Published online Dec 7, 2013. doi: 10.3748/wjg.v19.i45.8382
Revised: September 22, 2013
Accepted: October 19, 2013
Published online: December 7, 2013
Processing time: 138 Days and 11.5 Hours
AIM: To investigate the clinical characteristics, diagnosis, treatment, and prognosis of primary adenosquamous carcinoma (ASC) of the esophagus.
METHODS: A total of 4015 patients with esophageal carcinoma underwent surgical resection between January 1995 and June 2012 at the Cancer Hospital of Shantou University Medical College. In 37 cases, the histological diagnosis was primary ASC. Clinical data were retrospectively analyzed from these 37 patients, who underwent transthoracic esophagectomy with lymphadenectomy. The χ2 or Fisher’s exact test was used to compare the clinicopathological features between patients with ASC and those with squamous cell carcinoma (SCC). The Kaplan-Meier and Log-Rank methods were used to estimate and compare survival rates. A Cox proportional hazard regression model was used to identify independent prognostic factors.
RESULTS: Primary esophageal ASC accounted for 0.92% of all primary esophageal carcinoma cases (37/4015). The clinical manifestations were identical to those of other types of esophageal cancer. All of the 24 patients who underwent preoperative endoscopic biopsy were misdiagnosed with SCC. The median survival time (MST) was 21.0 mo (95%CI: 12.6-29.4), and the 1-, 3-, and 5-year overall survival rates were 67.5%, 29.4%, and 22.9%, respectively. In multivariate analysis, only adjuvant radiotherapy (HR = 0.317, 95%CI: 0.114-0.885, P = 0.028) was found to be an independent prognostic factor. The MST for ASC patients was significantly lower than that for SCC patients [21.0 mo (95%CI: 12.6-29.4) vs 46.0 mo (95%CI: 40.8-51.2), P = 0.001]. In subgroup analyses, the MST for ASC patients was similar to that for poorly differentiated SCC patients.
CONCLUSION: Primary esophageal ASC is a rare disease that is prone to be misdiagnosed by endoscopic biopsy. The prognosis is poorer than esophageal SCC but similar to that for poorly differentiated SCC patients.
Core tip: Primary adenosquamous carcinoma (ASC) of the esophagus is an uncommon malignant esophageal neoplasm containing coexisting elements of infiltrating squamous cell carcinoma and adenocarcinoma. The biological behavior and response to therapies of this disease have not been well studied. In the current study, we reported the largest ever single-center patient cohort undergoing surgical resection for primary esophageal ASC, and we investigated the clinical characteristics, diagnosis, treatment, and prognosis in these patients. These data will give us a better understanding of this disease and help select the proper strategy for therapy.
- Citation: Chen SB, Weng HR, Wang G, Yang JS, Yang WP, Liu DT, Chen YP, Zhang H. Primary adenosquamous carcinoma of the esophagus. World J Gastroenterol 2013; 19(45): 8382-8390
- URL: https://www.wjgnet.com/1007-9327/full/v19/i45/8382.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i45.8382
Primary adenosquamous carcinoma (ASC) of the esophagus is an uncommon malignant esophageal neoplasm containing coexisting elements of infiltrating squamous cell carcinoma (SCC) and adenocarcinoma (AC)[1]. To our knowledge, no more than 40 cases of primary esophageal ASC have been published in the English literature over the past 20 years[1-11]. Most of the previous studies concerning esophageal ASC were case reports, while only one series of 18 cases was previously reported by Yachida et al[1]. The biological behavior and response to therapies of this disease have not been well studied. In the current study, we present data of 37 patients with primary esophageal ASC who underwent surgical resection with systematic lymphadenectomy from a single cancer center. We investigate the clinical characteristics, diagnosis, treatment, and prognosis in these patients, and we further analyze the factors that are associated with survival.
This study was undertaken at the Cancer Hospital of Shantou University Medical College and was approved by the Ethics Committee of that hospital. A total of 4015 patients with esophageal carcinoma underwent surgical resection between January 1995 and June 2012. In 37 cases (0.92%, 37/4015), the histological diagnosis was primary ASC. The clinical records of these 37 patients were analyzed retrospectively.
The medical history was obtained from all patients, who then underwent a physical examination. A chest radiograph, barium meal, contrast-enhanced computed tomography scan of the chest and abdomen, complete blood count, blood biochemistry analyses, and liver and renal function evaluations were also performed. Twenty-four patients underwent esophagoscopy biopsy before surgery. The remaining 13 patients from the early period of the study did not undergo an endoscopic biopsy when typical features of esophageal cancer were confirmed by clinical manifestations, esophagographic examinations and computed tomography scan of the chest. All histological specimens were re-examined by expert pathologists.
All resection specimens, including the lymph nodes, were assessed by two expert pathologists (Wu MY and Tian DP). Specimen analysis was performed in a standardized fashion with prospective documentation of all of the assessed parameters. Tumors were classified as ASC when the features of SCC and AC could be identified by light microcopy, with each accounting for at least 20% of the area in the sections of the deepest portion of tumor penetration. Patients with mucoepidermoid carcinomas were not included in this series.
Follow-up was performed every 3 mo for the first year, every 6 mo for the second year and every 6-12 mo thereafter. During each follow-up visit, the patients received a clinical evaluation, blood biochemistry examination, including that of tumor markers (SCC antigen, carcinoembryonic antigen), ultrasonography, and X-ray examination. Computed tomography was performed every year. Endoscopic examinations were performed when necessary. Follow-up was continued up to January 2013 or until death, if this occurred earlier. The mean follow-up was 33.5 mo (range: 1-158 mo). One patient was lost to follow-up (2.7%).
Local and regional recurrences were defined, respectively, as recurrence at the anastomosis or at any site within the operative field. Distant metastases were defined as tumor growth outside the operative field. A diagnosis of recurrent disease was made when pathologically or radiologically confirmed.
Statistical analysis was performed using SPSS 13.0 software (SPSS Inc., Chicago, IL). The χ2 or Fisher’s exact test was used to compare the clinicopathological features between patients with ASC and SCC. Overall survival time was calculated from the date of operation to the date of death or the most recent follow-up. Univariate analysis of survival was performed using the Kaplan-Meier method to estimate survival probabilities in patient subgroups, with the entry factors of gender, age (≤ 60 years vs > 60 years), location, length of the primary lesion (≤ 4 cm vs > 4 cm), macroscopic tumor type, pT category, pN category, operation (radical/palliative), and radiotherapy (yes/no). The log-rank test was used to assess differences in survival between groups. Factors identified at a significance level of P < 0.2 in the univariate analysis were selected for inclusion in a multivariate Cox proportional hazard regression model. All of the performed statistical tests were two-sided, and a P value less than 0.05 was considered to be statistically significant. The treatment results in this group of patients were compared with those in patients with different histological grades of SCC who were treated during the same period.
The clinicopathological features of the 37 primary esophageal ASC patients are shown in Table 1. This study group contained 31 men and 6 women, ranging in age from 40 to 78 years (median: 60 years). The primary lesions were most often found in the middle third of the thoracic esophagus and had a median length of 4.0 cm (2.0-10.0 cm). The clinical manifestations were identical to those of other types of esophageal cancer, with dysphagia, retrosternal pain, and loss of body weight being the main presenting symptoms.
| Gender | Age (yr) | Location | Length (cm) | Macroscopic tumor type | pT | pN | Operation | Treatment | Result | Last follow up (mo) |
| M | 40 | Lt | 7 | Medullary | 4a | 1 | Radical | S + R | D | 4 |
| M | 51 | Mt | 7 | Medullary | 3 | 2 | Radical | S | D | 19 |
| F | 74 | Mt | 8 | Polypoid | 4a | 1 | Radical | S | A | 158 |
| M | 47 | Mt | 5 | Medullary | 3 | 1 | Radical | S + R | A | 114 |
| M | 64 | Mt | 7 | Medullary | 4a | 0 | Radical | S | D | 2 |
| F | 51 | Mt | 3 | Medullary | 3 | 0 | Radical | S | D | 74 |
| M | 61 | Ut | 4 | Medullary | 1b | 0 | Radical | S | D | 45 |
| M | 42 | Ut | 4 | Sclerotic | 4b | 0 | Palliative | S | D | 20 |
| M | 50 | Mt | 3 | Medullary | 3 | 0 | Radical | S | D | 42 |
| M | 56 | Mt | 6 | Ulcerative | 3 | 0 | Radical | S | D | 21 |
| M | 60 | Mt | 3 | Sclerotic | 3 | 0 | Radical | S + R | D1 | 140 |
| M | 63 | Lt | 4 | Medullary | 3 | 1 | Radical | S | D | 14 |
| M | 65 | Mt | 4 | Sclerotic | 3 | 0 | Radical | S | D | 26 |
| M | 67 | Mt | 10 | Intraluminal | 2 | 0 | Radical | S | D | 1 |
| M | 72 | Mt | 4 | Medullary | 3 | 1 | Radical | S | D | 11 |
| M | 56 | Mt | 4 | Medullary | 3 | 1 | Radical | S | D | 15 |
| M | 78 | Mt | 4 | Medullary | 2 | 1 | Radical | S | D | 12 |
| F | 66 | Lt | 2 | Ulcerative | 2 | 1 | Radical | S + R | D | 28 |
| M | 50 | Mt | 7 | Medullary | 4a | 2 | Radical | S + R | D | 11 |
| M | 40 | Mt | 5 | Medullary | 1b | 0 | Radical | S | D | 30 |
| M | 53 | Mt | 6 | Medullary | 3 | 1 | Radical | S | D | 25 |
| F | 54 | Mt | 4 | Medullary | 3 | 0 | Radical | S | D | 5 |
| M | 62 | Mt | 4 | Medullary | 3 | 3 | Palliative | S + C | D | 5 |
| M | 63 | Mt | 4 | Medullary | 3 | 0 | Radical | S | A | 85 |
| M | 63 | Ut | 6 | Medullary | 3 | 0 | Radical | S | D | 19 |
| M | 41 | Mt | 6 | Medullary | 3 | 1 | Radical | S + R | A | 80 |
| M | 47 | Mt | 6 | Medullary | 3 | 0 | Radical | S | D | 9 |
| M | 57 | Mt | 3 | Polypoid | 2 | 0 | Radical | S | A | 60 |
| M | 53 | Mt | 6 | Medullary | 3 | 3 | Radical | S | D | 10 |
| M | 65 | Mt | 3 | Medullary | 1b | 0 | Radical | S | D | 33 |
| M | 56 | Mt | 3 | Medullary | 3 | 1 | Radical | S + R | D | 32 |
| F | 45 | Mt | 3 | Medullary | 3 | 0 | Radical | S + R | L | 16 |
| M | 63 | Mt | 7 | Medullary | 3 | 1 | Radical | S + R | A | 34 |
| M | 63 | Lt | 2 | Medullary | 1b | 3 | Radical | S | D | 6 |
| M | 65 | Mt | 5 | Medullary | 3 | 1 | Radical | S + R | D | 20 |
| F | 73 | Mt | 5 | Medullary | 3 | 1 | Radical | S | A | 11 |
| M | 73 | Lt | 10 | Medullary | 4a | 2 | Palliative | S | D | 2 |
None of the 37 patients underwent chemotherapy or radiotherapy before surgery, and none had prior malignant disease or distant metastases on routine examination before surgery.
All 37 patients underwent transthoracic esophagectomy with two-field lymphadenectomy (the mediastinal and perigastric lymph nodes), including 34 cases of radical resection and three cases of palliative resection. A total of 480 lymph nodes were removed, and 69 had metastases. Twenty of the 37 patients (54.1%) proved postoperatively to have histologically confirmed lymph node metastases. According to the seventh edition of the American Joint Committee on Cancer staging system for esophageal cancer, there were fourteen stage pN1 cases, three stage pN2 cases, and three stage pN3 cases. Postoperative complications included pneumonia, pneumothorax, and esophagogastric anastomotic leak in each of two cases. No patients died during treatment in hospital and 30 d after surgery.
Adjuvant therapies were routinely recommended to patients with a locally advanced tumor or mediastinal lymph node metastases. However, not all patients complied with this recommendation. Twenty-four patients who underwent a radical operation were treated by surgery alone, and ten patients who underwent a radical operation were treated by surgery plus postoperative radiotherapy. None of the 34 patients who underwent a radical operation received adjuvant chemotherapy. One of the 3 patients who underwent a palliative operation received adjuvant chemotherapy (Cisplatin plus 5-Fu for two cycles) and died 5 mo after surgery. One palliatively operated patient died 2 mo after surgery and did not receive adjuvant therapy. The other palliatively operated patient did not receive adjuvant therapy for economic reasons and survived for 20 mo. Therapeutic radiation was delivered using 6 or 8 MV photons. A total dose of 50 Gy was delivered in 2 Gy fractions 5 d/wk.
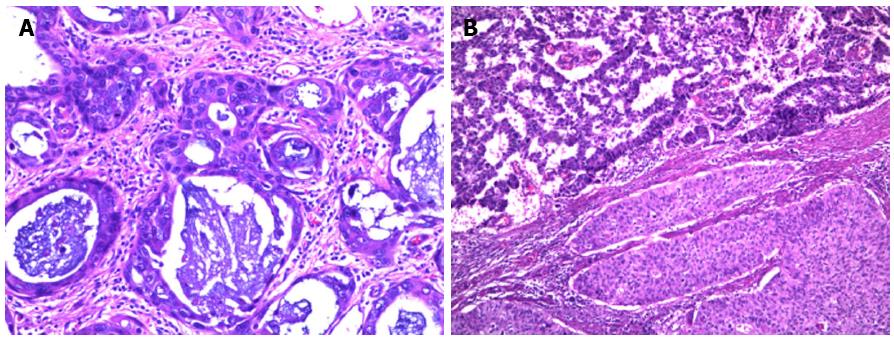
Grossly, ASC was indistinguishable from pure SCC. The macroscopic tumor was classified as intraluminal type in one case, polyploid type in two cases, ulcerative type in two cases, sclerotic type in three cases, and medullary type in 29 cases. Microscopically, ASC was characterized by a mixture of AC and SCC features (Figure 1). SCC could have different stages of squamous differentiation with or without intercellular bridges, individual cell keratinization, and cancer pearls. AC contained tubular or glandular structures with focal to abundant intracellular or extracellular mucin. The AC and SCC could be intermingled or have a fairly clear boundary. No intermediate cells such as the type observed in mucoepidermoid carcinoma were evident in ASC. In most tumors, SCC dominated in the mucosa, while AC was identified in the deeper portion of the tumor.
Twenty-four patients underwent esophagoscopy biopsy before surgery, and all of them were misdiagnosed with SCC.
By January 2013, with a mean follow-up of 33.5 mo (range: 1-158 mo), 29 patients had died, seven were still alive, and one was lost to follow-up.
The median survival time (MST) of the 37 patients was 21.0 mo (95%CI: 12.6-29.4), and the 1-, 3-, and 5-year overall survival rates were 67.5%, 29.4%, and 22.9%, respectively. One patient died of myocardial infarction 140 mo after the surgery.
Univariate analysis and multivariate analysis were performed to assess the relationship between the clinicopathological features and the prognosis of ASC patients. The variables related to survival in univariate analysis are shown in Table 2. Only location, macroscopic tumor type, pN category, and operation (radical/palliative) affected the overall survival (P < 0.05). Although the MST at 32.0 mo for the patients who received adjuvant radiotherapy was longer than that at 19.0 mo for the patients who did not, the difference was not significant (P = 0.066). In multivariate analysis, adjuvant radiotherapy (P = 0.028) was found to be an independent factor for the prediction of prognosis (Table 3). Patients who received adjuvant radiotherapy had a relatively better survival.
| Number of patients | Survival rate | MST (mo, 95%CI) | P value | |||
| 1-yr | 3-yr | 5-yr | ||||
| Gender | 0.302 | |||||
| Male | 31 | 64.5% | 25.8% | 18.4% | 20.0 (13.5-26.5) | |
| Female | 6 | 83.3% | 55.6% | 55.6% | 74.0 (0.0-149.4) | |
| Age (yr) | 0.448 | |||||
| ≤ 60 | 19 | 73.7% | 34.2% | 28.5% | 25.0 (11.6-38.4) | |
| > 60 | 18 | 60.6% | 24.2% | 16.2% | 19.0 (8.6-29.4) | |
| Location | 0.031 | |||||
| Ut | 3 | 100.0% | 33.3% | 0.0% | 20.0 (18.4-21.6) | |
| Mt | 29 | 68.8% | 34.5% | 30.2% | 25.0 (12.8-37.2) | |
| Lt | 5 | 40.0% | 0.0% | 0.0% | 6.0 (1.7-10.3) | |
| Length | 0.630 | |||||
| ≤ 4 cm | 19 | 73.7% | 34.4% | 23.0% | 28.0 (12.0-44.0) | |
| > 4 cm | 18 | 61.1% | 24.4% | 24.4% | 19.0 (7.4-30.6) | |
| Macroscopic tumor type | < 0.001 | |||||
| Medullary | 29 | 61.9% | 27.3% | 18.2% | 19.0 (11.7-26.3) | |
| Intraluminal | 1 | 0.0% | 0.0% | 0.0% | 1.0 | |
| Polyploid | 2 | 100.0% | 100.0% | 100.0% | - | |
| Ulcerative | 2 | 100.0% | 0.0% | 0.0% | 21.0 | |
| Sclerotic | 3 | 100.0% | 33.3% | 33.3% | 26.0 (16.4-35.6) | |
| pT category | 0.573 | |||||
| pT1 | 4 | 75.0% | 25.0% | 0.0% | 30.0 (3.5-56.5) | |
| pT2 | 4 | 50.0% | 25.0% | 25.0% | 12.0 (0.0-38.5) | |
| pT3 | 23 | 78.3% | 34.5% | 28.8% | 21.0 (12.4-29.6) | |
| pT4 | 6 | 33.3% | 16.7% | 16.7% | 4.0 (0.0-14.8) | |
| pN category | 0.002 | |||||
| pN0 | 17 | 76.5% | 38.2% | 25.5% | 30.0 (12.8-47.2) | |
| pN1 | 14 | 77.9% | 31.2% | 31.2% | 25.0 (9.9-40.1) | |
| pN2 | 3 | 33.3% | 0.0% | 0.0% | 11.0 (0.0-25.4) | |
| pN3 | 3 | 0.0% | 0.0% | 0.0% | 6.0 (4.4-7.6) | |
| Adjuvant radiotherapy | 0.066 | |||||
| No | 27 | 62.7% | 23.5% | 15.7% | 19.0 (11.7-26.3) | |
| Yes | 10 | 80.0% | 45.7% | 45.7% | 32.0 | |
| Operation | 0.025 | |||||
| Radical | 34 | 70.5% | 32.2% | 25.0% | 25.0 (15.4-34.6) | |
| Palliative | 3 | 33.3% | 0.0% | 0.0% | 5.0 (0.2-9.8) | |
| Prognostic factor | Hazard ratio | 95%CI | P value |
| Location | 2.177 | 0.797-5.951 | 0.129 |
| Macroscopic tumor type | 1.002 | 0.633-1.585 | 0.995 |
| pN category | 1.656 | 0.956-2.871 | 0.072 |
| Adjuvant radiotherapy | 0.317 | 0.114-0.885 | 0.028 |
| Operation | 3.718 | 0.782-17.679 | 0.099 |
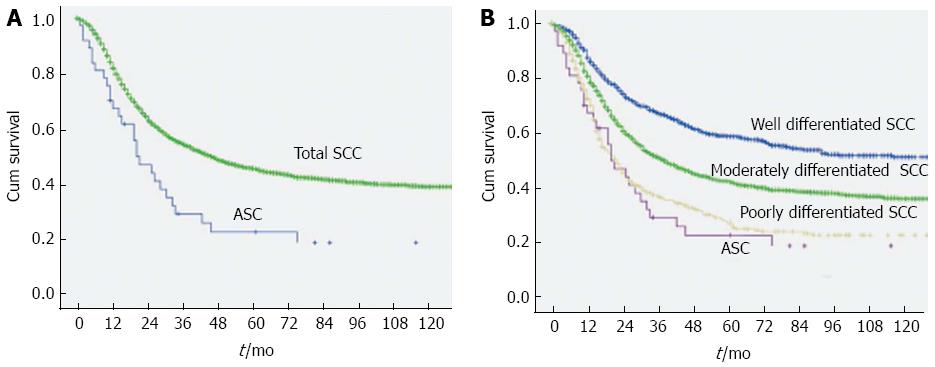
Finally, we assessed the prognosis between esophageal ASC and different histological grades of SCC. Three thousand seven hundred eighty five of 4015 esophageal carcinoma patients were histologically diagnosed with SCC. Of these patients, 3439 did not receive neoadjuvant therapy, including 1011 cases of well-differentiated SCC, 2007 cases of moderately differentiated SCC, and 421 cases of poorly differentiated SCC. All patients underwent transthoracic esophagectomy with two-field lymphadenectomy (the mediastinal and perigastric lymph nodes). One hundred twenty patients were lost to follow-up (3.5%). The clinicopathological features of patients with ASC or different histological grades of SCC are shown in Table 4. All of the factors were balanced between patients with ASC and SCC. The MST at 21.0 mo for ASC patients was significantly lower than that at 46.0 mo for SCC patients (P = 0.001) (Figure 2). We further sub-classified SCC into different histological grades and compared the prognosis with esophageal ASC. The MSTs for well-differentiated SCC, moderately differentiated SCC, and poorly differentiated SCC were 148.0, 39.0, and 22.0 mo, respectively (Table 5). The MST for ASC patients was significantly lower than that for well- (P < 0.001) and moderately differentiated SCC patients (P = 0.004), but there was no significant difference in the MST between ASC patients and poorly differentiated SCC patients (P = 0.536) (Figure 2).
| Variable | ASC | SCC | P value | |||
| Well differentiated (n = 1011) | Moderately differentiated (n = 2007) | Poorly differentiated (n = 421) | Total (n = 3439) | |||
| Gender | 0.191 | |||||
| Male | 31 | 748 | 1473 | 313 | 2534 | |
| Female | 6 | 263 | 534 | 108 | 905 | |
| Age (yr) | 0.056 | |||||
| ≤ 60 | 19 | 672 | 1347 | 270 | 2289 | |
| > 60 | 18 | 339 | 660 | 151 | 1150 | |
| Location | 0.647 | |||||
| Ut | 3 | 124 | 284 | 54 | 462 | |
| Mt | 29 | 746 | 1426 | 303 | 2475 | |
| Lt | 5 | 141 | 297 | 64 | 502 | |
| Tumor length | 0.182 | |||||
| ≤ 4 cm | 19 | 397 | 799 | 191 | 1387 | |
| > 4 cm | 18 | 614 | 1208 | 230 | 2052 | |
| pT category | 0.409 | |||||
| pTis | 0 | 10 | 17 | 5 | 32 | |
| pT1a | 0 | 18 | 35 | 10 | 63 | |
| pT1b | 4 | 39 | 88 | 22 | 149 | |
| pT2 | 4 | 202 | 350 | 80 | 632 | |
| pT3 | 23 | 562 | 1135 | 232 | 1929 | |
| pT4a | 5 | 139 | 278 | 68 | 485 | |
| pT4b | 1 | 41 | 84 | 24 | 149 | |
| pN category | 0.187 | |||||
| pN0 | 17 | 587 | 1049 | 212 | 1848 | |
| pN1 | 14 | 235 | 530 | 110 | 875 | |
| pN2 | 3 | 151 | 328 | 69 | 548 | |
| pN3 | 3 | 38 | 100 | 30 | 168 | |
| Adjuvant radiotherapy | 1.000 | |||||
| No | 27 | 729 | 1465 | 292 | 2486 | |
| Yes | 10 | 282 | 542 | 129 | 953 | |
| Operation | 0.755 | |||||
| Radical | 34 | 945 | 1851 | 385 | 3181 | |
| Palliative | 3 | 66 | 156 | 36 | 258 | |
| Cases | 1-yr OS | 3-yr OS | 5-yr OS | MST (mo, 95%CI) | |
| Well differentiated SCC | 1011 | 87.5% | 67.1% | 58.9% | 148.0 |
| Moderately differentiated SCC | 2007 | 81.0% | 51.5% | 42.5% | 39.0 (34.7-43.3) |
| Poorly differentiated SCC | 421 | 72.6% | 37.3% | 27.4% | 22.0 (18.3-25.7) |
| Total SCC | 3439 | 81.8% | 54.3% | 45.6% | 46.0 (40.8-51.2) |
| ASC | 37 | 67.5% | 29.4% | 22.9% | 21.0 (12.6-29.4) |
Twenty patients had complete recurrence and metastasis data. The first failure sites in these 20 cases included local/regional recurrences in six cases, local/regional recurrences with distant metastases in four cases, and distant metastases in 10 cases. The mean time from treatment to failure was 14.0 (2.0-65.0) mo. Metastases were detected in the lung in two cases, the brain in two cases, the liver in two cases, the bone in three cases, and distant lymph nodes in five cases.
ASC is a malignant tumor that has granular and squamous histological components. According to the guidelines for clinical and pathological studies of carcinomas of the esophagus established by the Japan Esophageal Society, ASC of the esophagus is defined as having at least 20% of the SCC and AC features on routine microscopic examination using hematoxylin and eosin staining[12]. Primary esophageal ASC was relatively uncommon, and in our study, it accounted for only 0.92% of all cases of primary esophageal carcinoma. Due to the low incidence of esophageal ASC, the biological behavior and response to therapies of this disease have not been well studied.
We report the largest ever single-center patient cohort undergoing surgical resection for primary esophageal ASC, having investigated the clinical characteristics, diagnosis, treatment, and prognosis in these patients. Most of the patients were male with a median age of 60 years. The primary lesions were most often found in the middle third of the thoracic esophagus, and progressive dysphagia, retrosternal pain, and loss of body weight were the main presenting symptoms, which were identical to those of other types of esophageal carcinoma.
The origin of primary esophageal ASC remains obscure. It has been suggested that both components of an ASC originate from the same clone[11]. Some authors believe that this type of tumor arises from esophageal gland cells or ductal cells[10]. However, it has also been speculated that ASC first develops as SCC and that glandular differentiation subsequently occurs in the tumor cell populations[1,8]. In our study group, most of the tumors exhibited carcinoma in situ differentiating in the mucosa adjacent to the tumors, while no cellular atypia or transition to tumor cells was found in the underlying esophageal glands or their ducts. These features indicated that esophageal ASC could originate from the covering squamous epithelium.
Because the clinical features of primary esophageal ASC are basically identical to those of other types of esophageal cancer, the diagnosis of this disease is dependent on histopathological examination. Esophagoscopy biopsy is the most frequently used method for diagnosis before treatment. However, in our study, all 24 patients who underwent esophagoscopy biopsy before surgery were misdiagnosed with SCC. In the postoperative pathological examinations, we found that SCC dominated in the mucosa in most tumors, while AC was identified in the deeper portion of the tumor. This difference could have contributed to the high misdiagnosis rate of esophageal ASC due to the small volume of the biopsy specimen. Deeper biopsies that include a greater tissue volume and are performed under an endoscope could help improve the diagnostic accuracy.
No standard treatment for esophageal ASC has been established because its incidence is low. Few studies have addressed the treatment of esophageal ASC. At present, the treatment for this condition is similar to that for other types of esophageal carcinoma because of the difficulty in establishing the definitive diagnosis preoperatively. Surgical resection is still an important method for resectable esophageal carcinoma with neoadjuvant chemoradiotherapy for a locally advanced disease[13-16]. In the current study, none of the 37 patients received neoadjuvant therapy, while 10 patients were treated by surgery plus postoperative radiotherapy. We found that adjuvant radiotherapy was an independent prognostic factor in multivariate analysis (P = 0.028). Patients who received adjuvant radiotherapy had a relatively better survival. The role of postoperative radiotherapy is still debated in esophageal cancer, and most previous studies have revealed no survival benefit with postoperative radiotherapy for esophageal SCC and AC[17,18]. In our study, although postoperative radiotherapy was found to be predictive of survival in the multivariate analysis, because the number of patients in this study was small, we think that more data should be collected to confirm this finding. Recently, neoadjuvant chemoradiotherapy had been increasingly used for the treatment of esophageal cancer patients, and it has shown a significant survival benefit[13-16]. It is suggested that further research should be conducted to establish the value of this new therapeutic approach for patients with esophageal ASC.
ASC has been confirmed to be more aggressive than “pure” AC and SCC in many other tumors, with frequent lymph node metastasis and poor prognosis[19-27]. Some case reports of esophageal ASC have also indicated that it has a highly aggressive biological behavior[5,7]. Another study of 18 cases reported by Yachida et al[1] found that esophageal ASC had a better prognosis than conventional SCC and AC, but their finding is likely due to the smaller size and lower stage of the tumors in that study. In our study, although the 54.1% rate of lymph node metastases was higher than that of esophageal SCC (46.3%, 1591/3439), no significant difference was observed (P > 0.05). Regarding the prognosis, the MST at 21.0 mo for ASC patients was significantly lower than that at 46.0 mo for the SCC patients in our study (P = 0.001). In the subgroup analyses, the MST for ASC patients was significantly lower than that for well- and moderately differentiated SCC patients (P < 0.001) but was similar to that for poorly differentiated SCC patients (P = 0.536). The poor prognosis of esophageal ASC could be caused by its aggressive biological behavior.
In summary, primary esophageal ASC is a rare disease that is prone to be misdiagnosed by endoscopic biopsy. The prognosis for patients undergoing surgical resection is poorer than that for most esophageal SCC patients but similar to that for poorly differentiated SCC patients. Further investigations are required to determine the biological behavior of this tumor and develop new therapeutic modalities to achieve further improvements in the clinical outcome.
We thank Dr. Ming-Yao Wu and Dr. Dong-Ping Tian from the Pathologic Department of Shantou University Medical College for their sincere help in this study.
Primary adenosquamous carcinoma (ASC) of the esophagus is an uncommon malignant esophageal neoplasm containing coexisting elements of infiltrating squamous cell carcinoma (SCC) and adenocarcinoma (AC). The biological behavior and response to therapies of this disease have not been well-studied due to its low incidence.
No more than 40 cases of primary esophageal ASC have been published in the English literature over the past 20 years. Most of the previous studies concerning esophageal ASC were case reports. The authors reported the largest ever single-center patient cohort of 37 cases undergoing surgical resection for primary esophageal ASC, and investigated the clinical characteristics, diagnosis, treatment, and prognosis.
This study was the largest that was conducted by a single institution to report the clinical characteristics, diagnosis, treatment, and prognosis of esophageal ASC. For the first time, the authors determined that esophageal ASC was prone to be misdiagnosed by endoscopic biopsy and that the prognosis for patients undergoing surgical resection is poorer than that for most esophageal SCC patients but similar to that for poorly differentiated SCC patients.
This is the largest study of primary esophageal ASC, and it will give the authors a better understanding of this disease and help the selection of the proper therapy strategy.
Esophageal ASC is an uncommon malignant esophageal neoplasm containing coexisting elements of infiltrating SCC and AC. According to the guidelines for clinical and pathological studies of carcinomas of the esophagus that were established by the Japan Esophageal Society, ASC of the esophagus is defined as having at least 20% each of the SCC and AC elements on routine microscopic examination using hematoxylin and eosin staining.
This is a well written and thorough coverage of this important topic. The authors investigated the clinical characteristics, diagnosis, treatment, and prognosis of 37 cases of ASC of the esophagus from 4015 esophageal carcinoma patients who received surgical resection between January 1995 and June 2012. They concluded that the prognosis of esophageal ASC is poorer than SCC. The paper is of interest for gastroenterologists.
P- Reviewers: Ding XW, Wang Y S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Ma S
| 1. | Yachida S, Nakanishi Y, Shimoda T, Nimura S, Igaki H, Tachimori Y, Kato H. Adenosquamous carcinoma of the esophagus. Clinicopathologic study of 18 cases. Oncology. 2004;66:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Noguchi T, Uchida Y, Fumoto S, Wada S, Sato T, Takeno S. Adenosquamous carcinoma arising in Barrett’s esophagus. Jpn J Thorac Cardiovasc Surg. 2002;50:537-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Pascal RR, Clearfield HR. Mucoepidermoid (adenosquamous) carcinoma arising in Barrett’s esophagus. Dig Dis Sci. 1987;32:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Ter RB, Govil YK, Leite L, Infantolino A, Ghabra M, Galan A, Katz PO. Adenosquamous carcinoma in Barrett’s esophagus presenting as pseudoachalasia. Am J Gastroenterol. 1999;94:268-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Bombí JA, Riverola A, Bordas JM, Cardesa A. Adenosquamous carcinoma of the esophagus. A case report. Pathol Res Pract. 1991;187:514-519; discussion 514-521. [PubMed] |
| 6. | Orsatti G, Corvalan AH, Sakurai H, Choi HS. Polypoid adenosquamous carcinoma of the esophagus with prominent spindle cells. Report of a case with immunohistochemical and ultrastructural studies. Arch Pathol Lab Med. 1993;117:544-547. [PubMed] |
| 7. | Sakata K, Ishida M, Hiraishi H, Sasai T, Watanabe N, Suzuki Y, Masuyama H, Terano A. Adenosquamous carcinoma of the esophagus after endoscopic variceal sclerotherapy: a case report and review of the literature. Gastrointest Endosc. 1998;47:294-299. [PubMed] |
| 8. | Zhao S, Xue Q, Ye B, Lu H, He J, Zhao H. Synchronous primary carcinosarcoma and adenosquamous carcinoma of the esophagus. Ann Thorac Surg. 2011;91:926-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Cirillo LC, Mainenti PP, Imbriaco M, Franco R, Gatta G, De Rosa G, Salvatore M. Synchronous primary adenocarcinoma and adenosquamous carcinoma of the esophagus. Eur Radiol. 2001;11:1964-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Lam KY, Loke SL, Ma LT. Histochemistry of mucin secreting components in mucoepidermoid and adenosquamous carcinoma of the oesophagus. J Clin Pathol. 1993;46:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | van Rees BP, Rouse RW, de Wit MJ, van Noesel CJ, Tytgat GN, van Lanschot JJ, Offerhaus GJ. Molecular evidence for the same clonal origin of both components of an adenosquamous Barrett carcinoma. Gastroenterology. 2002;122:784-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part II and III. Esophagus. 2009;6:71-94. [RCA] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4057] [Article Influence: 312.1] [Reference Citation Analysis (0)] |
| 14. | Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1263] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 15. | Platz TA, Nurkin SJ, Fong MK, Groman A, Flaherty L, Malhotra U, Levea CM, Yendamuri S, Warren GW, Nava HR. Neoadjuvant chemoradiotherapy for esophageal/gastroesophageal carcinoma. J Gastrointest Oncol. 2013;4:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Wang DB, Zhang X, Han HL, Xu YJ, Sun DQ, Shi ZL. Neoadjuvant chemoradiotherapy could improve survival outcomes for esophageal carcinoma: a meta-analysis. Dig Dis Sci. 2012;57:3226-3233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Chen J, Zhu J, Pan J, Zhu K, Zheng X, Chen M, Wang J, Liao Z. Postoperative radiotherapy improved survival of poor prognostic squamous cell carcinoma esophagus. Ann Thorac Surg. 2010;90:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oe sophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 19. | Voong KR, Davison J, Pawlik TM, Uy MO, Hsu CC, Winter J, Hruban RH, Laheru D, Rudra S, Swartz MJ. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol. 2010;41:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Filosso PL, Ruffini E, Asioli S, Giobbe R, Macri L, Bruna MC, Sandri A, Oliaro A. Adenosquamous lung carcinomas: a histologic subtype with poor prognosis. Lung Cancer. 2011;74:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Cagir B, Nagy MW, Topham A, Rakinic J, Fry RD. Adenosquamous carcinoma of the colon, rectum, and anus: epidemiology, distribution, and survival characteristics. Dis Colon Rectum. 1999;42:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Choi JW, Park HU. Adenosquamous carcinoma of the ascending colon: a case report and review of the literature. Ann Coloproctol. 2013;29:83-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Schick U, Pusztaszeri M, Betz M, Ghadjar P, Demiroz C, Kaanders JH, Ozsahin M. Adenosquamous carcinoma of the head and neck: report of 20 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Masoomi H, Ziogas A, Lin BS, Barleben A, Mills S, Stamos MJ, Zell JA. Population-based evaluation of adenosquamous carcinoma of the colon and rectum. Dis Colon Rectum. 2012;55:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Boyd CA, Benarroch-Gampel J, Sheffield KM, Cooksley CD, Riall TS. 415 patients with adenosquamous carcinoma of the pancreas: a population-based analysis of prognosis and survival. J Surg Res. 2012;174:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Wang J, Wang FW, Lagrange CA, Hemstreet GP. Clinical features and outcomes of 25 patients with primary adenosquamous cell carcinoma of the prostate. Rare Tumors. 2010;2:e47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Kobayashi M, Okabayashi T, Okamoto K, Namikawa T, Araki K. A clinicopathologic study of primary adenosquamous carcinoma of the liver. J Clin Gastroenterol. 2005;39:544-548. [PubMed] |