Published online Oct 28, 2013. doi: 10.3748/wjg.v19.i40.6934
Revised: August 4, 2013
Accepted: August 16, 2013
Published online: October 28, 2013
Processing time: 138 Days and 11.8 Hours
Patients with Stage IV cholangiocarcinoma are currently not considered to be surgical candidates and are typically offered systemic chemotherapy. Recently, several novel systemic chemotherapy regimens have allowed an initially unresectable cholangiocarcinoma to be resectable. The aim of this article is to present the usefulness of adjuvant surgery in a case of advanced cholangiocarcinoma that was successfully treated with gemcitabine. A 72-year-old man was diagnosed with distal cholangiocarcinoma with liver metastases (cT2N0M1, Stage IV). He underwent metal stent placement in the duodenum to alleviate jaundice. After 18 courses of chemotherapy using gemcitabine without severe drug toxicities, a computed tomography scan showed that the liver metastases in S6 and S7 had disappeared. The patient underwent subtotal stomach-preserving pancreaticoduodenectomy and lymph node dissection. The pathological stage was pT2N0M0, Stage IB. The patient underwent 6 cycles of adjuvant chemotherapy using gemcitabine. The patient is alive and well 6 years and 9 mo after the diagnosis.
Core tip: Patients with Stage IV cholangiocarcinoma are currently not considered to be surgical candidates and are typically offered systemic chemotherapy. Recently, several novel systemic chemotherapy regimens have allowed an initially unresectable cholangiocarcinoma to be resectable. In a patient with advanced extrahepatic cholangiocarcinoma, gemcitabine (GEM) induced a dramatic reduction of the tumor, which led to curative resection and a long-term survival of 6 years and 9 mo. This result suggests the possibility of advantages of using GEM for the treatment of advanced cholangiocarcinoma, and GEM-based chemotherapy could be performed more often for unresectable cholangiocarcinomas.
- Citation: Oshiro Y, Takahashi K, Sasaki R, Kondo T, Sakashita S, Ohkohchi N. Adjuvant surgery for advanced extrahepatic cholangiocarcinoma. World J Gastroenterol 2013; 19(40): 6934-6938
- URL: https://www.wjgnet.com/1007-9327/full/v19/i40/6934.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i40.6934
Cholangiocarcinoma continues to exhibit poor survival rates compared with other gastrointestinal malignancies[1-4]. Most cholangiocarcinoma patients are not surgical candidates. Patients with Stage IV cholangiocarcinoma are currently inoperable and are typically offered systemic chemotherapy. The most promising approaches involve the use of single agents such as gemcitabine (GEM), which has been shown to be effective against cholangiocarcinoma in phase II trials[5-7]. In these trials, the response rates for GEM ranged from 8% to 36%, and the overall survival (OS) ranged from 6.3 to 16 mo. We describe a rare case of stage IV cholangiocarcinoma with liver metastases that was initially deemed unresectable and became resectable after GEM chemotherapy and showed a favorable outcome.
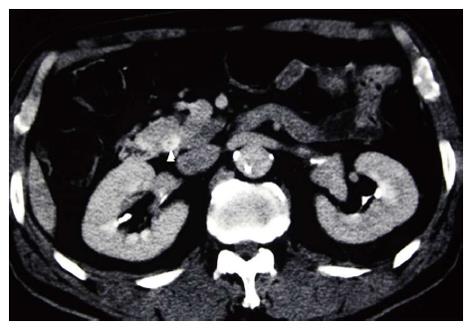
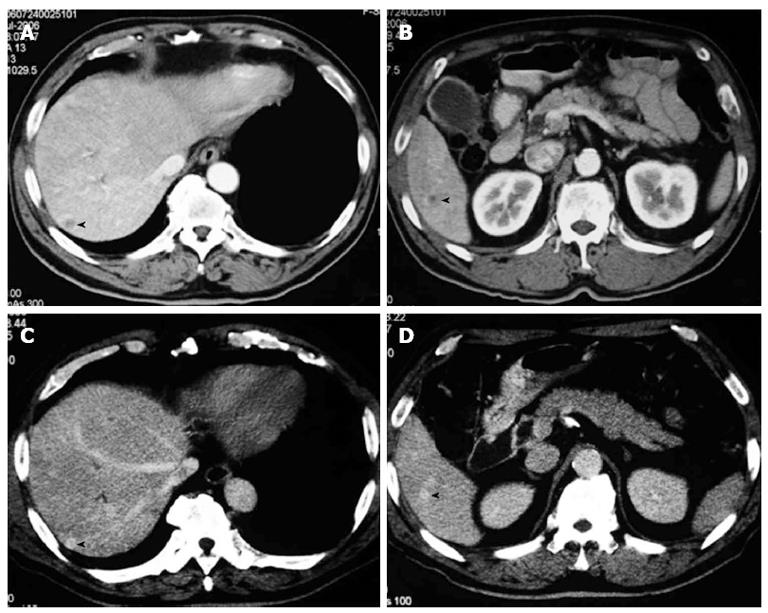
The patient was a 72-year-old man referred from a local hospital complaining of jaundice. The laboratory data on admission showed the following elevated values: total bilirubin (T-bil), 6.2 mg/dL (normal range, 0.2-1.2 mg/dL); lactic acid dehydrogenase, 243 U/L (124-232 U/L); alkaline phosphatase 354 U/L (120-320 U/L); and γ-glutamyl transpeptidase, 181 U/L (5-55 U/L). All the tumor markers tested were within the normal limits: carcinoembryonic antigen (CEA), 2.3 ng/mL (normal range, < 5.0 ng/mL), and carbohydrate antigen 19-9, 12.0 U/mL (< 37 U/mL). Abdominal computed tomography (CT) and ultrasonography showed mild dilatation of the common bile duct and bilateral dilation of the intrahepatic bile ducts. Abdominal computed tomography angiography (CTA) detected wall thickening in the distal common bile duct, and the lesion was enhanced by contrast (Figure 1). CT and CTA showed two liver metastases, which measured 8 mm (S6) and 8 mm (S7) in diameter (Figure 2). According to the Union Internationale Contre le Cancer (UICC) guidelines, the patient was diagnosed with lower cholangiocarcinoma (cT2N0M1, Stage IV)[8].
The patient underwent successful placement of a self-expandable metal duodenal stent to relieve jaundice. The patient received a total of 18 cycles of GEM. GEM was administered intravenously at a dose of 800 mg/m2 per day on days 1, 8, and 15 in a 28-d cycle. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria Grading System (Version 2.0, April 1999)[9]. Severe drug toxicities (grade 3 or 4) were not observed.
After 18 cycles of chemotherapy, CT showed that the two liver metastases in S6 and S7 disappeared. The tumor was clinically downstaged to Stage IB (cT2N0M0).
Four weeks after the completion of chemotherapy, the operation was successfully performed. Peritoneal lavage cytology demonstrated no cancer cells in the abdominal cavity. No microscopic invasion of the resected bile duct stump was observed in an intraoperative frozen specimen. The patient underwent curative resection consisting of SSPPD with D2 lymphadenectomy without resecting any other organs.
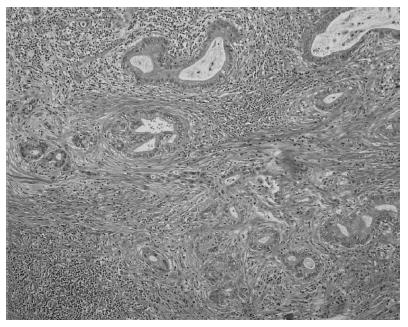
Tumor cells were detected in the distal bile duct upon microscopic examination (Figure 3). According to the UICC guidelines, the pathological classification of the tumor was cT2N0M0 Stage IB. The patient was discharged on postoperative day 61 in good condition. The patient subsequently received six cycles of adjuvant GEM chemotherapy similar to the preoperative regimen. The patient is alive at 6 years and 9 mo after the diagnosis and 5 years after the surgery.
The prognosis of patients with cholangiocarcinoma is poor, with a five-year survival rate of approximately 25% to 55%[1-4]. To overcome this clinical challenge, several strategies, including adjuvant chemotherapy, adjuvant radiotherapy, and adjuvant chemoradiotherapy have been considered for treating cholangiocarcinoma[10-13]. Few randomized clinical trials have evaluated the utility of adjuvant therapy following R0 resection of cholangiocarcinoma, and most of the current studies are small and retrospective. Therefore, no standard adjuvant modalities have been universally adopted for the treatment of cholangiocarcinoma, and the role of chemotherapy for unresectable cholangiocarcinoma has not been established. Although there has been no standard chemotherapy for cholangiocarcinoma, GEM has been the most actively used agent against cholangiocarcinoma. We treated a patient with advanced extrahepatic cholangiocarcinoma with liver metastases. The patient showed a dramatic response to GEM, which led to curative resection and long-term survival of more than 6 years. GEM may be an effective chemotherapeutic agent for treating cholangiocarcinoma, and a randomized clinical trial needs to be performed.
The feasibility of adjuvant surgery for cholangiocarcinoma has not been determined. Recently, in colorectal, gastric, and pancreatic cancer, several authors have reported “conversion surgery” or “adjuvant surgery”[14-16]. Suzuki et al[14] demonstrated that adjuvant surgery was effective in 20 advanced gastric cancer patients (Stage IV) based on liver or distant lymph node metastasis. The overall survival of patients in the partial response and curative resection groups was prolonged. The survival of patients with H or N factor was also prolonged when they received curative surgery. However, the survival of patients with P factor was not prolonged. Locally advanced pancreatic cancer may be a good indication for adjuvant surgery after sustained favorable responses to chemotherapy, even in patients with initially unresectable disease[16]. In 2013, Kato et al[17] reported that eight patients with initially unresectable advanced biliary tract cancer who underwent adjuvant surgery had significantly longer survival than 14 patients who were unable to undergo surgery. Of the eight patients in the surgery group, four patients had gallbladder carcinoma and four patients had intrahepatic cholangiocarcinoma. To our knowledge, from 1983 to 2013, in the field of bile duct cancer, only 16 cases in nine reports underwent adjuvant surgery, including the cases in the report[17-25] (Table 1). Of the 16 patients, 10 patients received GEM, 3 received S-1, 2 received GEM and S-1, 1 received GEM combined with cisplatin and fluorouracil, and 1 received cisplatin/interferon α-2b/doxorubicin/fluorouracil-combination chemotherapy. None of the 16 cases involved extrahepatic cholangiocarcinoma. To the best of our knowledge, this is the first report of adjuvant surgery for extrahepatic cholangiocarcinoma.
| Ref. | Diagnosis | Metastasis/invasion | Chemotherapy regimen | Response |
| Slupski et al[18] | IntraHepatic cholangiocarcinoma | Lung metastases | CDDP, 5-FU, IFN, doxorubicin | PR |
| Shirabe et al[19] | Gallbladder cancer | Para-aortic LNs | GEM, CDDP, 5-FU | PR |
| Kitajima et al[20] | Gallbladder cancer | Dissemination | S-1 | CR |
| Morimoto et al[21] | Gallbladder cancer | Liver metastasis | GEM | CR |
| Kanaji et al[22] | IntraHepatic cholangiocarcinoma | Dissemination | S-1 | CR |
| Kim et al[23] | IntraHepatic cholangiocarcinoma | Portal vein invasion | GEM | PR |
| Ohno et al[24] | Ampulla of vater cancer | Liver metastasis | GEM, S-1 | CR |
| Hasegawa et al[25] | Gallbladder cancer | Hepatic invasion | S-1, para-aortic LN | PR |
| Kato et al[17] | Intrahepatic cholangiocarcinoma | Hepatic vein invasion | GEM | SD |
| Intrahepatic cholangiocarcinoma | Hepatic vein invasion | GEM | PR | |
| Intrahepatic cholangiocarcinoma | Arterial invasion | GEM | SD | |
| Intrahepatic cholangiocarcinoma | Insufficient remnant liver volume | GEM | PR | |
| Gallbladder cancer | Arterial invasion | GEM | SD | |
| Gallbladder cancer | Arterial invasion | GEM | PR | |
| Gallbladder cancer | Arterial invasion portal vein invasion | GEM | SD | |
| Gallbladder cancer | Arterial invasion | GEM | SD |
Medical oncologists and surgeons have identified surgical candidates among patients with initially unresectable colorectal and gastric cancer who responded favorably to multimodal treatment[14,15]. In some cases, the addition of surgery resulted in increased long-term survival. Surgical resection coupled with multimodal treatment is called “adjuvant surgery.” Surgical resection can be classified as curative (no evidence of remaining disease after surgery) or palliative (remaining disease after surgery). Therefore, adjuvant surgery aims to be curative and not palliative after the response to chemotherapy[14].
In a strategy involving adjuvant surgery, adjuvant chemotherapy is considered necessary after the operation. In our patient, the liver metastases showed a surprising complete response without severe toxicity after GEM chemotherapy. Additionally, the patient received adjuvant chemotherapy using GEM as an outpatient and developed no adverse reactions. In previous phase II studies using single-agent GEM, major adverse reactions included neutropenia, leukopenia, and anemia were observed with little severe toxicity[5-7]. The results suggests that GEM is suitable for outpatients because of its mild toxicity.
The UK ABC-02 study defined the standard of care for unresectable advanced biliary tract cancer[26]. Valle et al[26] reported that cisplatin with GEM (GEMC) was associated with a significant survival advantage compared with GEM alone. The median OS was 11.7 mo for GEMC and 8.1 mo for GEM alone[26]. A Japanese trial of 83 patients using the same treatment regimens as UK ABC-02 showed the median survival and overall response rate of GEMC vs GEM alone were 11.2 mo vs 7.7 mo and 19.5% vs 11.9%, respectively. These results were consistent with the results of the UK ABC-02 study. GEMC was found to be effective and well tolerated, which indicates that it could also be a standard regimen for Japanese patients[27].
In conclusion, in a patient with advanced extrahepatic cholangiocarcinoma, GEM induced a dramatic reduction of the tumor, which led to curative resection. The patient was still living 6 years and 9 mo after the study. The results suggest possible advantages of using GEM for the treatment of advanced cholangiocarcinoma. GEM-based chemotherapy could be more commonly administered for unresectable cholangiocarcinoma. Furthermore, “adjuvant surgery” (i.e., R0 resection) may significantly contribute to curing cholangiocarcinoma. An evidence-based consensus should be developed on potentially resectable cholangiocarcinoma with liver metastases in each hospital.
P- Reviewer Erichsen R S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Nagorney DM, Donohue JH, Farnell MB, Schleck CD, Ilstrup DM. Outcomes after curative resections of cholangiocarcinoma. Arch Surg. 1993;128:871-877; discussion 871-877. [PubMed] |
| 2. | DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 1017] [Article Influence: 56.5] [Reference Citation Analysis (1)] |
| 3. | Sasaki R, Takahashi M, Funato O, Nitta H, Murakami M, Kawamura H, Suto T, Kanno S, Saito K. Prognostic significance of lymph node involvement in middle and distal bile duct cancer. Surgery. 2001;129:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Oshiro Y, Sasaki R, Kobayashi A, Murata S, Fukunaga K, Kondo T, Oda T, Ohkohchi N. Prognostic relevance of the lymph node ratio in surgical patients with extrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2011;37:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Penz M, Kornek GV, Raderer M, Ulrich-Pur H, Fiebiger W, Lenauer A, Depisch D, Krauss G, Schneeweiss B, Scheithauer W. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol. 2001;12:183-186. [PubMed] |
| 6. | Tsavaris N, Kosmas C, Gouveris P, Gennatas K, Polyzos A, Mouratidou D, Tsipras H, Margaris H, Papastratis G, Tzima E. Weekly gemcitabine for the treatment of biliary tract and gallbladder cancer. Invest New Drugs. 2004;22:193-198. [PubMed] |
| 7. | Okusaka T, Ishii H, Funakoshi A, Yamao K, Ohkawa S, Saito S, Saito H, Tsuyuguchi T. Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2006;57:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Union Internationale Contre le Cancer. TNM classification of malignant tumours. 7th ed. New York, NY: Wiley-Liss 2009; . |
| 9. | National Institute of Health. NCI: Common Toxicity Criteria, Version 2.0. Available from: http://ctep.info.nih.gov/reporting/ctc.html. |
| 10. | Gerhards MF, van Gulik TM, González González D, Rauws EA, Gouma DJ. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Todoroki T, Ohara K, Kawamoto T, Koike N, Yoshida S, Kashiwagi H, Otsuka M, Fukao K. Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys. 2000;46:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 134] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Kelley ST, Bloomston M, Serafini F, Carey LC, Karl RC, Zervos E, Goldin S, Rosemurgy P, Rosemurgy AS. Cholangiocarcinoma: advocate an aggressive operative approach with adjuvant chemotherapy. Am Surg. 2004;70:743-748; discussion 743-748. [PubMed] |
| 13. | McMasters KM, Tuttle TM, Leach SD, Rich T, Cleary KR, Evans DB, Curley SA. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg. 1997;174:605-608; discussion 605-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 145] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Suzuki T, Tanabe K, Taomoto J, Yamamoto H, Tokumoto N, Yoshida K, Ohdan H. Preliminary trial of adjuvant surgery for advanced gastric cancer. Oncol Lett. 2010;1:743-747. [PubMed] |
| 15. | Power DG, Kemeny NE. Chemotherapy for the conversion of unresectable colorectal cancer liver metastases to resection. Crit Rev Oncol Hematol. 2011;79:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Kato K, Kondo S, Hirano S, Tanaka E, Shichinohe T, Tsuchikawa T, Matsumoto J. Adjuvant surgical therapy for patients with initially-unresectable pancreatic cancer with long-term favorable responses to chemotherapy. J Hepatobiliary Pancreat Sci. 2011;18:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Kato A, Shimizu H, Ohtsuka M, Yoshidome H, Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Kimura F, Miyazaki M. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol. 2013;20:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Slupski MW, Szczylik C, Jasinski MK. Unexpected response to systemic chemotherapy in case of primarily nonresectable advanced disseminated intrahepatic cholangiocarcinoma. World J Surg Oncol. 2007;5:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Shirabe K, Tomoyuki Abe Kiyoshi Kajiyama, Kazuya Akahoshi. [The Survival Impact of Chemotherapy in the Patients with Gall Bladder Cancer-A pilot study]. J Jpn Biliary Association. 2008;22:41-46. |
| 20. | Kitajima K, Kobayashi S, Shiba H, Uwagawa T, Ishida Y, Aiba K, Kawakami M, Yanaga K. Successful treatment of advanced gallbladder cancer with an anticancer drug S-1: assessment based on intratumoral gene. Int J Clin Oncol. 2008;13:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Morimoto H, Ajiki T, Takase S, Fujita T, Matsumoto T, Mita Y, Matsumoto I, Fujino Y, Suzuki Y, Kuroda Y. Resection of gallbladder cancer with hepatic metastasis after chemotherapy with gemcitabine. J Hepatobiliary Pancreat Surg. 2008;15:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Kanaji S, Kobayashi I, Fujita T, Ueno K, Tsuchida S, Kawasaki K, Ohno M, Osawa M, Fujino Y, Tominaga M. [A case of curatively resected biliary tract cancer with peritoneal dissemination through effective response to chemotherapy of S-1]. Gan To Kagaku Ryoho. 2009;36:1337-1339. [PubMed] |
| 23. | Kim SH, Kim IH, Kim SW, Lee SO. Repetitive response to gemcitabine that led to curative resection in cholangiocarcinoma. World J Gastroenterol. 2009;15:4593-4595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Ohno T, Koguchi H, Miura A, Tanaka Y, Endo M, Matsunaga S, Hasegawa I, Kato A, Tokuda Y, Sakakibara K. [A case of advanced ampullary carcinoma successfully resected after primary chemotherapy with s-1 and gemcitabine]. Gan To Kagaku Ryoho. 2009;36:999-1002. [PubMed] |
| 25. | Hasegawa N, Abei M, Sasaki R, Pak S, Moriwaki T, Minami Y, Fukuda K, Hirai S, Shoda J, Ohkouchi N. [A case with Stage IVb advanced gallbladder cancer curatively resected following effective S-1 chemotherapy]. J Jpn Biliary Association. 2010;24:723-728. |
| 26. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3165] [Article Influence: 211.0] [Reference Citation Analysis (1)] |
| 27. | Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, Nagino M, Kondo S, Nagaoka S, Funai J. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 570] [Cited by in RCA: 568] [Article Influence: 37.9] [Reference Citation Analysis (0)] |