INTRODUCTION
Laparoscopic pancreatectomy was first reported in the early 1990s. Since then, a variety of procedures have been introduced, such as staging of pancreatic malignancy, debridement for acute necrotizing pancreatitis, cystoenterostomy for pancreatic pseudocysts, pancreatic tumor enucleation, and pancreaticoduodenectomy[1-4]. Laparoscopic distal pancreatectomy (LDP) is widely accepted by the surgical community due to its advantages of decreased postoperative pain, earlier normalization of bowel function, and shorter length of hospitalization compared to open surgery[5-8]. However, the complication of pancreatic leakage after LDP remains a challenge because it may lead to a series of events including intraperitoneal abscess, subsequent sepsis, pseudoaneurysm formation, and occasional fatal hemorrhage[9]. Dealing with these complications by reoperation is particularly difficult due to adhesion formation and the generally fragile postoperative condition of the patient.
CASE REPORT
A 48-year-old man was admitted because of the incidental discovery of a pancreatic mass during a routine medical check-up. Abdominal computed tomography (CT) showed a low-density lesion of 1.5 cm × 1.3 cm with arterial enhancement in the pancreatic tail (Figure 1A). The patient underwent spleen-preserving LDP in April 2009. During the procedure, the splenic artery and vein were meticulously dissected from the pancreatic tissue and preserved. Branches from these two vessels to the pancreatic parenchyma were either severed by a harmonic scalpel or carefully ligated and separated. The pancreas was transected by an endoscopic linear stapler and the stump continuously sutured. A Jackson-Pratt drainage tube was placed in close proximity to the pancreatic stump. The procedure which lasted for 160 min was smooth. Postoperative pathological examination diagnosed a pancreatic insulinoma.
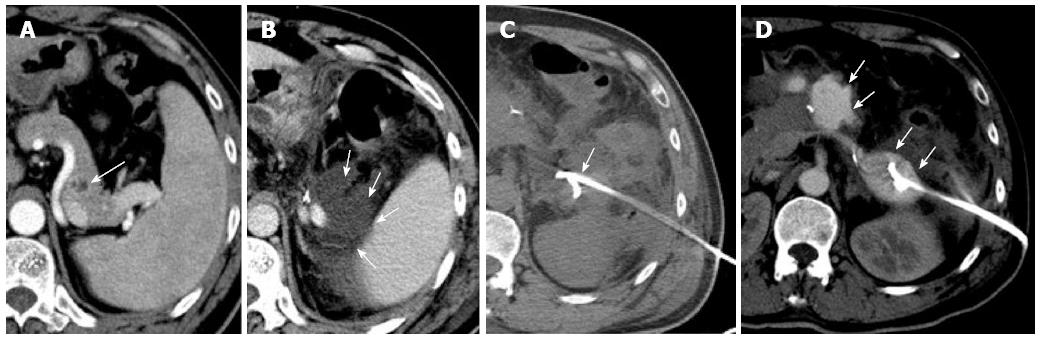
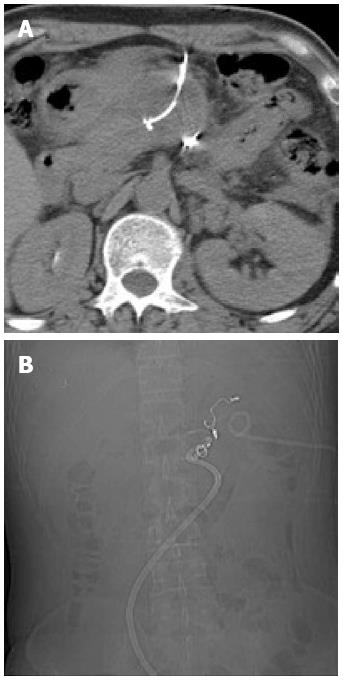
Figure 1 Computed tomography.
A: Distal pancreatic tumor; B: Hydrops adjacent to the pancreatic stump; C: A pigtail catheter (percutaneous catheter drainage tube 1) was placed into the left anterior pararenal space under computed tomography (CT) guidance; D: Abdominal contrast-enhanced CT revealed extravasation of contrast medium adjacent to the pancreatic stump.
The patient complained of abdominal fullness at 4 d after the operation, with physical examination revealing fever (up to 38.8 °C), tachycardia, and left abdominal tenderness. Amylase-rich abdominal fluid (4389 IU/L) draining from the Jackson-Pratt tube was detected. An effusion was demonstrated on both abdominal ultrasonography and CT beside the pancreatic stump (Figure 1B). Pancreatic leakage from the stump was therefore diagnosed according to International Study Group for Pancreatic Fistula criteria[10]. It was also noted that the patient’s sclera and skin were yellow in hue. Laboratory findings showed obvious elevation of serum bilirubin (total/direct bilirubin: 6.7/3.4 mg/dL) without evidence of biliary obstruction on imaging studies.
CT-guided percutaneous catheter drainage (PCD) was performed using Seldinger’s method: A 12-Fr locking pigtail catheter (Angiotech, Stenlose, Denmark) was placed into the left anterior pararenal space (Figure 1C). Thick brown amylase-rich (19000 IU/L) fluid of about 50 mL in volume was drained out daily; drainage continued for 6 d after which the daily drainage decreased to < 10 mL. The patient’s body temperature returned to normal 2 d after the PCD procedure. His white blood cell count and serum bilirubin level declined to the normal range in subsequent laboratory examinations. The patient was discharged with two drainage tubes in place on postoperative day 15.
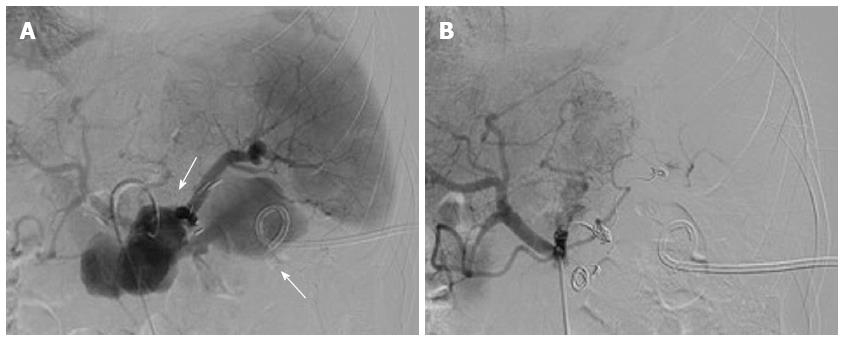
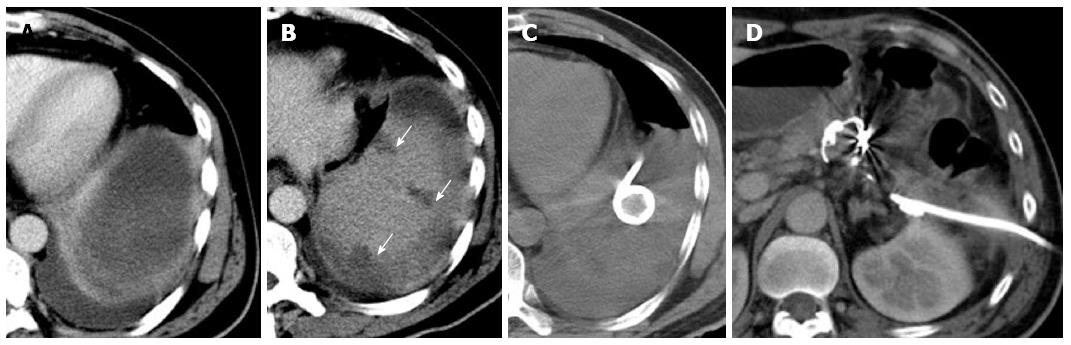
Six days later, the patient was readmitted due to persistent epigastric pain. On readmission, his vital signs were stable. There was tenderness and rebound pain at the upper quadrant of his abdomen with moderate muscle guarding. Sentinel bleeding was suspected because bloody fluid was observed in the PCD tube. The patient underwent an emergency abdominal CT scan that showed extravasation of contrast medium beside the pancreatic stump (Figure 1D). Follow-up arteriography revealed a splenic arterial wall irregularity at the proximal portion 3 cm from the celiac trunk, suggesting a pseudoaneurysm (Figure 2A). Under interventional radiology, the pseudoaneurysm was occluded at both proximal and distal sites with microcoils (Figure 2B). There was no continuous intra-abdominal bleeding after embolization, however, leakage of pancreatic juice and bacterial infection did not cease despite administration of octreotide and antibiotics. On postoperative day 25, the patient complained of epigastric pain and fever again, whereas his abdominal drainage decreased to < 5 mL/d. Enhanced abdominal CT revealed a peripancreatic effusion of considerable size (Figure 3A) and multiple low-density splenic shadows (Figure 3B). A second PCD was subsequently performed for drainage of the peripancreatic fluid collection (Figure 3C). Dark brown turbid fluid of a total volume of 200 mL/d was drained out by the two PCD catheters (PCD tube 1: 20 mL/d, PCD tube 2: 180 mL/d) and the patient’s symptoms were alleviated promptly. After 10 d, drainage from the two catheters began to decrease gradually and re-examination by CT showed apparent absorption of the peripancreatic effusion (Figure 3D). The second PCD tube was removed on postoperative day 40.
Figure 2 Celiac trunk angiography.
A: Celiac trunk angiography revealed pseudoaneurysm of the splenic artery; B: Celiac trunk angiography revealed no extravasation of contrast medium after embolization of the splenic artery with microcoils.
Figure 3 Examination.
A: Contrast-enhanced abdominal computed tomography revealed a peripancreatic effusion; B: Multiple focal ischemic lesions of the spleen; C: A pigtail catheter (percutaneous catheter drainage tube 2) was placed to drain the peripancreatic fluid collection; D: Absorption of the peripancreatic effusion.
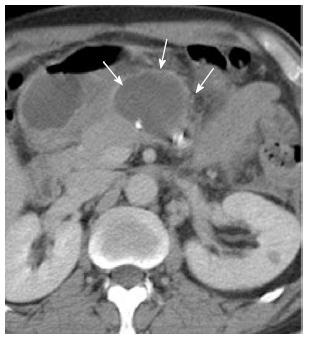
One week later, the patient suffered another episode of epigastric pain, fever, and leukocytosis despite cessation of drainage from PCD 1. Abdominal CT revealed an encapsulated fluid collection in front of the pancreatic stump (Figure 4) and the third PCD was inserted under CT guidance (Figure 5). Continuous intra-abdominal lavage was established between the two drainage catheters (PCD tube 1 and PCD tube 3) afterwards. As a result, the encapsulated peripancreatic fluid collection was absorbed within 1 mo after the third PCD was inserted and the two catheters were eventually removed. The patient was discharged on postoperative day 80. The patient was well on subsequent follow-up and remained so until April 2013.
Figure 4 Encapsulated fluid collection anterior to the pancreatic stump.
Figure 5 A pigtail catheter (percutaneous catheter drainage tube 3) was placed anterior to the pancreatic stump to drain the effusion.
A: Percutaneous catheter drainage (PCD) tube 3; B: PCD tubes 1 and 3.
DISCUSSION
LDP has been increasingly utilized as management for various lesions of the distal pancreas (e.g., neuroendocrine tumors and cystic lesions) since development of the procedure towards the turn of the century. For benign lesions of the left pancreas, spleen-preserving LDP (SPLDP) has been proposed in order to reduce risks associated with splenectomy[11,12]. In SPLDP, the blood supply to the spleen can be maintained by either preserving the splenic artery and vein[13] or preserving collaterals of short gastric and left gastroepiploic arteries and veins while severing the splenic vessels (the Warshaw’s method)[14]. The pancreatic lesion of the presented case, which was visualized as a hypodense mass on arterial enhancement of preoperative CT, was diagnosed as a neuroendocrine tumor. Tumor margins were clear with no apparent involvement of the splenic artery and vein, therefore, SPLDP was performed as surgical treatment.
The most common and clinically relevant complication of LDP is pancreatic leakage, which may lead to a series of sequelae including intra-abdominal abscess formation, sepsis, and even fatal bleeding. Although the incidence of pancreatic leakage after distal pancreatectomy has declined in the past decade, various high-volume centers have reported an occurrence rate of 5%-30% in recent studies[15-17]. A literature review revealed no significant variation in the incidence of pancreatic leakage after LDP and SPLDP[18-20]. Various techniques have been used to prevent pancreatic leakage, such as transecting the pancreatic parenchyma with endo-GI, or endo-GI closure with subsequent suturing, wound sealing with either fibrin-glue or an omental plug, and postoperative administration of octreotide. However, none of these aforementioned methods produced results of efficacious superiority.
On postoperative day 4, the patient complained of marked abdominal fullness. Physical examination revealed fever, tachycardia, and left abdominal tenderness. Amylase-rich fluid meeting the criteria for pancreatic leakage was detected from the Jackson-Pratt tube[10]. Fluid effusion was revealed adjacent to the pancreatic stump on ultrasonography and abdominal CT. The patient was noted to have jaundice on both physical and laboratory examinations. Given that there was no evidence of biliary obstruction, sepsis associated with pancreatic leakage was determined to be a likely etiology.
Percutaneous catheter drainage has proven effective in evacuating intra-abdominal abscesses in the past decade, with a reported resolution rate of 50%-80%[21-24]. In the present case, the left anterior pararenal space was considered an ideal route for percutaneous drainage, and PCD was performed with resultant rapid mitigation of fever, leukocytosis, and jaundice.
Pseudoaneurysm formation and bleeding, as a fatal complication following pancreatic surgery, occurs in 2%-4% of patients with pancreatic leakage[25]. Such bleeding usually presents as a sudden or intermittent event in the late postoperative stage. According to the literature, most patients with pseudoaneurysm hemorrhage develop some form of sentinel bleeding prior to major onset[26]. Immediate diagnostic intervention of sentinel bleeding is essential to save patients from a foreseeable massive hemorrhage. Angiography has been established as the management of choice for sentinel bleeding, because it can simultaneously determine the origin of the hemorrhage and provide selective embolization[9]. In the present patient, the cause of pseudoaneurysm formation was considered to be exposure of underlying parenchymal vasculature to the leaked pancreatic juice, with subsequent regional infection. Furthermore, we speculate that there may have been several weak points on the trunk of the splenic artery postoperatively, particularly the origins of small branches from the splenic artery to the pancreatic parenchyma, which were severed via harmonic scalpel without ligation. These points might have been sensitive to erosion by pancreatic juice and thus were particularly prone to pseudoaneurysm formation. Therefore, we recommend that every visible branch between the splenic artery and pancreatic parenchyma should be ligated and severed. It is also questionable whether this patient’s intra-abdominal bleeding was related to postoperative drainage catheter retention. However, iatrogenic etiology involving a pigtail catheter was deemed unlikely due to its coiled tip, which reduces friction between the catheter and surrounding tissue.
Based on the fact that radiological intervention has been widely recommended for pseudoaneurysm occlusion with a high success rate (67%-100%)[9,27-29], transcatheter microcoil embolization of the splenic artery was performed in our patient. Both the distal and proximal portions of the pseudoaneurysm were blocked and hemorrhage was effectively controlled. Occlusion of the splenic artery, the main arterial blood supply of the spleen, might have led to splenic infarction and secondary infection. Post-embolization CT revealed multiple patchy ischemic changes in the spleen, without obvious progression of splenic infarction on subsequent CT. Delayed follow-up at 2 years postoperatively indicated that perfusion of the spleen recovered, apparently compensated for by adequate circulatory collateralization. It is therefore reasonable to assume that the short gastric and gastroepiploic arteries are selectively preserved during SPLDP even when the splenic vessels are preserved.
The patient’s hemorrhage ceased after splenic artery embolization. However, pancreatic leakage and sepsis continued and resulted in recurrence of abdominal abscess formation. According to Gervais et al[22], repeated percutaneous drainage had a success rate of 91% in evacuating recurrent abdominal abscesses and was effective in obviating surgery in 56% of patients. In our case, two peripancreatic drainage catheters were inserted under interventional radiological guidance and peritoneal lavage was performed between the two tubes. Eventually, the pancreatic leakage ceased and the infection was controlled without further episodes of intra-abdominal bleeding.
In conclusion, apart from surgery, interventional radiological approaches are viable treatment options for post-LDP pancreatic leakage and ensuing secondary complications with safety and less invasiveness.