Published online Oct 28, 2013. doi: 10.3748/wjg.v19.i40.6919
Revised: August 16, 2013
Accepted: September 4, 2013
Published online: October 28, 2013
Processing time: 126 Days and 14 Hours
AIM: To compare the outcome of upper gastrointestinal bleeding (UGIB) between patients receiving restrictive and liberal transfusion.
METHODS: PubMed, EMBASE, and Cochrane Library databases were employed to identify all relevant randomized controlled trials regarding the outcome of UGIB after restrictive or liberal transfusion. Primary outcomes were death and rebleeding. Secondary outcomes were length of hospitalization, amount of blood transfused, and hematocrit and hemoglobin at discharge or after expansion.
RESULTS: Overall, 4 papers were included in this meta-analysis. The incidence of death was significantly lower in patients receiving restrictive transfusion than those receiving liberal transfusion (OR: 0.52, 95%CI: 0.31-0.87, P = 0.01). The incidence of rebleeding was lower in patients receiving restrictive transfusion than those receiving liberal transfusion, but this difference did not reach any statistical significance (OR: 0.26, 95%CI: 0.03-2.10, P = 0.21). Compared with those receiving liberal transfusion, patients receiving restrictive transfusion had a significantly shorter length of hospitalization (standard mean difference: -0.17, 95%CI: -0.30--0.04, P = 0.009) and a significantly smaller amount of blood transfused (standard mean difference: -0.74, 95%CI: -1.15--0.32, P = 0.0005) with a lower hematocrit and hemoglobin level at discharge or after expansion.
CONCLUSION: Restrictive transfusion should be employed in patients with UGIB.
Core tip: Current international consensus recommends restrictive transfusion for upper gastrointestinal bleeding. However, this recommendation is largely based on expert opinions. We have performed the present meta-analysis of randomized controlled trials, which potentially supported the superiority of restrictive over liberal transfusion for upper gastrointestinal bleeding.
-
Citation: Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS. Restrictive
vs liberal transfusion for upper gastrointestinal bleeding: A meta-analysis of randomized controlled trials. World J Gastroenterol 2013; 19(40): 6919-6927 - URL: https://www.wjgnet.com/1007-9327/full/v19/i40/6919.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i40.6919
Acute upper gastrointestinal bleeding is a common cause for emergency hospitalization with a relatively high annual incidence of 50-200/100000 population and a mortality of 10%-30%[1-3]. Red blood cell transfusion is often required in such patients due to the reduction of tissue perfusion after acute blood loss[4-6]. Current international consensus on the management of upper gastrointestinal bleeding recommends that the threshold for initiating blood transfusion is a hemoglobin level of 70 g/L or less in patients with nonvariceal upper gastrointestinal bleeding[5] and a hemoglobin level of 80 g/L or less in patients with variceal bleeding[6]. However, these recommendations are largely based on expert opinions or international guidelines regarding transfusion requirement in critically ill patients without upper gastrointestinal bleeding[7-9]. Accordingly, the grade of evidence for these recommendations is low[5,6].
A previous Cochrane Collaboration systematic review of three randomized controlled trials has shown a tendency in decreasing the mortality of patients with upper gastrointestinal bleeding after restrictive transfusion[10,11]. But a small number of participants and a high proportion of missing data limit the significance of these findings[10,11]. These authors concluded that their review might not provide useful data regarding outcomes following red blood cell transfusion for acute upper gastrointestinal bleeding[10,11]. Recently, several large-scale observational studies demonstrated that blood transfusion after nonvariceal upper gastrointestinal bleeding might increase the rate of mortality and rebleeding[12,13]. More recently, a large and well-organized randomized controlled trial showed a significant benefit of restrictive transfusion strategy in improvement of outcome in patients with upper gastrointestinal bleeding[14].
Herein, we performed an updated meta-analysis of randomized controlled trials to compare the outcome of upper gastrointestinal bleeding between patients treated with restrictive and liberal transfusion. Primary outcomes were death and rebleeding. Secondary outcomes were length of hospitalization, amount of blood transfused, and hematocrit and hemoglobin at discharge or after expansion.
This work was performed according to the PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions[15].
(1) randomized controlled trials were included, if they compared the outcome of upper gastrointestinal bleeding between patients treated with restrictive and liberal blood transfusion; (2) no publication date, publication language, or publication status was restricted; (3) basic studies were excluded; (4) comments, editorials, or letters were excluded; (5) introductions for Cochrane groups were excluded; (6) narrative reviews, systematic reviews, or meta-analyses were excluded; (7) study design reports, case reports, non-comparative case series were excluded; (8) non-randomized comparative studies were excluded; and (9) randomized controlled trials unrelated to our topics were excluded.
Studies were identified using a search strategy in the PubMed, EMBASE, and Cochrane Library databases. Search items were listed as follows: (“gastrointestinal” [All Fields] OR “digestive” [All Fields] OR “peptic” [All Fields] OR “alimentary tract” [All Fields] OR “esophageal” [All Fields] OR “esophagus” [All Fields] OR “gastric” [All Fields] OR “stomach” [All Fields] OR “duodenal” [All Fields] OR “duodenum” [All Fields]) AND (“hemorrhage” [All Fields] OR “haemorrhage” [All Fields] OR “bleeding” [All Fields] OR “bleed” [All Fields] OR “melena” [All Fields] OR “melaema” [All Fields] OR “hematemesis” [All Fields] OR “haematemesis” [All Fields]) AND (“transfusion” [All Fields]) AND (“blood” [All Fields] OR “red cell” [All Fields]) AND (“randomized” [All Fields] OR “randomized” [All Fields] OR “randomly” [All Fields]). The last search was performed on January 5, 2013. Study eligibility was independently judged by two authors. In cases of disagreement between the two authors, they would be discussed with another author. When two or more studies were conducted by one affiliation, only the studies with more complete data and more extensive interval of enrollment were included in the meta-analysis.
We developed a data extraction sheet, including the authors, journal, publication year, whether articles were published in peer-reviewed journals or not, regions where the study was conducted, period of enrollment, study design, target population, study endpoints, eligibility criteria, sample size, and demographic data (age and sex), source of upper gastrointestinal bleeding, hematocrit, and hemoglobin of two groups. Data were independently extracted by two authors. In cases of disagreement between the two authors, they would be discussed with another author. We used Google to translate the information in the non-English language full-texts.
Quality of included randomized controlled trials was scored by Jadad composite scale[16,17], in which the description of randomization, blinding, and withdrawals were assessed. The quality scale ranged from 0 to 5 points. If the score was 2 or less, the quality of the study would be considered lower. If the score was beyond 2, the quality of the study would be high.
Data were collected, using Microsoft Office Excel 2003. For the dichotomous variables (i.e., incidence of death and rebleeding), number of events and total patients were extracted from these included studies. Then, OR with 95%CI was calculated. For the continuous variables (i.e., length of hospitalization, amount of blood transfusion, and hematocrit and hemoglobin), mean value and standard deviation were extracted from these included studies. Then, standard mean difference with 95%CI was calculated. Finally, the OR and standard mean difference of each study were pooled, using both fixed-effects (Mantel-Haenszel method)[18] and random-effects model (DerSimonian-Laird method)[19]. When heterogeneity was not significant, the pooled data using fixed-effects model were considered appropriate. When significant heterogeneity was observed, only the pooled data using random-effects model were considered appropriate. Heterogeneity between studies was assessed by using the I2 statistic (I2 > 50% was considered as having substantial heterogeneity) and the χ2 test (P < 0.10 was considered to represent significant statistical heterogeneity)[20]. Funnel plots were not performed due to a small number of included studies. Sensitivity analyses were done to assess the reliability of meta-analysis. All analyses were conducted using the statistical package Review Manager version 5.1 (Copenhagen, The Nordic Cochrane Center, The Cochrane Collaboration, 2011).
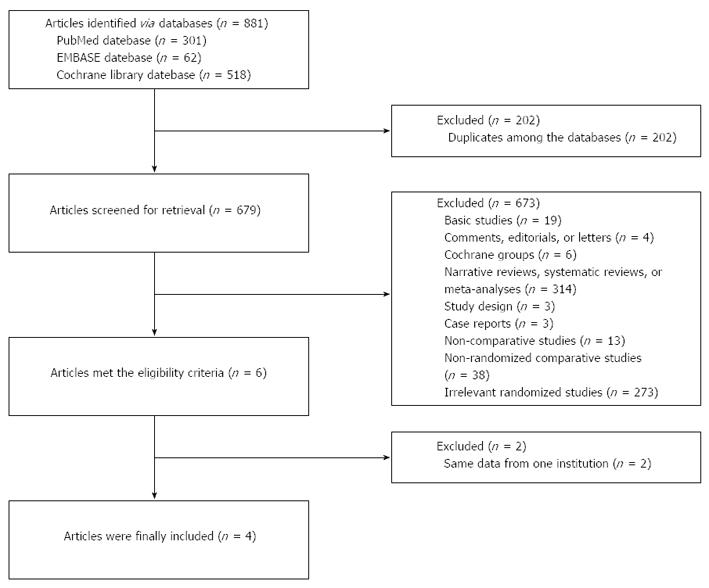
Overall, 881 papers were initially identified by the search strategy. Among them, six papers met eligibility criteria[14,21-25]. Of note, three papers were reported by the same one affiliation[14,21-22]. Among them, two papers with a smaller number of patients were excluded[21,22]. Thus, four randomized controlled trials were finally included in the meta-analysis[14,23-25] (Figure 1), in which 982 patients with upper gastrointestinal bleeding were treated with restrictive or liberal blood transfusion.
All of four randomized controlled trials were single-center studies, and were published in peer-reviewed journals between 1986 and 2013 (Table 1). Three randomized controlled trials were published in three English-language priority journals[14,24,25], and another one was published in one Spanish-language journal[23]. Target population was patients with upper gastrointestinal bleeding from variceal or non-variceal source. Of note, a total of 60 patients were randomized in one study[23], but only 27 patients were finally observed (restrictive transfusion, n = 14, and liberal transfusion, n = 13). The detailed eligibility criteria of these studies were described in Table 2. The baseline characteristics were comparable between the two groups (Table 3).
| Ref. | Design | Regions | Target population | Intervention | Outcome measures |
| Blair et al[25] | Single center RCT | London, United Kingdom | Acute severe UGIB; no esophageal varices | Early transfusion group (liberal transfusion): at least 2 units of blood No transfusion group (restrictive transfusion): no blood transfusion unless hemoglobin fell below 8 g/dL or they were shocked | Observed results: coagulation results (impedance clotting time, kaolin cephalin clotting time); haematological results (hematocrit); eventual blood transfused; number of rebleed; number of death |
| Elizalde et al[24] | Single center RCT | Barcelona, Spain | Liver cirrhosis with an acute variceal bleeding episode | PRC group (liberal transfusion): 2 units of packed red cells PPL group (restrictive transfusion): 500 mL of a 5% plasma protein solution | Observed items: hemodynamic measurements (cardiopulmonary pressures, cardiac output, wedged and free hepatic venous pressures, mean arterial blood pressure, systemic vascular resistance); hormonal measurements (plasma renin activity, aldosterone levels, norepinephrine); rheological parameters (plasma volume, blood viscosity) |
| Villarejo et al[23] | Single center RCT | Buenos Aires, Argentina | Acute digestive hemorrhage with stable haemodynamics | Treatment group (restrictive transfusion): patients underwent normovolemic hemodilution with crystalloid solutions, and the hematocrit value was maintained as ≥ 21% and < 28%; red cell transfusion was given if angina, shock, hemodynamic instability, or hematocrit < 21% Control group (liberal transfusion): the target of transfusion in these patients was the hematocrit value of ≥ 28% | Observed results: organ failure, hospital stay, APACHE II score, red cell transfused, hematocrit, haemoglobin |
| Villanueva et al[14] | Single center RCT | Barcelona, Spain | Upper gastrointestinal bleeding | Liberal transfusion: the hemoglobin threshold for transfusion was 9 g/dL, with a target range for the post-transfusion hemoglobin level of 9-11 g/dL Restrictive transfusion: the hemoglobin threshold for transfusion was 7 g/dL, with a target range for the post-transfusion hemoglobin level of 7-9 g/dL | Primary endpoints: the rate of death from any cause within the first 45 d Secondary endpoints: the rate of further bleeding and the rate of in-hospital complications |
| Ref. | Eligibility criteria |
| Blair et al[25] | Consecutive patients with acute severe upper gastrointestinal haemorrhage were prospectively randomized on arrival to receive during their first 24 h in hospital |
| Only known cases of oesophageal varices were excluded as they frequently have abnormal coagulation due to liver disease | |
| Elizalde et al[24] | The study population consisted of patients with cirrhosis of the liver admitted for the management of an acute variceal bleeding episode |
| Only patients in whom hemostasia had been achieved within the previous 24-72 h by means of endoscopic sclerotherapy, and who were still anemic (hematocrit < 30%) and normovolemic as defined by clinical parameters (systolic pressure > 100 mmHg, right atrial pressure > 2 cm H2O, heart rate < 100 beats/min, and urine output > 0.5 mL/kg per hour) were eligible for the study | |
| Age < 18 or > 80 yr, renal failure as defined as serum creatinine > 2 mg/dL, portal thrombosis, diffuse or multinodular hepatocellular carcinoma, arterial hypertension, peripheral vasculopathy, previous surgical or transjugular intrahepatic portosystemic shunt, bacterial infection, and use of vasoactive drugs to prevent or treat portal hypertension-related bleeding were considered exclusion criteria | |
| Villarejo et al[23] | Inclusion criteria: acute high digestive haemorrhage with stable haemodynamics and any aetiology; age > 15 yr old |
| Exclusion criteria: history of angina; shock not responsive to volume expansion; requirement of surgery; established renal insufficiency; poliglobulina; bleeding diathesis; acute or chronic liver dysfunction; chronic anemia; pregnancy; sepsis; acute or chronic respiratory failure; haematocrit < 20% on admission; religious objection to transfusion | |
| Villanueva et al[14] | Patients older than 18 yr of age who had hematemesis (or bloody nasogastric aspirate), melena, or both, as confirmed by the hospital staff, were considered for inclusion |
| Patients were excluded if they declined to undergo a blood transfusion | |
| Additional exclusion criteria: massive exsanguinating bleeding, an acute coronary syndrome, symptomatic peripheral vasculopathy, stroke, transient ischemic attack, or transfusion within the previous 90 d; a recent history of trauma or surgery; lower gastrointestinal bleeding; a previous decision on the part of the attending physician that the patient should avoid specific medical therapy; and a clinical Rockall score of 0 with a hemoglobin level >12 g/dL |
| Ref. | Groups | No. Patients | Age (yr) | Sex (male:female) | Source of bleeding | Hematocrit atadmission | Hemoglobin atadmission (g/dL) |
| Blair et al[25] | Restrictive transfusion | 26 | 60 ± 4.5 | 2:1 | Gastric ulcer (n = 4); duodenal ulcer (n = 13); carcinoma (n = 2); Mallory-Weiss tear (n = 3); not visualized (n = 4) | 29 ± 1.6 | NA |
| Liberal transfusion | 24 | 64 ± 3.6 | 2:1 | Gastric ulcer (n = 2); duodenal ulcer (n = 17); carcinoma (n = 1); Mallory-Weiss tear (n = 2); not visualized (n = 2) | 28 ± 1.2 | NA | |
| Elizalde et al[24] | Restrictive transfusion | 8 | 60 ± 4 | 5/3 | Bleeding from esophageal varices (n = 7); bleeding from gastric varices in the fundus of the stomach (n = 1) | 27.0 ± 1.3 | 91.5 ± 6.8 |
| Liberal transfusion | 8 | 64 ± 2 | 4/4 | Bleeding from esophageal varices (n = 7); bleeding from gastric varices in the fundus of the stomach (n = 1) | 27.0 ± 1.3 | 90.5 ± 3.96 | |
| Villarejo et al[23] | Restrictive transfusion | 14 | 56.8 ± 12.8 | 9/5 | Mallory Weiss (n = 2); erosive gastritis (n = 14); erosive gastroduodenitis (n = 4); Forrest gastric ulcer (n = 10); Forrest duodenal ulcer (n = 10); erosive duodenitis (n = 1) | 26.9 ± 4.29 | 8.76 ± 2.47 |
| Liberal transfusion | 13 | 45.3 ± 14.6 | 9/4 | 28.3 ± 5.59 | 8.85 ± 2.53 | ||
| Villanueva et al[14] | Restrictive transfusion | 444 | NA | NA | Peptic ulcer (n = 228); gastroesophageal varices (n = 101); Mallory-Weiss tears (n = 25); erosive gastritis or esophagitis (n = 38); neoplasms (n = 16); other (n = 36) | NA | 9.6 ± 2.2 |
| Liberal transfusion | 445 | NA | NA | Peptic ulcer (n = 209); gastroesophageal varices (n = 109); Mallory-Weiss tears (n = 30); erosive gastritis or esophagitis (n = 29); neoplasms (n = 20); other (n = 48) | NA | 9.4 ± 2.4 |
In the four studies, double-blinding technique was not feasible due to the nature of interventions. Two studies were scored as 3 points and considered to be of higher quality, and another two studies were scored as 1 point and considered to be of lower quality (Table 4).
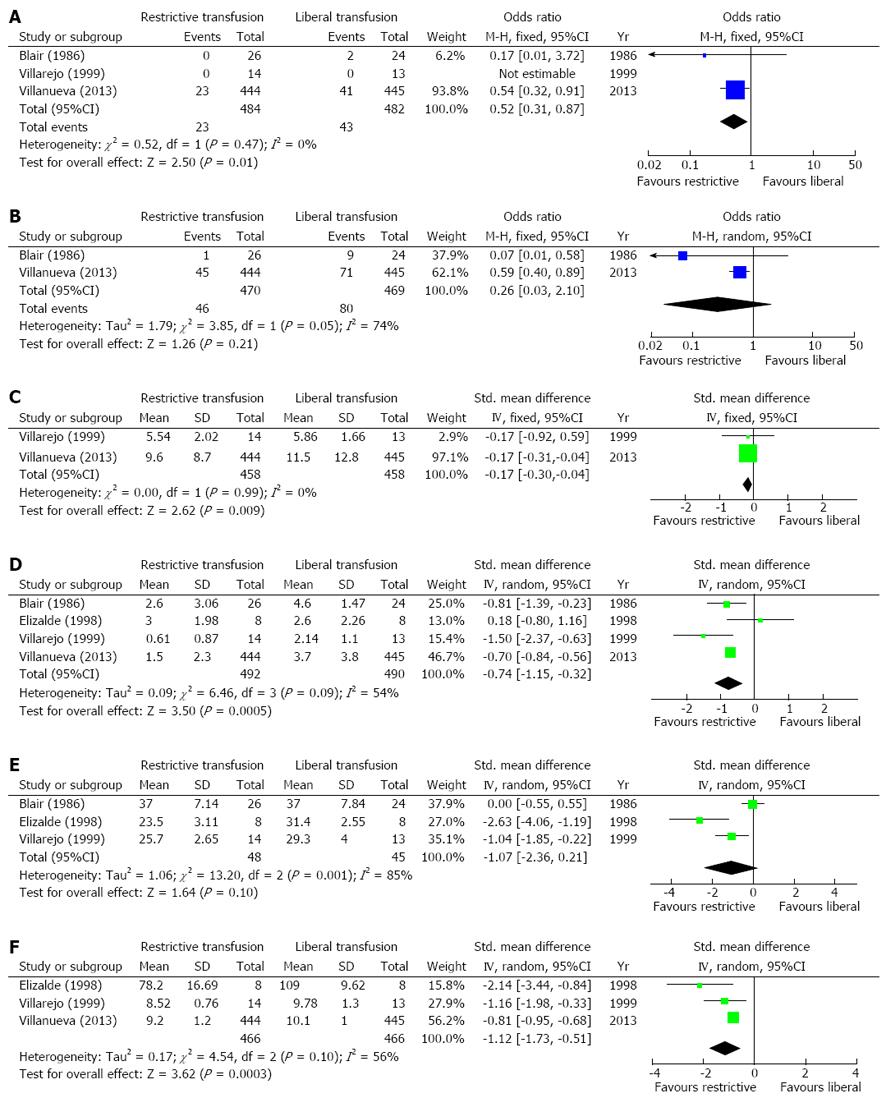
Three studies reported the incidence of death in two groups[14,23,25]. One study did not observe any death during the study period in both groups[23], so the OR of this study was not estimable. Another two studies showed a higher incidence of death in patients receiving liberal transfusion[14,25]. Heterogeneity among the three studies was not significant (I2 = 0%; P = 0.47). Using a fixed-effect model, the pooled OR was significant (OR = 0.52, 95%CI: 0.31-0.87, P = 0.01) (Figure 2A).
Two studies reported the incidence of rebleeding in two groups[14,25]. Both studies showed a higher incidence of rebleeding in patients receiving liberal transfusion. Heterogeneity among the two studies was significant (I2 = 74%; P = 0.05). Using a random-effect model, the pooled OR was not significant (OR = 0.26, 95%CI: 0.03-2.10, P = 0.21) (Figure 2B).
Two studies reported the length of hospitalization in two groups[14,23]. Heterogeneity among the two studies was not significant (I2 = 0%; P = 0.99). Using a fixed-effect model, the pooled standard mean difference was significant (standard mean difference: -0.17, 95%CI: -0.30--0.04, P = 0.009) (Figure 2C).
Four studies reported the amount of blood transfused in two groups[14,23-25]. Heterogeneity among the four studies was significant (I2 = 54%; P = 0.09). Using a random-effect model, the pooled standard mean difference was significant (standard mean difference: -0.74, 95%CI: -1.15--0.32, P = 0.0005) (Figure 2D).
Three studies reported the value of hematocrit at discharge or after expansion in two groups[23-25]. Heterogeneity among the three studies was significant (I2 = 85%; P = 0.001). Using a random-effect model, the pooled standard mean difference was not significant (standard mean difference: -1.07, 95%CI: -2.36-0.21, P = 0.10) (Figure 2E).
Three studies reported the hemoglobin concentration at discharge or after expansion in two groups[14,23,24]. Heterogeneity among the three studies was significant (I2 = 56%; P = 0.10). Using a random-effect model, the pooled standard mean difference was significant [standard mean difference: -1.12, 95%CI: -1.73--0.51, P = 0.0003] (Figure 2F).
Sensitivity analyses were performed after one study with a high proportion of loss to follow-up was excluded[23]. Results of all meta-analyses were consistent with those of previous meta-analyses. For death, the pooled OR was 0.52 (95%CI: 0.31-0.87, P = 0.01). For length of hospitalization, the pooled standard mean difference was -0.17 (95%CI: -0.31--0.04, P = 0.01). For amount of blood transfused, the pooled standard mean difference was -0.64 (95%CI: -0.97--0.30, P = 0.0002). For hematocrit, the pooled standard mean difference was -1.23 (95%CI: -3.79-1.34, P = 0.35). For hemoglobin, the pooled standard mean difference was -1.31 (95%CI: -2.57--0.06, P = 0.04).
This updated meta-analysis of randomized controlled trials showed the following important findings. First, restrictive transfusion could significantly decrease the incidence of death in patients with upper gastrointestinal bleeding. The survival benefit of restrictive transfusion might be attributed to a lower incidence of further bleeding and transfusion-related adverse events. Second, two randomized controlled trials were included in our meta-analysis and unanimously supported the effectiveness of restrictive transfusion in decreasing the incidence of rebleeding. Our meta-analysis demonstrated a trend in decreasing the incidence of rebleeding in patients treated with restrictive transfusion, but it did not reach any statistical significance. This unexpected finding could be explained by the fact that a random-effect model was employed due to a significant heterogeneity among studies, and the number of patients included in the two randomized controlled trials was substantially different. Third, patients treated with restrictive transfusion had a significantly shorter length of hospitalization and a smaller amount of blood transfused, although the value of hematocrit and hemoglobin at discharge or after expansion was lower in patients treated with restrictive transfusion than in those with liberal transfusion. Generally, these findings accorded with the current international consensus recommendation in which restrictive transfusion should be employed in patients with upper gastrointestinal bleeding. However, we should acknowledge that the threshold of restrictive transfusion strategy was various among these included studies. Accordingly, further studies should be warranted to elucidate the accurate threshold of transfusion in these patients.
The strengths of our study were as follows. First, only randomized controlled trials were included into our meta-analysis. Second, a comprehensive literature search was performed by searching three databases. Third, no publication language was restricted. One Spanish-language full text paper was retrieved by contacting the journal secretary and was translated by Google[23]. Fourth, a random-effect model was employed to produce a conservative result with wider confidence intervals, as the heterogeneity among studies was significant[19].
Several limitations of our study should be fully emphasized. First, the publication date of the four studies spanned from 1986 to 2013. This was a relatively long time during which many diagnostic techniques and treatment modalities had been improved. But it should be noted that heterogeneity among studies was not significant for death. Second, three earlier randomized controlled trials had a relatively small sample size[23-25]. By comparison, the latest randomized controlled trial had a larger number of participants included[14]. Therefore, the weight of this trial was larger than that of other studies in meta-analyses. In future, more randomized controlled trials are needed to perform a further meta-analysis regarding this topic. Third, one randomized controlled trial had a relatively high proportion of loss to follow-up, thereby increasing the possibility of selection bias[23]. Fourth, one randomized controlled trial primarily aimed to observe the hemodynamic effects of an acute increase in blood hemoglobin levels[24]. Thus, the data regarding the survival and rebleeding could not be extracted. Fifth, results of one large randomized controlled trial were partially published in two previously published abstracts[21,22]. A total of 277 patients with cirrhosis with acute variceal bleeding were enrolled in this study[14]. By contrast, the outcome of 147 participants with cirrhosis with acute variceal bleeding was described in one abstract, and the outcome of 214 participants was reported in another abstract[21,22]. Importantly, no interim analysis or so called “early stopping rule” was planned in the study. Accordingly, the misbehavior that the authors looked at the data and reported the partial results before the study was completed would introduce the chance of falsely rejecting the null hypothesis (i.e., type I error). Finally, we could not do a meta-analysis according to the different source of bleeding due to a small number of studies included.
In conclusions, this meta-analysis provides preliminary evidence to support that the restrictive transfusion should be employed in patients with upper gastrointestinal bleeding, although the limitation of our study is obvious. Further well designed and conducted randomized controlled trials should be warranted to confirm whether or not restrictive transfusion should be beneficial in different sources of upper gastrointestinal bleeding.
We are indebted to Mariela García Muñoz (Secretary Acta Gastroenterológica LatinoAmericana, Organo oficial de la Sociedad Argentina de Gastroenterología) for literature retrieval.
Acute upper gastrointestinal bleeding is a common cause for emergency hospitalization with a relatively high morbidity and mortality. Red blood cell transfusion is often required in such patients due to the reduction of tissue perfusion after acute blood loss. Current international consensus recommends restrictive transfusion for upper gastrointestinal bleeding. However, this recommendation is largely based on expert opinions.
Recently, several large-scale observational studies demonstrated that blood transfusion after nonvariceal upper gastrointestinal bleeding might increase the rate of mortality and rebleeding. More recently, a large and well-organized randomized controlled trial showed a significant benefit of restrictive transfusion strategy in improvement of outcome in patients with upper gastrointestinal bleeding.
A previous Cochrane Collaboration systematic review of three randomized controlled trials has shown a tendency in decreasing the mortality of patients with upper gastrointestinal bleeding after restrictive transfusion. But a small number of participants and a high proportion of missing data limit the significance of these findings. These authors concluded that their review might not provide useful data regarding outcomes following red blood cell transfusion for acute upper gastrointestinal bleeding. Thus, authors performed an updated meta-analysis of randomized controlled trials to compare the outcome of upper gastrointestinal bleeding between patients treated with restrictive and liberal transfusion. Results of their meta-analysis demonstrated that patients receiving restrictive transfusion had a lower incidence of death and rebleeding, a shorter length of hospitalization, and a smaller amount of blood transfused than those receiving liberal transfusion.
Evidence suggested that restrictive transfusion should be employed in patients with upper gastrointestinal bleeding.
Restrictive transfusion for acute upper gastrointestinal bleeding will become a hot topic in recent years. This study is very interesting. It is valuable to be published.
P- Reviewers Pan WS, Yan SL S- Editor Gou SX L- Editor A E- Editor Wang CH
| 1. | Lewis JD, Bilker WB, Brensinger C, Farrar JT, Strom BL. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: relationship to sales of nonsteroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol. 2002;97:2540-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Sung JJ, Tsoi KK, Ma TK, Yung MY, Lau JY, Chiu PW. Causes of mortality in patients with peptic ulcer bleeding: a prospective cohort study of 10,428 cases. Am J Gastroenterol. 2010;105:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. 2012;107:1190-1195; quiz 1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (1)] |
| 4. | Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359:928-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1030] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 7. | Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3537] [Cited by in RCA: 3083] [Article Influence: 181.4] [Reference Citation Analysis (0)] |
| 8. | Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3699] [Cited by in RCA: 3352] [Article Influence: 128.9] [Reference Citation Analysis (0)] |
| 9. | Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet JP, Toledano BJ, Robillard P. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 733] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 10. | Hearnshaw S, Brunskill S, Doree C, Hyde C, Travis S, Murphy MF. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database Syst Rev. 2009;CD006613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Jairath V, Hearnshaw S, Brunskill SJ, Doree C, Hopewell S, Hyde C, Travis S, Murphy MF. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database Syst Rev. 2010;CD006613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Hearnshaw SA, Logan RF, Palmer KR, Card TR, Travis SP, Murphy MF. Outcomes following early red blood cell transfusion in acute upper gastrointestinal bleeding. Aliment Pharmacol Ther. 2010;32:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Restellini S, Kherad O, Jairath V, Martel M, Barkun AN. Red blood cell transfusion is associated with increased rebleeding in patients with nonvariceal upper gastrointestinal bleeding. Aliment Pharmacol Ther. 2013;37:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1069] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [PubMed] |
| 16. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [PubMed] |
| 17. | Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 2653] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 18. | Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719-748. [PubMed] |
| 19. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] |
| 20. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46470] [Article Influence: 2112.3] [Reference Citation Analysis (3)] |
| 21. | Colomo A, Hernandez-Gea V, Madoz P, Carles A, Alvarez-Urturi C, Poca M, Concepcion M, Gordillo J, Soriano G, Torras X. Hemodynamic changes and transfusion strategies in cirrhotic patiens with acute variceal bleeding. Hepatology. 2009;50:403A Available from: http: //onlinelibrary.wiley.com/o/cochrane/clcentral/articles/718/ CN-00739718/frame.html. |
| 22. | Colomo A, Hernandez-Gea V, Muñiz-Diaz E, Madoz P, Aracil C, Álvarez-Urturi C, Jordi G, Soriano G, Torras X, Sáinz S. Transfusion strategies in patients with cirrhosis and acute gastrointestinal bleeding. Hepatology. 2008;48:413A Available from: http: //onlinelibrary.wiley.com/o/cochrane/clcentral/articles/965/CN-00691965/frame.html. |
| 23. | Villarejo F, Rizzolo M, Lópéz E, Domeniconi G, Arto G, Apezteguia C. Acute anemia in high digestive hemorrhage. Margins of security for their handling without transfusion of red globules. Acta Gastroenterol Latinoam. 1999;29:261-270. [PubMed] |