Published online Dec 7, 2013. doi: 10.3748/wjg.v19.i45.8219
Revised: November 4, 2013
Accepted: November 12, 2013
Published online: December 7, 2013
Processing time: 81 Days and 20.3 Hours
Helicobacter pylori (H. pylori) infection might initiate and contribute to the progression of lymphoma from gastric mucosa-associated lymphoid tissue (MALT). Increasing evidence shows that eradication of H. pylori with antibiotic therapy can lead to regression of gastric MALT lymphoma and can result in a 10-year sustained remission. The eradication of H. pylori is the standard care for patients with gastric MALT lymphoma. Cytotoxin-associated gene A (CagA) protein, one of the most extensively studied H. pylori virulence factors, is strongly associated with the gastric MALT lymphoma. CagA possesses polymorphisms according to its C-terminal structure and displays different functions among areas and races. After being translocated into B lymphocytes via type IV secretion system, CagA deregulates intracellular signaling pathways in both tyrosine phosphorylation-dependent and -independent manners and/or some other pathways, and thereby promotes lymphomagenesis. A variety of proteins including p53 and protein tyrosine phosphatases-2 are involved in the malignant transformation induced by CagA. Mucosal inflammation is the foundational mechanism underlying the occurrence and development of gastric MALT lymphoma.
Core tip: Cytotoxin-associated gene A (CagA) protein encoded by cag pathogenicity island of Helicobacter pylori is a bacterium-derived oncoprotein and is strongly associated with the gastric mucosa-associated lymphoid tissue (MALT) lymphoma. After being translocated into B cells via type IV secretion system in ATP-dependent manner, CagA deregulates several pathways in both tyrosine phosphorylation-dependent and -independent manners, and thereby promotes lymphomagenesis. Two important proteins, p53 and protein tyrosine phosphatases-2, are involved in the malignant transformation induced by CagA. In addition, mucosal inflammation is the foundational mechanism underlying the occurrence and development of gastric MALT lymphoma. However, the exact mechanism by which CagA promotes oncogenesis needs further clarification.
-
Citation: Wang HP, Zhu YL, Shao W. Role of
Helicobacter pylori virulence factor cytotoxin-associated gene A in gastric mucosa-associated lymphoid tissue lymphoma. World J Gastroenterol 2013; 19(45): 8219-8226 - URL: https://www.wjgnet.com/1007-9327/full/v19/i45/8219.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i45.8219
Helicobacter pylori (H. pylori), a spiral-shaped, microaerophilic, Gram-negative bacterium, infects approximately 50% of humans worldwide. H. pylori is associated with chronic active gastritis and peptic ulcers, is a risk factor for gastric cancer[1] and has been ranked as a class I carcinogen by the International Agency for Research on Cancer since 1994[2,3]. H. pylori infection might initiate and contribute to the progression of lymphoma from gastric mucosa-associated lymphoid tissue[4,5]. Clinical observations have shown that the eradication of H. pylori with antibiotic therapy can lead to regression of gastric mucosa-associated lymphoid tissue (MALT) lymphoma in 77.5%-94.0% of patients[6-9] and can result in a 10-year sustained remission in up to 64% of cases[10]. The eradication of H. pylori is the standard care for patients with gastric MALT. The results of a population-based study showed that the incidence of H. pylori-positive gastric MALT lymphoma had reduced sharply in the era of anti-H. pylori intervention[11]. This review summarizes the role of H. pylori cytotoxin-associated gene A (CagA) in the development and/or maintenance of gastric MALT lymphoma.
The CagA protein, encoded by the cytotoxin-associated gene (cag) pathogenicity island, is one of the most important H. pylori virulence factors[12,13] and is causally linked to gastric MALT lymphoma. Fischbach et al[14] and Eck et al[15] determined that seropositivity of CagA was present in 89.0%-95.5% of patients with gastric MALT lymphoma, as tested by enzyme-linked immunosorbent assay and Western blot. The seroprevalence rate exceeded the prevalence of chronic active gastritis in the German population. The serological discovery of cagA-positive H. pylori isolates does not necessarily reflect the current colonization of the gastric mucosa because the immunoglobulin (Ig)A/IgG represents a past immune response. Mucosal-derived antibodies play an important part in the mucosal immune response. CagA-specific mucosal IgA and IgG antibodies occur in almost all patients with H. pylori-associated gastric MALT lymphoma[16,17]. Sumida et al[18] showed that in t(11;18)(q21;q21)-negative gastric MALT lymphoma patients, concentrations of anti-CagA IgG were significantly higher in the H. pylori-dependent cases than in the H. pylori-independent cases, and the H. pylori-dependent cases had a better therapeutic effect. The CagA protein can be detected in B lymphocytes in people infected with cagA-positive H. pylori strains[19]. Kuo further explored that CagA can be detected in the malignant B cells of H. pylori-associated gastric MALT lymphoma. The expression of CagA was evaluated using immunohistochemistry and confirmed using immunoblot analyses[20]. These findings suggest that gastric MALT lymphoma is associated with H. pylori strains expressing the CagA protein. Ohnishi and colleagues transfected C57BL/6 mice with a cagAHs (humanized cagA gene) expression vector throughout the body or predominantly in the stomach to generate transgenic mice[21]. They performed immunoprecipitation, immunoblotting, histological examinations and other analyses of the gastric mucosa from 72-wk-old cagAHs mice and determined that CagA induced abnormal proliferation of the gastric epithelial cells and hematopoietic cells, which was followed by the development of gastrointestinal carcinomas and lymphomas of B-cell origin. These results indicate that CagA is involved in the development of gastric MALT lymphoma, which provide the first direct evidence that CagA functions as a bacterium-derived oncoprotein in mammals[21].
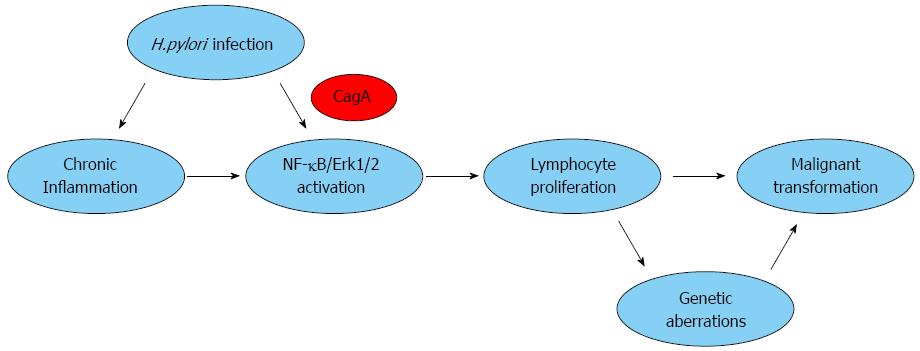
Lymphomas are malignant tumors that originate in the lymphatic system. Lymphocytes proliferate in respond to the stimulation of persistent antigens and repeated infections in patients with immune deficiencies. The deregulation of the cell cycle and apoptosis is important in the pathogenesis of lymphoma. Lymphocytes that lack self-control divide faster than normal cells or survive longer than they should, proliferating in response to antigenic stimulation, which leads to the occurrence of unlimited proliferation and eventual lymphoma. Lymphocytes and lymphoid tissues do not normally exist in the stomach[22]. The onset of gastric MALT lymphoma is preceded by the acquisition of MALTs as a result of sustained H. pylori infection, which initiates the inflammatory lymphoproliferation[23,24]. The persistence of bacterial colonization, acting as immunologic stimuli, results in the recruitment of immune lymphocytes that migrate to and infiltrate the site of H. pylori infection in the stomach, which induce and sustain an actively proliferating B-cell population. Eventually, the formation of acquired lymphoid follicles and mucosal associated lymphoid tissues develop[25-27] (Figure 1). Much attention has been focused on the role of CagA in malignant transformation of the B cells. CagA may deregulate the host intracellular signaling transduction and lower the threshold for neoplastic transformation[28].
CagA is encoded by the cagA gene within the cag pathogenicity island, a chromosomal region that simultaneously encodes a type IV secretion system (T4SS) specializing in the transfer of CagA from bacteria to the target cells in an ATP-dependent manner[29,30]. CagA is the only known effector protein that is translocated by a T4SS[31-33]. Analyses of the DNA sequence and molecular phylogenic trees show that the CagA protein comprises a solid structured N-terminal region[31,34] and a variable, intrinsically disordered C-terminal region that is different among strains and exhibits scaffold/hub functions that are responsible for the morphogenetic activity of CagA[35]. The C-terminal domain contains repeated tandem five-amino-acid motifs of glutamic acid-proline-isoleucine-tyrosine-alanine (EPIYA). Within the variable region of CagA, there are different intervening sequences between the EPIYA motifs. One copy of EPIYA plus an intervening sequence is identified as an EPIYA segment. The tyrosine residues on the EPIYA (Y) motifs undergo tyrosine phosphorylation. Both the number and type of the EPIYA motifs determine the outcomes of cellular and gastric lesions[36,37]. Four unique types of EPIYA motifs (A, B, C and D) have been described based on their flanking amino acid sequences, which contribute to the CagA sequence polymorphism and geographical difference among strains. Almost all CagA contains both EPIYA-A and EPIYA-B motifs. The EPIYA-C motif is usually present in one to three repeats forming the typical Western CagA configuration of ABC, ABCC and ABCCC subtypes. In contrast, the EPIYA-D motif rarely repeats and thus prevalent East Asian CagA strains are ABD combinations[38]. The EPIYA-C and EPIYA-D motifs act as phosphorylation sites[39]. It is reported that increased number of EPIYA-C could enhance the binding ability to protein-tyrosine phosphatase-2 (SHP-2)[40]. Compared with EPIYA-C, EPIYA-D experiences a greater degree of tyrosine phosphorylation and stronger SHP-2-binding affinity, which leads to increased oncogenic potential[41,42]. Epidemiological data identified that the incidence rate of gastric MALT lymphoma is higher in East Asia than in Western countries[11,43-45]. East Asian might be prone to gastric MALT lymphoma at least partly, if not all, because most H. pylori strains are cagA-positive and nearly 90% of CagA carry EPIYA-D motif, 83.6% of which are of EPIYA-ABD genotype[46].
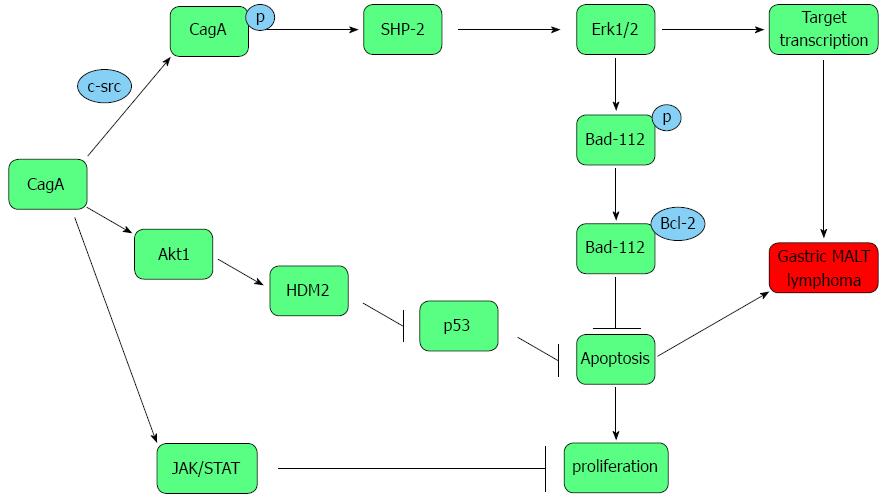
Tyrosine phosphorylation-dependent pathway: CagA was directly injected from bacteria into attached gastric epithelial cells by a T4SS. Lin et al[19] further showed that the translocation of the CagA protein into human B lymphocytes could occur through the T4SS. The delivered CagA activates and stimulates the B lymphocytes, initiating the first step of the B-cell malignant stimulation. In host cells, CagA undergoes tyrosine phosphorylation by c-src/Lyn kinase on specific tyrosine residues of the EPIYA motifs[19,47]. The phosphorylated CagA deregulates the intracellular signaling pathways and initiates the malignant transformation of B lymphocytes. CagA specifically binds to intracellular target molecules, including the SHP-2 [Src homology 2 (SH2) domain containing phosphotyrosine phosphatase 2][39,41,48,49]. SHP-2, encoded by PTPN11, is a protein tyrosine phosphatase (PTP) and plays a vital role in normal hematopoiesis. SHP2 has two tandem SH2 domains, a PTP domain and a carboxyl-terminal tail which contains multiple tyrosine phosphorylation sites and is rich in praline motifs. In the inactive state, the N-terminal SH2 domain binds the PTP domain and hampers access of potential substrates to the active site. Thus, SHP-2 is auto-inhibited. In contrast, the N-terminal SH2 domain is free from the PTP domain by binding to target phospho-tyrosyl residues, catalytically activating the enzyme by relieving this auto-inhibition[50]. Mutation in PTPN11, an identified cellular proto-oncogene[51], or aberrant SHP-2 expression/activity positively correlates with the hyperproliferation of leukemic hematopoiesis[50,52]. SHP-2 functions as a vital adaptor protein in CagA signaling pathway[48,49]. However, PTPN11/SHP-2 has dual roles in different cell types and its oncogenic role is tissue specific[53]. Most recent experimental data suggest PTPN11/SHP-2 as a tumor suppressor in hepatocarcinogenesis[54]. The above pathway depends on tyrosine phosphorylation of CagA.
Zhu et al[47] transiently transfected a recombinant retrovirus encoding an inserted cagA into conditionally immortalized B lymphocytes. The expressed and phosphorylated CagA was detected in the transfected B cells by Western blot and co-immunoprecipitation analyses, and CagA/SHP-2 complex was detected. The transfection of B lymphocytes with cagA significantly increased extracellular signal-regulated kinase1/2 (Erk1/2) phosphorylation, which is negatively regulated by MKP-1 and MKP-6, resulting in the phosphorylation of Bad at Serine 112 of CagA. Erk1/2, activated by CagA, can hamper apoptosis of B lymphocytes by inducing phosphorylation of Bad at Ser-112. cagA transfection did not alter the levels of the pro-apoptotic Bcl-2 and Bax. Immunofluorescence staining analysis displayed that CagA-activated Erk1/2 could translocate simultaneously to the cytoplasm and the nucleus, whereas serum-stimulated activated Erk1/2 was located only in the cytoplasm. The evidence indicates that the CagA-activated Erk1/2 can block apoptosis by activating the downstream target molecules, promoting the development of lymphoma. Lin et al[19] suggested that CagA translocation, following the phosphorylation of CagA which subsequently binds to and activates endogenous SHP-2, induces the activation of Erk1/2 and mitogen activated protein kinase and the up-regulation of the anti-apoptotic proteins Bcl-2 and Bcl-XL in human B lymphocytes. The step prevents human B lymphocytes from apoptosis, allowing the lymphocytes to acquire survival ability, which contributes to the pathogenesis of lymphoma.
Tyrosine phosphorylation-independent pathway: Umehara et al[55] determined that CagA may block the cell cycle progression in the Ba/F3 and gastric epithelial cancer AGS cells, and inhibit the B lymphocyte apoptosis by impairing the p53 and JAK/STAT pathway. The enforced expression of CagA in the interleukin (IL)-3-dependent B-lymphoid cells functions as a G1 inhibitor, suppressing cell proliferation through the inhibition of JAK-STAT pathway and resulting in significant retardation of the G1- to S-phase cell-cycle transition. The IL-3 signal is mainly transmitted by the sequential activations of JAK and STAT. CagA offsets hydroxyurea-induced B-cell apoptosis by disturbing the tumor suppressor p53 accumulation. CagA inhibits the expression of p53 at the level of transcription. Meanwhile, cagA-positive H. pylori may be involved in the initial stage of gastric MALT lymphoma development, whereas it might not be necessary in the maintenance stage of lymphoma cell proliferation. CagA blocks apoptosis, promoting the accumulation of genetically abnormal cells that should otherwise be removed from the tissue. In IL-3-dependent B cells including BaF3, inhibitors of deoxyribonucleotide synthesis such as hydroxyurea induce apoptosis in a p53-dependent manner, whereas, DNA-damaging agents such as X-irradiation and cisplatin induce cell death in a p53-independent manner[56]. Interestingly, oxidative stress has been reported to be contributed to a variety of gastric disorders such as gastritis and ulcer diseases, especially gastric cancer[57,58]. Upon H. pylori infection and colonization, CagA might stimulate the response of gastric epithelial cells to oxidative stress and produce, mainly from neutrophils, reactive oxygen species (ROS) and/or reactive nitrogen species (RNS). Excessive ROS/RNS causes dysfunction of antioxidant defense mechanism in gastric mucosal, leading to DNA damage, accelerating cell death including apoptosis and subsequent cell proliferation, and resulting in the pathogenesis of gastric disorders as well as carcinogenesis[58]. Meanwhile, additional ROS and RNS may decline the expression of Runt domain transcription factor 3 (RUNX3), a marker of oxidative stress, which could restore after H. pylori eradication[58]. Therefore, RUNX3 acts as a tumor suppressor and is involved in H. pylori CagA-dependent gastric carcinogenesis. Moreover, some other molecules have been reported to be correlated with CagA-induced gastric carcinogenesis. Murine double minute 2 (MDM2) might promote pathogenesis of gastric cancer through inactivating the apoptotic and cell cycle arrest function of p53[59]. Yet, the role of RUNX3, MDM2 as well as oxidative stress production in CagA-induced gastric MALT lymphoma has been unclear and should be elucidated by further exploration. The malignant transformation from cagA+H. pylori infection into gastric MALT lymphoma should involve multiple steps. CagA has phosphorylation-dependent and -independent activities, and the biological effects of CagA in mammals depend on the cellular context. An imbalance between apoptosis and proliferation is involved in the pathogenesis and development of H. pylori-dependent gastric MALT lymphoma[60]. CagA inhibits apoptosis and impairs survival in the B cells, resulting in the transformation of MALT lymphoma[20] (Figure 2).
Mucosal inflammation is the basic mechanism underlying the occurrence and development of gastric MALT lymphoma. Infection with H. pylori induces inflammatory and immune responses in the gastric mucosa. The incapability of the host immune response to clear the bacterial pathogen results in a persistent infection and the subsequent development of chronic gastric inflammation[61]. The T-helper 17 (Th17) cells, whose hallmark cytokine is IL-17A, are important for the clearance of extracellular bacteria[62], and they play a role in infection control and precarcinogenesis. IL-17A may contribute to inflammation-associated carcinogenesis[62,63]. B7-H2 is among the newer members of the B7 family and is known to have a co-stimulatory function on T cell activity[64]. Recent in vitro and in vivo studies showed that H. pylori down-regulates B7-H2 (the positive co-stimulators required for an efficient effector T cell response) in a CagA-dependent manner in gastric epithelial cells (GECs). CagA-dependent B7-H2 down-regulation in GECs suppresses the Th17-mediated immune response, contributing to outcomes of chronic gastric inflammation and persistent H. pylori colonization[65]. This process may be involved in gastric carcinogenesis, but the relationship with the development of gastric MALT lymphoma remains unclear. The activation of nuclear factor kappa-light-chain-enhancer of activated B cells and the up-regulation of IL-8 induced by H. pylori infections in B lymphocytes lead to the malignant transformation of B cells in a SHP-2-dependent and CagA-independent mechanism[66-68]. There is no direct evidence associating CagA, inflammation and gastric MALT lymphoma.
In recent years, microRNAs (miRNAs), a class of small non-coding RNAs that can modulate gene expression at the post-transcriptional level, have been implicated in H. pylori-dependent gastric carcinogenesis[69,70]. Much data suggest that miRNAs are important in fundamental cellular processes such as proliferation and apoptosis, and miRNAs can function as tumor promoters or suppressors[71]; the role of the miRNAs in the association between CagA and gastric MALT lymphoma remains unclear. Gastric MALT lymphoma is considered one of the best models of how infectious pathogens and genetic events lead to oncogenesis[72,73]. CagA functions as a typical bacterium-derived oncoprotein in gastric MALT lymphoma pathogenesis, but the molecular mechanism of CagA underlying the development of gastric MALT lymphoma should be further elucidated.
P- Reviewers: Handa O, Sugimoto M S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Ma S
| 1. | Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 398] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3187] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 3. | Rieder G, Hofmann JA, Hatz RA, Stolte M, Enders GA. Up-regulation of inducible nitric oxide synthase in Helicobacter pylori-associated gastritis may represent an increased risk factor to develop gastric carcinoma of the intestinal type. Int J Med Microbiol. 2003;293:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Zucca E, Bertoni F, Roggero E, Bosshard G, Cazzaniga G, Pedrinis E, Biondi A, Cavalli F. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid-tissue lymphoma of the stomach. N Engl J Med. 1998;338:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 157] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Ferrucci PF, Zucca E. Primary gastric lymphoma pathogenesis and treatment: what has changed over the past 10 years? Br J Haematol. 2007;136:521-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Nakamura S, Sugiyama T, Matsumoto T, Iijima K, Ono S, Tajika M, Tari A, Kitadai Y, Matsumoto H, Nagaya T. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 7. | Ferreri AJ, Govi S, Ponzoni M. The role of Helicobacter pylori eradication in the treatment of diffuse large B-cell and marginal zone lymphomas of the stomach. Curr Opin Oncol. 2013;25:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, Tomao S, Stolte M, Morini S, Vaira D. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, Wündisch T, Neubauer A, Raderer M, Savio A. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut. 2007;56:1685-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Wündisch T, Dieckhoff P, Greene B, Thiede C, Wilhelm C, Stolte M, Neubauer A. Second cancers and residual disease in patients treated for gastric mucosa-associated lymphoid tissue lymphoma by Helicobacter pylori eradication and followed for 10 years. Gastroenterology. 2012;143:936-942; quiz e13-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Luminari S, Cesaretti M, Marcheselli L, Rashid I, Madrigali S, Maiorana A, Federico M. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol. 2010;21:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis. Helicobacter. 2010;15:163-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Hatakeyama M. SagA of CagA in Helicobacter pylori pathogenesis. Curr Opin Microbiol. 2008;11:30-37. [PubMed] |
| 14. | Fischbach W, Jung T, Goebeler-Kolve M, Eck M. Comparative analysis of the Helicobacter pylori status in patients with gastric MALT-type lymphoma and their respective spouses. Z Gastroenterol. 2000;38:627-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Eck M, Schmausser B, Haas R, Greiner A, Czub S, Müller-Hermelink HK. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology. 1997;112:1482-1486. [PubMed] |
| 16. | Schmausser B, Eck M, Greiner A, Kraus M, Müller-Hermelink HK. Mucosal humoral immune response to CagA shows a high prevalence in patients with gastric MALT-type lymphoma. Virchows Arch. 2000;436:115-118. [PubMed] |
| 17. | Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1345] [Article Influence: 58.5] [Reference Citation Analysis (1)] |
| 18. | Sumida T, Kitadai Y, Hiyama T, Shinagawa K, Tanaka M, Kodama M, Masuda H, Ito M, Tanaka S, Yoshihara M. Antibodies to Helicobacter pylori and CagA protein are associated with the response to antibacterial therapy in patients with H. pylori-positive API2-MALT1-negative gastric MALT lymphoma. Cancer Sci. 2009;100:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Lin WC, Tsai HF, Kuo SH, Wu MS, Lin CW, Hsu PI, Cheng AL, Hsu PN. Translocation of Helicobacter pylori CagA into Human B lymphocytes, the origin of mucosa-associated lymphoid tissue lymphoma. Cancer Res. 2010;70:5740-5748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Kuo SH, Chen LT, Lin CW, Wu MS, Hsu PN, Tsai HJ, Chu CY, Tzeng YS, Wang HP, Yeh KH. Detection of the Helicobacter pylori CagA protein in gastric mucosa-associated lymphoid tissue lymphoma cells: clinical and biological significance. Blood Cancer J. 2013;3:e125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Ohnishi N, Yuasa H, Tanaka S, Sawa H, Miura M, Matsui A, Higashi H, Musashi M, Iwabuchi K, Suzuki M. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA. 2008;105:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 465] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 22. | Zucca E, Bertoni F, Roggero E, Cavalli F. The gastric marginal zone B-cell lymphoma of MALT type. Blood. 2000;96:410-419. [PubMed] |
| 23. | Nishikawa K, Nakamura M, Takahashi S, Matsui H, Murayama SY, Matsumoto T, Yamada H, Tsuchimoto K. Increased apoptosis and angiogenesis in gastric low-grade mucosa-associated lymphoid tissue-type lymphoma by Helicobacter heilmannii infection in C57/BL6 mice. FEMS Immunol Med Microbiol. 2007;50:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Yoon SS, Hochberg EP. Chemotherapy is an effective first line treatment for early stage gastric mucosa-associated lymphoid tissue lymphoma. Cancer Treat Rev. 2006;32:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Wu YY, Tsai HF, Lin WC, Hsu PI, Shun CT, Wu MS, Hsu PN. Upregulation of CCL20 and recruitment of CCR6+ gastric infiltrating lymphocytes in Helicobacter pylori gastritis. Infect Immun. 2007;75:4357-4363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Isaacson PG, Spencer J. Gastric lymphoma and Helicobacter pylori. Important Adv Oncol. 1996;111-121. [PubMed] |
| 27. | Kim JS, Chung SJ, Choi YS, Cheon JH, Kim CW, Kim SG, Jung HC, Song IS. Helicobacter pylori eradication for low-grade gastric mucosa-associated lymphoid tissue lymphoma is more successful in inducing remission in distal compared to proximal disease. Br J Cancer. 2007;96:1324-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2013;Aug 24; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 573] [Article Influence: 52.1] [Reference Citation Analysis (1)] |
| 29. | Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. [PubMed] |
| 30. | Noto JM, Peek RM. The Helicobacter pylori cag Pathogenicity Island. Methods Mol Biol. 2012;921:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Backert S, Tegtmeyer N. Helicobacter pylori CagA tertiary structure reveals functional insights. Cell Host Microbe. 2012;12:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648-14653. [PubMed] |
| 33. | Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, Reece CA, Bukanov NO, Drazek ES, Roe BA, Berg DE. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37-53. [PubMed] |
| 34. | Hayashi T, Senda M, Morohashi H, Higashi H, Horio M, Kashiba Y, Nagase L, Sasaya D, Shimizu T, Venugopalan N. Tertiary structure-function analysis reveals the pathogenic signaling potentiation mechanism of Helicobacter pylori oncogenic effector CagA. Cell Host Microbe. 2012;12:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Nesić D, Miller MC, Quinkert ZT, Stein M, Chait BT, Stebbins CE. Helicobacter pylori CagA inhibits PAR1-MARK family kinases by mimicking host substrates. Nat Struct Mol Biol. 2010;17:130-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 36. | Zhang C, Xu S, Xu D. Risk assessment of gastric cancer caused by Helicobacter pylori using CagA sequence markers. PLoS One. 2012;7:e36844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Ferreira RM, Machado JC, Leite M, Carneiro F, Figueiredo C. The number of Helicobacter pylori CagA EPIYA C tyrosine phosphorylation motifs influences the pattern of gastritis and the development of gastric carcinoma. Histopathology. 2012;60:992-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Hatakeyama M. Anthropological and clinical implications for the structural diversity of the Helicobacter pylori CagA oncoprotein. Cancer Sci. 2011;102:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Higashi H, Tsutsumi R, Fujita A, Yamazaki S, Asaka M, Azuma T, Hatakeyama M. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci USA. 2002;99:14428-14433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 456] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 40. | Figueiredo C, Van Doorn LJ, Nogueira C, Soares JM, Pinho C, Figueira P, Quint WG, Carneiro F. Helicobacter pylori genotypes are associated with clinical outcome in Portuguese patients and show a high prevalence of infections with multiple strains. Scand J Gastroenterol. 2001;36:128-135. [PubMed] |
| 41. | Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 587] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 42. | Miura M, Ohnishi N, Tanaka S, Yanagiya K, Hatakeyama M. Differential oncogenic potential of geographically distinct Helicobacter pylori CagA isoforms in mice. Int J Cancer. 2009;125:2497-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol. 2012;47:92-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Capelle LG, de Vries AC, Looman CW, Casparie MK, Boot H, Meijer GA, Kuipers EJ. Gastric MALT lymphoma: epidemiology and high adenocarcinoma risk in a nation-wide study. Eur J Cancer. 2008;44:2470-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Stolte M, Bayerdörffer E, Morgner A, Alpen B, Wündisch T, Thiede C, Neubauer A. Helicobacter and gastric MALT lymphoma. Gut. 2002;50 Suppl 3:III19-III24. [PubMed] |
| 46. | Chen CY, Wang FY, Wan HJ, Jin XX, Wei J, Wang ZK, Liu C, Lu H, Shi H, Li DH. Amino acid polymorphisms flanking the EPIYA-A motif of Helicobacter pylori CagA C-terminal region is associated with gastric cancer in east China: experience from a single center. J Dig Dis. 2013;14:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Zhu Y, Wang C, Huang J, Ge Z, Dong Q, Zhong X, Su Y, Zheng S. The Helicobacter pylori virulence factor CagA promotes Erk1/2-mediated Bad phosphorylation in lymphocytes: a mechanism of CagA-inhibited lymphocyte apoptosis. Cell Microbiol. 2007;9:952-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 783] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 49. | Tsutsumi R, Higashi H, Higuchi M, Okada M, Hatakeyama M. Attenuation of Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J Biol Chem. 2003;278:3664-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 50. | Mohi MG, Neel BG. The role of Shp2 (PTPN11) in cancer. Curr Opin Genet Dev. 2007;17:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 51. | Chan RJ, Feng GS. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007;109:862-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 292] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 52. | Xu R, Yu Y, Zheng S, Zhao X, Dong Q, He Z, Liang Y, Lu Q, Fang Y, Gan X. Overexpression of Shp2 tyrosine phosphatase is implicated in leukemogenesis in adult human leukemia. Blood. 2005;106:3142-3149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Li S, Hsu DD, Wang H, Feng GS. Dual faces of SH2-containing protein-tyrosine phosphatase Shp2/PTPN11 in tumorigenesis. Front Med. 2012;6:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Jiang C, Hu F, Tai Y, Du J, Mao B, Yuan Z, Wang Y, Wei L. The tumor suppressor role of Src homology phosphotyrosine phosphatase 2 in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2012;138:637-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Umehara S, Higashi H, Ohnishi N, Asaka M, Hatakeyama M. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene. 2003;22:8337-8342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 56. | Palacios C, Gutierrez del Arroyo A, Silva A, Collins MK. The role of p53 in death of IL-3-dependent cells in response to cytotoxic drugs. Oncogene. 2000;19:3556-3559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Handa O, Naito Y, Yoshikawa T. Redox biology and gastric carcinogenesis: the role of Helicobacter pylori. Redox Rep. 2011;16:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr. 2012;50:35-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 59. | Nakajima N, Ito Y, Yokoyama K, Uno A, Kinukawa N, Nemoto N, Moriyama M. The Expression of Murine Double Minute 2 (MDM2) on Helicobacter pylori-Infected Intestinal Metaplasia and Gastric Cancer. J Clin Biochem Nutr. 2009;44:196-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Sibony M, Jones NL. Recent advances in Helicobacter pylori pathogenesis. Curr Opin Gastroenterol. 2012;28:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Müller A, Oertli M, Arnold IC. H. pylori exploits and manipulates innate and adaptive immune cell signaling pathways to establish persistent infection. Cell Commun Signal. 2011;9:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 62. | Kabir S. The role of interleukin-17 in the Helicobacter pylori induced infection and immunity. Helicobacter. 2011;16:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 63. | Czinn SJ, Blanchard T. Vaccinating against Helicobacter pylori infection. Nat Rev Gastroenterol Hepatol. 2011;8:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Aicher A, Hayden-Ledbetter M, Brady WA, Pezzutto A, Richter G, Magaletti D, Buckwalter S, Ledbetter JA, Clark EA. Characterization of human inducible costimulator ligand expression and function. J Immunol. 2000;164:4689-4696. [PubMed] |
| 65. | Lina TT, Pinchuk IV, House J, Yamaoka Y, Graham DY, Beswick EJ, Reyes VE. CagA-dependent downregulation of B7-H2 expression on gastric mucosa and inhibition of Th17 responses during Helicobacter pylori infection. J Immunol. 2013;191:3838-3846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Brandt S, Kwok T, Hartig R, König W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci USA. 2005;102:9300-9305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 420] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 67. | Ohmae T, Hirata Y, Maeda S, Shibata W, Yanai A, Ogura K, Yoshida H, Kawabe T, Omata M. Helicobacter pylori activates NF-kappaB via the alternative pathway in B lymphocytes. J Immunol. 2005;175:7162-7169. [PubMed] |
| 68. | Sun SC. Non-canonical NF-κB signaling pathway. Cell Res. 2011;21:71-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 920] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 69. | Feng Y, Wang L, Zeng J, Shen L, Liang X, Yu H, Liu S, Liu Z, Sun Y, Li W. FoxM1 is overexpressed in Helicobacter pylori-induced gastric carcinogenesis and is negatively regulated by miR-370. Mol Cancer Res. 2013;11:834-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 70. | Thorns C, Kuba J, Bernard V, Senft A, Szymczak S, Feller AC, Bernd HW. Deregulation of a distinct set of microRNAs is associated with transformation of gastritis into MALT lymphoma. Virchows Arch. 2012;460:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 794] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 72. | Sagaert X, Van Cutsem E, De Hertogh G, Geboes K, Tousseyn T. Gastric MALT lymphoma: a model of chronic inflammation-induced tumor development. Nat Rev Gastroenterol Hepatol. 2010;7:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | Witkowska M, Smolewski P. Helicobacter pylori infection, chronic inflammation, and genomic transformations in gastric MALT lymphoma. Mediators Inflamm. 2013;2013:523170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |