Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.8056
Revised: July 17, 2013
Accepted: July 23, 2013
Published online: November 28, 2013
Processing time: 291 Days and 17.8 Hours
AIM: To evaluate the potential use of colonoscopy and endoluminal ultrasonic biomicroscopy (eUBM) to track the progression of mouse colonic lesions.
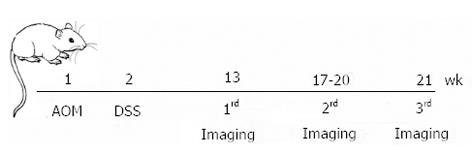
METHODS: Ten mice were treated with a single azoxymethane intraperitoneal injection (week 1) followed by seven days of a dextran sulfate sodium treatment in their drinking water (week 2) to induce inflammation-associated colon tumors. eUBM was performed simultaneously with colonoscopy at weeks 13, 17-20 and 21. A 3.6-F diameter 40 MHz mini-probe catheter was used for eUBM imaging. The ultrasound mini-probe catheter was inserted into the accessory channel of a pediatric flexible bronchofiberscope, allowing simultaneous acquisition of colonoscopic and eUBM images. During image acquisition, the mice were anesthetized with isoflurane and kept in a supine position over a stainless steel heated surgical waterbed at 37 °C. Both eUBM and colonoscopic images were captured and stored when a lesion was detected by colonoscopy or when the eUBM image revealed a modified colon wall anatomy. During the procedure, the colon was irrigated with water that was injected through a flush port on the mini-probe catheter and that acted as the ultrasound coupling medium between the transducer and the colon wall. Once the acquisition of the last eUBM/colonoscopy section for each animal was completed, the colons were fixed, paraffin-embedded, and stained with hematoxylin and eosin. Colon images acquired at the first time-point for each mouse were compared with subsequent eUBM/colonoscopic images of the same sites obtained in the following acquisitions to evaluate lesion progression.
RESULTS: All 10 mice had eUBM and colonoscopic images acquired at week 13 (the first time-point). Two animals died immediately after the first imaging acquisition and, consequently, only 8 mice were subjected to the second eUBM/colonoscopy imaging acquisition (at the second time-point). Due to the advanced stage of colonic tumorigenesis, 5 animals died after the second time-point image acquisition, and thus, only three were subjected to the third eUBM/colonoscopy imaging acquisition (the third time-point). eUBM was able to detect the four layers in healthy segments of colon: the mucosa (the first hyperechoic layer moving away from the mini-probe axis), followed by the muscularis mucosae (hypoechoic), the submucosa (the second hyperechoic layer) and the muscularis externa (the second hypoechoic layer). Hypoechoic regions between the mucosa and the muscularis externa layers represented lymphoid infiltrates, as confirmed by the corresponding histological images. Pedunculated tumors were represented by hyperechoic masses in the mucosa layer. Among the lesions that decreased in size between the first and third time-points, one of the lesions changed from a mucosal hyperplasia with ulceration at the top to a mucosal hyperplasia with lymphoid infiltrate and, finally, to small signs of mucosal hyperplasia and lymphoid infiltrate. In this case, while lesion regression and modification were observable in the eUBM images, colonoscopy was only able to detect the lesion at the first and second time-points, without the capacity to demonstrate the presence of lymphoid infiltrate. Regarding the lesions that increased in size, one of them started as a small elevation in the mucosa layer and progressed to a pedunculated tumor. In this case, while eUBM imaging revealed the lesion at the first time-point, colonoscopy was only able to detect it at the second time-point. All colonic lesions (tumors, lymphoid infiltrate and mucosal thickening) were identified by eUBM, while colonoscopy identified just 76% of them. Colonoscopy identified all of the colonic tumors but failed to diagnose lymphoid infiltrates and increased mucosal thickness and failed to differentiate lymphoid infiltrates from small adenomas. During the observation period, most of the lesions (approximately 67%) increased in size, approximately 14% remained unchanged, and 19% regressed.
CONCLUSION: Combining eUBM with colonoscopy improves the diagnosis and the follow-up of mouse colonic lesions, adding transmural assessment of the bowel wall.
Core tip: This paper employed imaging methods, endoluminal ultrasonic biomicroscopy (eUBM) associated to colonoscopy, in a longitudinal study to evaluate the progression of chemically-induced colonic lesions in mice, during a period of two months. The eUBM method complemented colonoscopy and enhanced the study, once the ultrasonic images allowed the detection of lesions underneath the epithelium. Potential future application of eUBM combined with colonoscopy could be in the monitoring of therapeutic efficacy of chemotherapeutic drugs in vivo.
- Citation: Soletti RC, Alves KZ, Britto MA, Matos DG, Soldan M, Borges HL, Machado JC. Simultaneous follow-up of mouse colon lesions by colonoscopy and endoluminal ultrasound biomicroscopy. World J Gastroenterol 2013; 19(44): 8056-8064
- URL: https://www.wjgnet.com/1007-9327/full/v19/i44/8056.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i44.8056
Colorectal cancer (CRC) has a high incidence in the world as it is the third most common cancer in men and women in developed countries[1]. It is estimated that more than 142000 people in the United States will be diagnosed with CRC in 2013[2]. In Europe, CRC is detected in approximately 413000 people each year, half of whom die during the course of the disease. Despite its high incidence and mortality rates, the majority of CRC-related deaths could be prevented through the implementation of powerful tools for CRC early detection and staging.
Currently, colonoscopy is the recommended screening method for CRC screening and follow-up, but it has some limitations. Studies have demonstrated that the detection of adenomas, serrated polyps and sessile serrated adenomas differs significantly among endoscopists[3]. Furthermore, colorectal neoplasms of a diminutive size (smaller than 10 mm) or nonpolypoid shape may be more easily overlooked during a routine colonoscopy[4-6]. The miss rate for CRC lesions may explain the high proportion (3.3%-12.4%) of proximal CRC that is diagnosed shortly after a clearing colonoscopy[7,8]. Therefore, the efforts of some research groups are focused on the development of other imaging methods that complement the results of a colonoscopy.
High frequency endoscopic ultrasonography (EUS) is a relatively new technique in which an ultrasonography probe is inserted into the accessory channel of a regular endoscope. EUS has the capacity to look deep below the lining of the colon and is a useful modality for transmural assessment of the bowel wall[9,10]. Usually, the ultrasound transducers used in EUS instrumentations operate at low frequencies (7.5-12 MHz), but higher ultrasound frequencies are also employed by using a mini-probe[11-13]. Higher ultrasound frequencies increase EUS resolution, allowing for the staging of colon tumors and the visualization of small colonic lesions. The use of high frequency mini-probe ultrasound for the diagnosis of mucosal and submucosal colorectal lesions and for the guidance of lesion resection has already been proposed as a safe and effective technique[13,14]. However, the role for high frequency mini-probe ultrasound in the routine diagnosis of colonic lesions has not been fully established[15-18].
Animal models of diseases can be used to develop and evaluate new diagnostic tools before they are applied clinically. The development of non-invasive experimental imaging modalities allows for the study of the same animal over time, enabling the investigation of disease development and therapeutic interventions. Mouse models of chemically induced CRC are highly reproducible, can be tested on animals with different genetic backgrounds and recapitulate human CRC. The use of an effective and valuable mouse model of chemically induced CRC can help investigators understand colonic tumorigenesis and to probe novel diagnostic platforms for use in clinical practice[19].
Our group has previously used ultrasonic biomicroscopic (UBM) instrumentation, operating at 45 MHz, for in vitro imaging of chemically induced mouse CRC[20] to demonstrate that UBM is a feasible tool to identify the layers of mouse colon with adequate contrast between them and with sufficient resolution. Afterwards, endoluminal UBM (eUBM), operating at 40 MHz, was performed along with a colonoscopy, and simultaneous eUBM and colonoscopic images were generated in vivo[21].
Recently, studies have verified the efficacy of UBM as a tool for longitudinal studies in mice: Harmon and co-workers[22] validated the use of 40 MHz extracorporeal UBM for carotid plaque development in mice; Tiwari et al[23] used a 40 MHz UBM for longitudinal monitoring of infliximab treatment efficacy in a mouse model of pancreatic cancer; Fernández-Domínguez et al[24] also used a 40 MHz UBM in a longitudinal study to evaluate the progression of fatty liver disease in mice; and Campos-Junior et al[25] analyzed the efficacy of UBM in the evaluation of induced ovarian follicular growth and ovulation in mice. Despite growing evidence confirming the efficacy of high frequency ultrasound in the monitoring of lesion progression, the ability of eUBM to diagnose colonic tumoral development in animal models has not yet been studied.
The present work comprises the use of eUBM instrumentation associated with colonoscopy in a longitudinal study to evaluate the progression of chemically induced colonic lesions in mice.
Ten mice [Mus musculus (Linnaeus, 1758)] of both genders, with an average age of 7 wk, an average weight of 25 g, and p53+/+ and p53+/- (heterozygous for tumor suppressor gene Trp53), were used. The mice were originally purchased from The Jackson Laboratory (Bar Harbor, ME, United States) and kept in the 129/SvJ background. We used the p53+/- mice because Trp53 mutations accelerate tumorigenesis in several tissues, including the colon[26].
The animals were maintained at room temperature with the appropriate circadian cycle and diet. The Guide for Care and Use of Laboratory Animals (National Institutes of Health) was also considered.
Colon tumors were induced using a protocol (DAHEICB 042) approved by the Animal Care and Use Committee of the Biological Science Institute/Federal University of Rio de Janeiro. The studies involving colon imaging, such as eUBM combined with colonoscopy, were conducted under a protocol (71/08) approved by the Ethical Committee for Laboratory Animal Research/Federal University of Rio de Janeiro.
Inflammation-related colon tumors were induced using azoxymethane (AOM) and dextran sulfate sodium (DSS)[27-29]. AOM is a colon-specific carcinogen that can be combined with DSS, a mucosal-irritant agent, to mimic inflammation-associated colon carcinogenesis[29,30]. The animals were subjected to a single intraperitoneal (ip) injection of AOM (A5486; Sigma Aldrich, St. Louis, MO, United States) with a concentration of 12.5 mg/kg. One week after AOM administration, the mice were fed with water containing 3% DSS salt, 36000-50000 Da (02160110; MP Biomedicals, Santa Ana, CA, United States), for 1 wk. All of the mice received solid food and water ad libitum, with regular water given after the week of DSS intake.
Briefly, images were generated by employing a 3.6-F diameter 40 MHz mini-probe catheter (Atlantis® SR Pro Coronary Imaging Catheter; Boston Scientific Corporation, Natick, MA, United States) mechanically driven by a motordrive unit (MD5; Boston Scientific Corporation, Natick, MA, United States). The ultrasonic transducer rotates 360° around its axis, providing cross-sectional ultrasound images of the colon wall. More details concerning the eUBM instrumentation are described in Alves et al[21].
Colonoscopy was used simultaneously with eUBM and served to guide the mini-probe through the colon. The ultrasound mini-probe catheter was inserted into the accessory channel of a pediatric flexible bronchofiberscope (FB120P; Fujinon, Tokyo, Japan), allowing simultaneous acquisition of colonoscopy and eUBM images. The bronchofiberscope has a total length of 920 mm and outer diameters of 2.8 and 2.7 mm for the flexible and distal-end portions, respectively.
To ensure that the colonoscopy and eUBM techniques acquired simultaneous images from the same region, the ultrasonic transducer, at the mini-probe imaging core tip, was positioned outside of the distal end accessory channel extremity, while still as close as possible to the bronchofiberscope extremity. The mini-probe telescoping shaft section was used to advance and retract the imaging core, placing it in the correct position.
During image acquisition, the mice were anesthetized with isoflurane (Cristália; São Paulo, Brazil) at 1.5% in 1.5 L/min oxygen, using a laboratory animal anesthesia system (EZ-7000; Euthanex, Palmer, PA, United States). The animals were kept in a supine position over a stainless steel heated surgical waterbed at 37 °C using the T/Pump System (Gaymar, Orchard Park, NY, United States). Before the examination, an enema was performed with 1 mL of water to remove feces. Subsequently, the flexible bronchofiberscope containing the ultrasound mini-probe catheter was introduced into the descending colon. Both eUBM and colonoscopy images were captured simultaneously and stored when a lesion was detected by colonoscopy or when the eUBM image revealed a modified colon wall anatomy. During the procedure, the colon was irrigated with water that was injected through a flush port of the mini-probe catheter and that acted as the ultrasound coupling medium between the transducer and the colon wall.
The sequential evaluation of colonic lesions by simultaneous in vivo eUBM and colonoscopic imaging started at 13 wk after AOM administration and was performed at three different time-points, according to Figure 1: the first one at week 13, the second one between weeks 17 and 20 and the last one at week 21.
Colon lesion images acquired at the first time-point for each mouse were compared with subsequent eUBM/colonoscopic images of the same sites obtained in the following acquisitions. After the last eUBM examination, the images of each lesion were separated for subsequent comparison with histopathology.
Once the acquisition of the last image for each animal was completed, each anesthetized mouse was euthanized by cervical dislocation. The distal colon was excised, cleaned and fixed in 4% formaldehyde for 16 h before paraffin embedding. The paraffin-embedded tissues were cross-sectioned (5 μm) stepwise transversally to the colon longitudinal axis and stained with hematoxylin and eosin. All stained sections were analyzed by light microscopy and compared with the ultrasonic images, whose frames were obtained from the same lesions observed with the eUBM and/or colonoscopy.
The time-points for image acquisition of each animal are presented in Table 1. All 10 mice had eUBM and colonoscopic images acquired at week 13. Two animals died immediately after the first imaging acquisition and, consequently, only eight mice were subjected to the second eUBM/colonoscopy imaging acquisition. Due to the advanced stage of colonic tumorigenesis, five animals died after the second time-point image acquisition, and thus, only three were subjected to the third eUBM/colonoscopy imaging acquisition.
| Weeks after AOM administration | |||
| Mouse number | 1st eUBM | 2nd eUBM | 3rd eUBM |
| 1 | 13 | - | - |
| 2 | 13 | - | - |
| 3 | 13 | 18 | - |
| 4 | 13 | 20 | - |
| 5 | 13 | 20 | - |
| 6 | 13 | 20 | - |
| 7 | 13 | 20 | - |
| 8 | 13 | 17 | 21 |
| 9 | 13 | 17 | 21 |
| 10 | 13 | 17 | 21 |
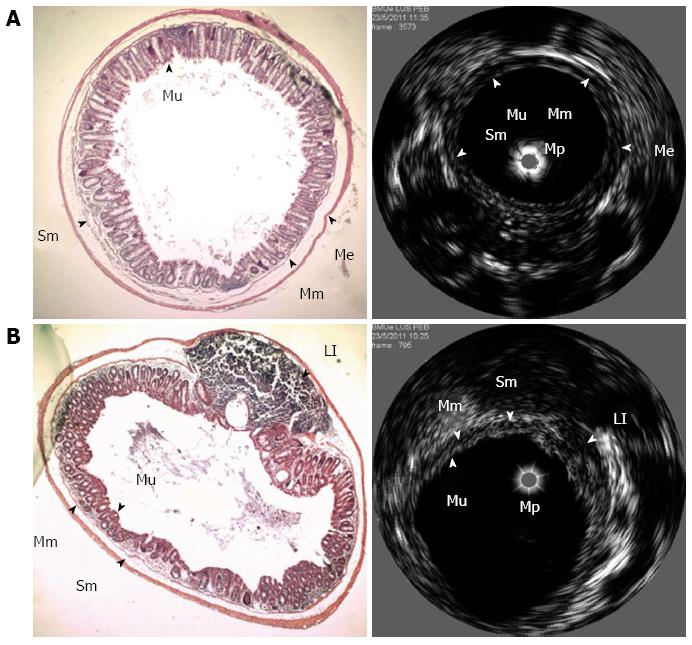
An example of interrelated eUBM and histological images of a healthy section from the mouse colon is presented in Figure 2A. The mucosal layer is seen as a hyperechoic circular layer (the first hyperechoic layer moving away from the mini-probe axis), followed by a hypoechoic layer representing the muscularis mucosae. The submucosa corresponds to a hyperechoic layer, followed by the muscularis externa, the second hypoechoic layer. At the center of the lumen is the ultrasound mini-probe, represented by a gray circle. An eUBM image of a colonic lymphoid infiltrate, represented by a hypoechoic region between the mucosa and muscularis externa layers, is presented in Figure 2B with the corresponding histological image.
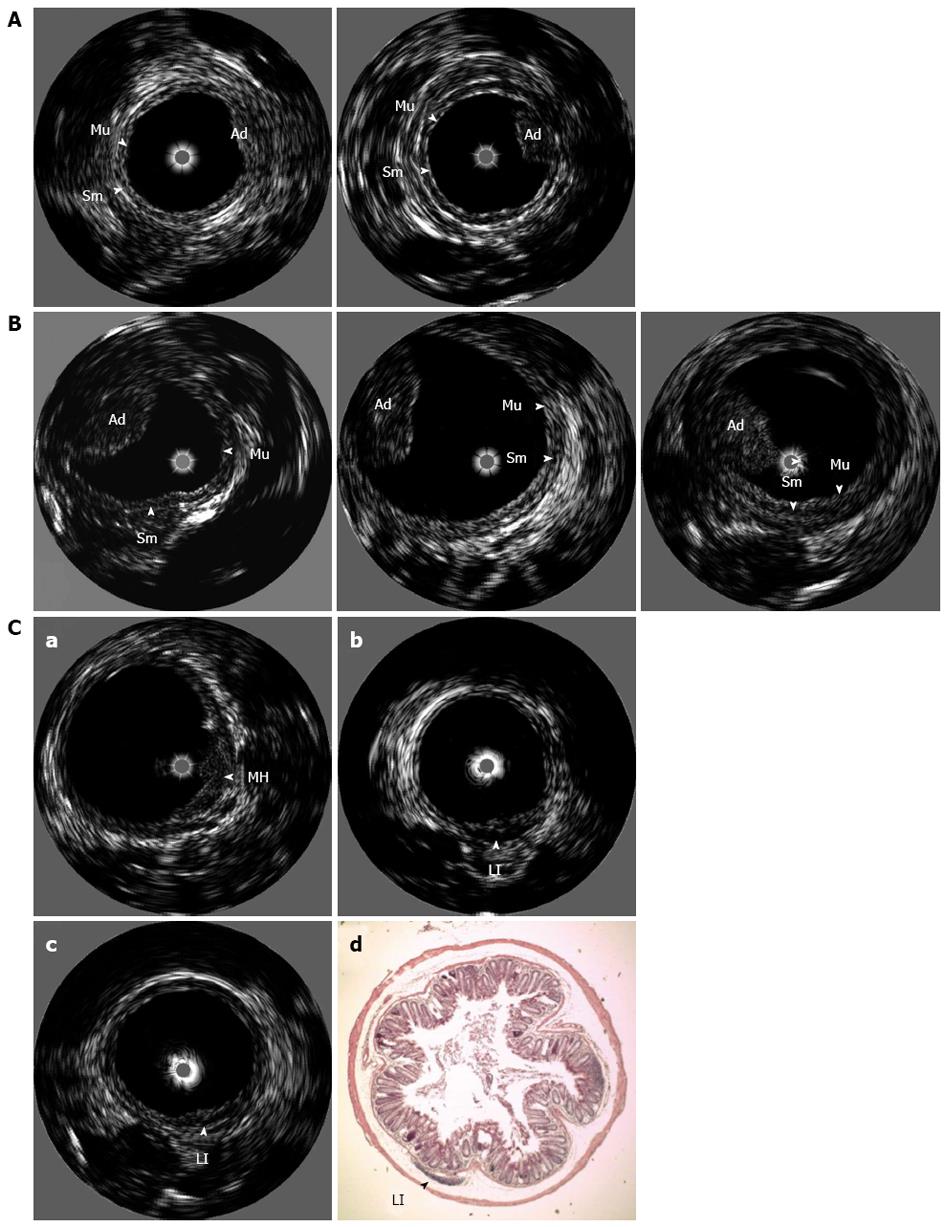
An example of an eUBM image of a pedunculated tumor, whose size increased during the 6 wk between the first and second time-points, is presented in Figure 3A. At the first eUBM exam, a small elevation in the mucosa layer is seen, indicating an early adenoma. At this time, colonoscopy was unable to visualize the lesion. Six weeks later, eUBM showed that the adenoma had increased in size, and the lesion was then observed in the colonoscopic image. Figure 3B presents an eUBM image of a pedunculated adenoma, whose size remained virtually unchanged between the first and third image acquisitions. The adenoma was also visualized in all of the colonoscopy sections. Finally, a sequence of three eUBM images of a lesion that decreased in size during the observation period is depicted in Figure 3C. This lesion was identified at the first eUBM exam (Figure 3C-a) as a mucosal hyperplasia with ulceration at the top. The ulceration was also visualized by colonoscopy. Four weeks later, at the second eUBM exam (Figure 3C-b), the mucosal hyperplasia had decreased, and a hypoechoic area underneath the mucosa was observed, indicating the emergence of an inflammatory infiltrate. At this point, colonoscopy showed no alterations in this colonic section. At the last eUBM exam (Figure 3C-c), both the mucosal hyperplasia and lymphoid infiltrate had almost completely disappeared. Histological analysis of the same section confirmed the presence of the remaining diminutive lymphoid infiltrate section (Figure 3C-d).
Colonic lesions detected by either the last eUBM or colonoscopy, and confirmed by post mortem histology, are indicated in Table 2. Altogether, eUBM identified all of the lesions (tumors, lymphoid infiltrate and mucosal thickening), while colonoscopy identified just 76% of them. Colonoscopy identified all colonic tumors but failed to diagnose lymphoid infiltrates and increased mucosal thickness and failed to differentiate lymphoid infiltrates from small adenomas.
| Animals | Animal lesion | Lesion detection | Lesion progression | ||||||||||
| eUBM | Colonoscopy | Size | Lesion type | ||||||||||
| N° | Yes | No | Yes | No | Obs | ↑ | ↓ | = | Tu | LI | MT | ||
| 1 | L1-1 | 1 | √ | √ | √ | √ | √ | ||||||
| 2 | L1-2 | 1 | √ | √ | √ | √ | √ | ||||||
| 3 | L1-3 | 2 | √ | √ | √ | √ | |||||||
| L2-3 | √ | √ | √ | √ | |||||||||
| 4 | L1-4 | 2 | √ | √ | √ | √ | |||||||
| L2-4 | √ | √ | √ | √ | |||||||||
| L3-4 | √ | √ | √ | √ | |||||||||
| L4-4 | √ | √ | √ | ||||||||||
| 5 | L1-5 | 2 | √ | √ | √ | √ | |||||||
| L2-5 | √ | √ | √ | √ | |||||||||
| 6 | L1-6 | 2 | √ | √ | √ | √ | |||||||
| L2-6 | √ | √ | √ | √ | |||||||||
| L3-6 | √ | √ | √ | √ | |||||||||
| L4-6 | √ | √ | √ | √ | |||||||||
| 7 | L1-7 | 2 | √ | √ | √ | √ | |||||||
| L2-7 | √ | √ | √ | √ | |||||||||
| 8 | L1-8 | 3 | √ | √ | √ | √ | |||||||
| L2-8 | √ | √ | √ | √ | |||||||||
| L3-8 | √ | √ | √ | √ | |||||||||
| 9 | L1-9 | 3 | √ | √ | √ | √ | |||||||
| 10 | L1-10 | 3 | √ | √ | √ | √ | |||||||
Additionally, the lesion progression outcomes, based on eUBM image analysis, are presented in Table 2. During the observation period, most of the lesions (approximately 67%) increased in size, approximately 14% remained unchanged and 19% regressed.
This report describes the use of a eUBM imaging system for the detection and follow-up of mouse colonic lesions. The simultaneous use of eUBM with colonoscopy was able to detect, diagnose and analyze the progression of tumoral and non-tumoral lesions in a CRC mouse model. Our group has previously demonstrated that two UBM systems, one operating at 45 MHz and the other at 40 MHz, could diagnose mouse colonic lesions in vitro[20] and in vivo[21], respectively. Here, we have demonstrated that a variety of colon lesions can be detected by eUBM in a minimally invasive way. In contrast to histopathological analysis, eUBM can be employed to make repeated measures on the same animal, facilitating the investigation of pathological processes and therapies.
Similar to the previous work, the ultrasound images obtained with eUBM also allowed for the visualization of normal colonic layers: the mucosa, muscularis mucosae, submucosa and muscularis externa (Figure 2A), as well as colon alterations, such as lymphoid infiltrates, ulcerations and tumors (Figure 2B and Figure 3). Confirming our previous findings, lymphoid infiltrates appear as hypoechoic regions underneath a hyperechoic layer representing the mucosa. Colon tumors appear as hyperechoic masses above the mucosa layer. This characterization is of great importance because it could be used to distinguish small adenomatous polyps from lymphoid hyperplasias, both seen by colonoscopy as mucosal elevations.
The correct detection and diagnosis of colonic neoplasias during a colonoscopy is essential for CRC prevention. The ranges for adenoma detection rates during a routine colonoscopy could vary up to 37% among endoscopists[3], increasing the chances to misdiagnose CRC. Most postcolonoscopy cancers have a small macroscopic appearance[31,32] and in these cases, the simultaneous use of eUBM with colonoscopy could aid in accurately detecting submucosal invasion in colonic lesions.
The small elevation in the mucosa layer observed with eUBM and registered in Figure 3A was not detected by colonoscopy. Perhaps, this fact was due to the poor bronchofiberscope image quality and could be overcome with high-resolution scopes designed specifically for work with rat and mouse models of colonic diseases[33]. These high-resolution scopes are usually rigid telescopes and a working channel is formed in a space between an operating sheath and the telescope external wall. Although the bronchofiberscope used in the present work is unable to produce high-resolution images, it has the advantage of being flexible. According to the authors’ experience, this facility of the bronchofiberscope is important to position the eUBM mini-probe tip close to a lesion, which improves the lesion visualization, or at the center of the colon lumen in order to generate circular eUBM images of the colon.
According to the results obtained with this longitudinal evaluation of inflammation-associated colon tumor progression, most of the lesions increased in size, mimicking human cancer development. All tumoral lesions were diagnosed at the first analysis (13 wk after AOM administration), even when the size was very diminutive. The diameter of the smallest detected tumoral lesion was 0.45 mm, and eUBM was able to identify even smaller structures, such as mucosal elevations with a height of 0.1 mm. Of all the lesions detected by eUBM, approximately 15% of them (four lesions) showed a reduction in size. Of these lesions, two were lymphoid infiltrates, whose size reduction indicated inflammation resolution; one was an increase in mucosal thickness that regressed; and the last one was a small adenoma whose tumoral mass decreased. Besides pedunculated and depressed lesions, we have also detected flat lesions in animal models of CRC and current work is being conducted using p53 knockout mice, which develop flat lesions with a higher incidence than wild type mice[34], to evaluate the eUBM sensitivity.
The data presented here suggest that the use of high-resolution endoluminal ultrasound is a valuable tool to evaluate the progression of colonic lesions. eUBM detected alterations in mouse colonic lesion morphology and in adenoma volume throughout the examination period. Longitudinal high-resolution ultrasound measurements could be helpful in the monitoring of therapeutic efficacy of chemotherapeutic drugs in vivo. Additionally, this technique allows for the study of lesion progression in animal models, providing detailed insights into the biology of tumor development.
The potential of the eUBM technique to differentiate malignant from non-malignant lesions is yet to be implemented. Nowadays, the technique of narrow band imaging (NBI) has the capacity to diagnose colon lesion malignancy in real time based in mucosal and superficial vascular structures imaging enhancement[35]. However, eUBM has the potential to detect lesion penetration depth through submucosal layers. Both methodologies have their advantages and limitations and could be performed simultaneously to complement each other.
Another advantage of longitudinal eUBM imaging is the possibility to use ultrasound contrast agents to target specific molecules involved in tumor development, such as the angiogenic promoter vascular endothelial growth factor, providing a minimally invasive tool for molecular diagnosis. This new modality of molecular imaging is now being tested in preclinical models with successful results in the characterization of tumor response to anti-angiogenic treatment[36-38].
In summary, the simultaneous use of eUBM with colonoscopy enhances the ability to correctly diagnose and follow-up colonic lesions, offering rapid imaging acquisition and distinct advantages because high-resolution transmural imaging of the bowel wall improves lesion detection and cost-effectiveness.
The authors are thankful to Cefas Augusto de Medeiros Paiva and Lucas Lobianco de Matheo for mouse handling and Alyson do Rosário Júnior for technical support.
Colonoscopy is the recommended screening method for colorectal cancer screening and follow-up, but it fails to detect some small or nonpolypoid lesions. Therefore, the development of other imaging methods that complement the results of colonoscopy is extremely important. The authors have previously show that endoluminal ultrasonic biomicroscopy (eUBM) associated to colonoscopy improves the detection and diagnose of inflammatory and tumoral colonic lesions in animal models. Here the authors analyze the capacity of eUBM to evaluate the progression of chemically-induced colonic lesions in mice.
The use of eUBM to diagnose mucosal and submucosal colorectal lesions and to guide lesion resection has already been proposed as a safe and effective clinical technique. However, its significant role in the routine diagnosis of colonic lesions has not yet been established. The use of animal models contributes in the development and evaluation of new diagnostic tools before they are completely clinically applied.
A step forward of previous work done by our group, which now includes the longitudinal study of lesion progression.
The current results suggest that the use of eUBM simultaneously to colonoscopy enhances the ability to correctly diagnose and follow up colonic lesions. In addition to its potential clinical application, eUBM can aid investigators to study colonic tumorigenesis processes and to evaluate novel therapeutic agents for colorectal cancer.
eUBM, also known as high frequency endoscopic ultrasonography is a relatively new technique in which an ultrasound probe is inserted into the accessory channel of a regular endoscope. eUBM is an useful modality for transmural assessment of the bowel wall.
The authors describe the evaluation of the potential use of colonoscopy and eUBM to track the progression of mouse colonic lesions. This is a clinically very interesting study.
P- Reviewer: Ikematsu H S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
| 1. | American Cancer Society. Colorectal Cancer Facts & Figures 2011-2013. Atlanta: American Cancer Society 2011; . |
| 2. | American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society 2013; . |
| 3. | Hetzel JT, Huang CS, Coukos JA, Omstead K, Cerda SR, Yang S, O’Brien MJ, Farraye FA. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 266] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 4. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [PubMed] |
| 5. | Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, Sautereau D, Boustière C, Grimaud JC, Barthélémy C. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Munroe CA, Lee P, Copland A, Wu KK, Kaltenbach T, Soetikno RM, Friedland S. A tandem colonoscopy study of adenoma miss rates during endoscopic training: a venture into uncharted territory. Gastrointest Endosc. 2012;75:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 404] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 8. | Rondagh EJ, Bouwens MW, Riedl RG, Winkens B, de Ridder R, Kaltenbach T, Soetikno RM, Masclee AA, Sanduleanu S. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc. 2012;75:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9-20. [PubMed] |
| 10. | Schizas AM, Williams AB, Meenan J. Endosonographic staging of lower intestinal malignancy. Best Pract Res Clin Gastroenterol. 2009;23:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Stergiou N, Haji-Kermani N, Schneider C, Menke D, Köckerling F, Wehrmann T. Staging of colonic neoplasms by colonoscopic miniprobe ultrasonography. Int J Colorectal Dis. 2003;18:445-449. [PubMed] |
| 12. | Hünerbein M, Totkas S, Ghadimi BM, Schlag PM. Preoperative evaluation of colorectal neoplasms by colonoscopic miniprobe ultrasonography. Ann Surg. 2000;232:46-50. [PubMed] |
| 13. | Hurlstone DP, Brown S, Cross SS, Shorthouse AJ, Sanders DS. High magnification chromoscopic colonoscopy or high frequency 20 MHz mini probe endoscopic ultrasound staging for early colorectal neoplasia: a comparative prospective analysis. Gut. 2005;54:1585-1589. [PubMed] |
| 14. | Waxman I, Saitoh Y, Raju GS, Watari J, Yokota K, Reeves AL, Kohgo Y. High-frequency probe EUS-assisted endoscopic mucosal resection: a therapeutic strategy for submucosal tumors of the GI tract. Gastrointest Endosc. 2002;55:44-49. [PubMed] |
| 15. | Schulzke JD. Does miniprobe endoscopic ultrasound have a role in the diagnostic repertoire for colorectal cancer? Int J Colorectal Dis. 2003;18:450. [PubMed] |
| 16. | Uradomo LT, Darwin PE. Evaluation of subepithelial abnormalities of the appendix by endoscopic ultrasound. Diagn Ther Endosc. 2009;2009:295379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Chen TH, Lin CJ, Wu RC, Ho YP, Hsu CM, Lin WP, Tseng YP, Chen CH, Chiu CT. The application of miniprobe ultrasonography in the diagnosis of colorectal subepithelial lesions. Chang Gung Med J. 2010;33:380-388. [PubMed] |
| 18. | Haji A, Ryan S, Bjarnason I, Papagrigoriadis S. High-frequency mini-probe ultrasound as a useful adjunct in the management of patients with malignant colorectal polyps. Colorectal Dis. 2013;15:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 419] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 20. | Alves KZ, Borges HL, Soletti RC, Viana AL, Petrella LI, Soldan M, Chagas VL, Schanaider A, Machado JC. Features of in vitro ultrasound biomicroscopic imaging and colonoscopy for detection of colon tumor in mice. Ultrasound Med Biol. 2011;37:2086-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Alves KZ, Soletti RC, de Britto MA, de Matos DG, Soldan M, Borges HL, Machado JC. In vivo endoluminal ultrasound biomicroscopic imaging in a mouse model of colorectal cancer. Acad Radiol. 2013;20:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Harmon EY, Fronhofer V, Keller RS, Feustel PJ, Brosnan MJ, von der Thüsen JH, Loegering DJ, Lennartz MR. Ultrasound biomicroscopy for longitudinal studies of carotid plaque development in mice: validation with histological endpoints. PLoS One. 2012;7:e29944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Tiwari S, Egberts JH, Korniienko O, Köhler L, Trauzold A, Glüer CC, Kalthoff H. Assessment of anti-inflammatory tumor treatment efficacy by longitudinal monitoring employing sonographic micro morphology in a preclinical mouse model. BMC Med Imaging. 2011;11:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Fernández-Domínguez I, Echevarria-Uraga JJ, Gómez N, Luka Z, Wagner C, Lu SC, Mato JM, Martínez-Chantar ML, Rodríguez-Cuesta J. High-frequency ultrasound imaging for longitudinal evaluation of non-alcoholic fatty liver disease progression in mice. Ultrasound Med Biol. 2011;37:1161-1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Campos-Junior PH, Silva CA, Grazia JG, Soares MB, Santos RR, Viana JH. Use of ultrasound biomicroscopy to evaluate induced ovarian follicular growth and ovulation in mice. Lab Anim. 2011;45:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Borges HL, Bird J, Wasson K, Cardiff RD, Varki N, Eckmann L, Wang JY. Tumor promotion by caspase-resistant retinoblastoma protein. Proc Natl Acad Sci USA. 2005;102:15587-15592. [PubMed] |
| 27. | Ward JM, Yamamoto RS, Brown CA. Pathology of intestinal neoplasms and other lesions in rats exposed to azoxymethane. J Natl Cancer Inst. 1973;51:1029-1039. [PubMed] |
| 28. | Reddy BS, Narisawa T, Weisburger JH. Colon carcinogenesis in germ-free rats with intrarectal 1,2-dimethylhydrazine and subcutaneous azoxymethane. Cancer Res. 1976;36:2874-2876. [PubMed] |
| 29. | Tanaka T. Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog. 2009;8:5. [PubMed] |
| 30. | Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39:87-92. [PubMed] |
| 31. | Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol. 2006;4:1259-1264. [PubMed] |
| 32. | Le Clercq C, Rondagh E, Riedl R, Bosman FT, Beets GL, Hameeteman W, Masclee A, Sanduleanu S. Interval colorectal cancers frequently have subtle macroscopic appearance: a 10 year-experience in an academic center. Gastroenterology. 2011;140:S-112-S-113. |
| 33. | Olson TJP, Halbeg RB. Experimental small animal colonoscopy. Colonoscopy. 2011;19:309-327. |
| 34. | Chang WC, Coudry RA, Clapper ML, Zhang X, Williams KL, Spittle CS, Li T, Cooper HS. Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis. 2007;28:2375-2381. [PubMed] |
| 35. | Lee MM, Enns R. Narrow band imaging for the detection of neoplastic lesions of the colon. Can J Gastroenterol. 2009;23:15-18. [PubMed] |
| 36. | Săftoiu A. State-of-the-art imaging techniques in endoscopic ultrasound. World J Gastroenterol. 2011;17:691-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Rix A, Lederle W, Siepmann M, Fokong S, Behrendt FF, Bzyl J, Grouls C, Kiessling F, Palmowski M. Evaluation of high frequency ultrasound methods and contrast agents for characterising tumor response to anti-angiogenic treatment. Eur J Radiol. 2012;81:2710-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Greco A, Mancini M, Gargiulo S, Gramanzini M, Claudio PP, Brunetti A, Salvatore M. Ultrasound biomicroscopy in small animal research: applications in molecular and preclinical imaging. J Biomed Biotechnol. 2012;2012:519238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |