivo visualization of trophozoites in patients with amoebic colitis by using a newly developed endocytoscope. Gastrointest Endosc. 2010;72:643-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |