Published online Oct 28, 2013. doi: 10.3748/wjg.v19.i40.6894
Revised: September 4, 2013
Accepted: September 15, 2013
Published online: October 28, 2013
Processing time: 186 Days and 21.3 Hours
AIM: To determine the expression of the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) and the Ku70/Ku80 heterodimer (Ku 70/80) in gastric carcinoma.
METHODS: Gastric biopsies were obtained from 146 gastric carcinoma patients [Helicobacter pylori (H. pylori)-negative: 89 and H. pylori-positive: 57] and 34 from normal subjects (H. pylori-negative: 16 and H. pylori-positive: 18) via surgery and endoscopic detection from April 2011 to August 2012 at the First Affiliated Hospital of Nanchang University. Pathological diagnosis and classification were made according to the criteria of the World Health Organization and the updated Sydney system. An ‘‘in-house’’ rapid urease test and modified Giemsa staining were employed to detect H. pylori infection. The expression of DNA-PKcs and the Ku 70/80 protein was detected by immunohistochemistry.
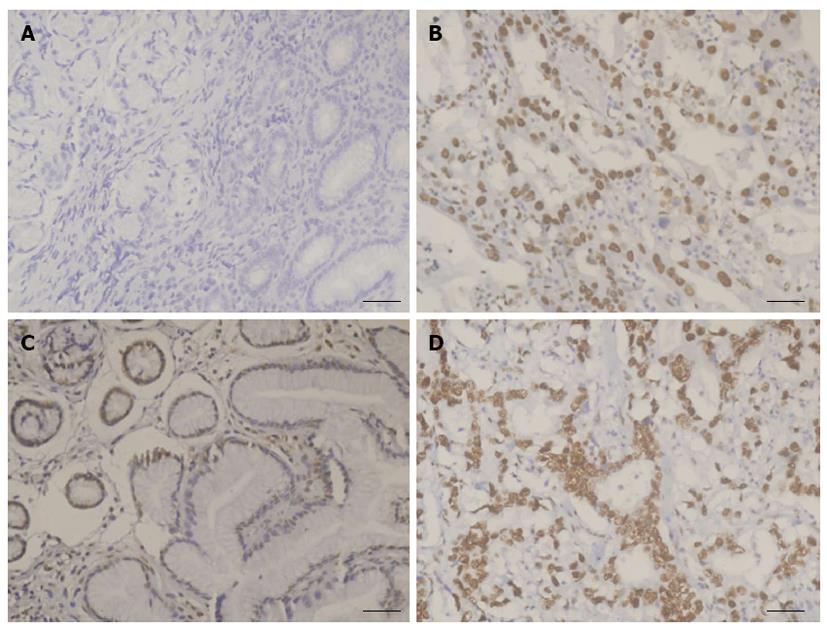
RESULTS: Overall, the positive rates of both DNA-PKcs and Ku 70/80 were significantly increased in gastric cancer (χ2 = 133.04, P < 0.001 for DNA-PKcs and χ2 = 13.06, P < 0.01 for Ku) compared with normal gastric mucosa. There was hardly any detectable expression of DNA-PKcs in normal gastric mucosa, and the positive rate of DNA-PKcs protein expression in patients with a normal gastric mucosa was 0% (0/34), whereas the rate in gastric cancer (GC) was 93.8% (137/146). The difference between the two groups was statistically significant. Additionally, the positive rate of Ku 70/80 was 79.4% (27/34) in normal gastric mucosa and 96.6% (141/146) in gastric cancer. The DNA-PKcs protein level was significantly increased in gastric cancer (Mann-Whitney U = 39.00, P < 0.001), compared with normal gastric mucosa. In addition, there was a significant difference in the expression of Ku 70/80 (Mann-Whitney U = 1117.00, P < 0.001) between gastric cancer and normal gastric mucosa. There was also a significant difference in Ku70/80 protein expression between GC patients with and without H. pylori infection (P < 0.05). Spearman analysis showed a negative correlation between tumor differentiation and DNA-PKcs expression (r = -0.447, P < 0.05). Moreover, Ku70/80 expression was negatively correlated with both clinical stage (r = -0.189, P < 0.05) and H. pylori colonization (r = -0.168, P < 0.05).
CONCLUSION: Overall, this research demonstrated that enhanced DNA-PKcs and Ku 70/80 expression may be closely associated with gastric carcinoma.
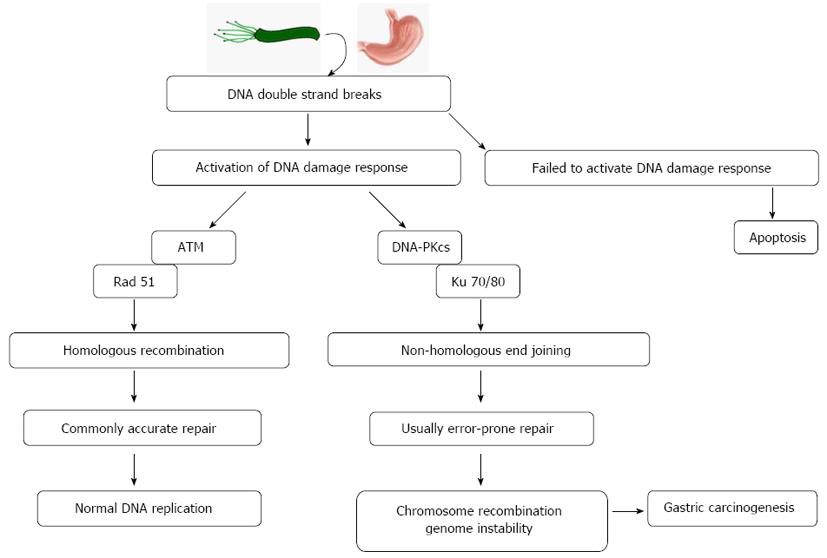
Core tip: This is the first study to clarify the two key promoters of the non-homologous end joining (NHEJ) pathway, DNA-dependent protein kinase (DNA-PKcs) and Ku 70/80, in human gastric carcinoma tissues. The present study found an upregulated expression of DNA-PKcs and Ku 70/80 in gastric cancer tissues compared with normal gastric mucosa, which suggests that there is an enhanced function of NHEJ in gastric carcinogenesis. As NHEJ is an error-prone and non-specific repair mechanism and can be induced before homologous recombination, its excessive activation is capable of regulating cell cycle arrest, cell apoptosis, chromosome recombination and genome instability.
- Citation: Li W, Xie C, Yang Z, Chen J, Lu NH. Abnormal DNA-PKcs and Ku 70/80 expression may promote malignant pathological processes in gastric carcinoma. World J Gastroenterol 2013; 19(40): 6894-6901
- URL: https://www.wjgnet.com/1007-9327/full/v19/i40/6894.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i40.6894
Gastric carcinoma is a worldwide malignant disease with high incidence and mortality, and it is characterized by genome instability through severe DNA damage caused by various factors, including Helicobacter pylori (H. pylori) infection, heredity and living habits[1,2]. The catalytic subunit of the DNA-dependent protein kinase (DNA-PKcs) and the Ku 70/Ku 80 heterodimer (Ku 70/80) can instantly combine into DNA-dependent protein kinase (DNA-PK), which is a crucial promoter of the non-homologous end joining (NHEJ) pathway[3,4]. First, DNA-PKcs receives a DNA damage signal and launches a damage response. Next, Ku 70/80 binds to the damaged DNA ends and then attracts DNA-PKcs to form DNA-PK, which can trigger NHEJ repair activities[4,5]. Accumulating evidence has shown that dysregulation of DNA-PKcs and Ku 70/80 is associated with pathological processes in various tumors[6]. The expression changes and biological function of DNA-PKcs and Ku 70/80 in gastric cancer (GC) are still unclear. It is possible that DNA-PKcs and Ku 70/80 play a dual role in tumorigenicity, as both a rapid repair method and error-prone mechanism. The inability to activate the promoter of this repair pathway could increase the mutation rate within the genome, promoting malignant cellular changes associated with carcinogenesis. Malignant tissues induce unbalanced NHEJ activities to handle metabolic stress and promote tumor infiltration. However, normal repair activities within tumor tissues may also lead to cell death and genome instability, as well as create a microenvironment that is predisposed to cancer. To determine a possible pathological role of DNA-PKcs and Ku 70/80-mediated DNA repair pathways in human gastric cancer tissues and to verify whether H. pylori infection disturbs the regular repair function of gastric mucosal epithelial cells through a series of DNA damage responses and the NHEJ repair pathway, we measured the expression of DNA-PKcs and Ku 70/80 by immunohistochemistry in biopsies or surgical specimens of 180 patients with or without gastric carcinoma.
Gastric samples of patients who underwent upper gastroduodenoscopy from January 2007 to September 2009 at The First Affiliated Hospital of Nanchang University were retrospectively reviewed and examined. A total of 180 patients (76 females and 104 males, with a mean age of 55.37 ± 14.05 years) were enrolled in this study, including 34 patients with a normal gastric mucosa (NGM, H. pylori-negative: 16 cases and H. pylori-positive: 18 cases), and 146 with gastric carcinoma (GC, H. pylori-negative: 89 cases and H. pylori-positive: 57 cases). There was no significant difference in the age or gender distribution among these groups. None of the patients had been treated with any regimens to eradicate H. pylori infection. This study was approved ethically by the First Affiliated Hospital of Nanchang University. All of the patients gave written informed consent to participate in the study.
An ‘‘in-house’’ rapid urease test (RUT) and modified Giemsa staining were employed for detecting H. pylori infection. The effectiveness of RUT is more than 95% (data not shown). The modified Giemsa staining was carried out in a double-blind fashion. An H. pylori infection was diagnosed as positive only if both of the methods produced positive results. An H. pylori-negative diagnosis was confirmed if both of the methods yielded negative results[7].
Gastric samples were obtained from patients who underwent endoscopy of the upper gastrointestinal tract. All of the biopsies were taken from the gastric antrum and the lesions of individual patients. The tissues used for histological analysis were fixed in 10% formaldehyde in Ca2+ and Mg2+ free phosphate-buffered saline (PBS) overnight at 4 °C before paraffin embedding. Paraffin sections of 4 μm were cut with a microtome and stored at room temperature. Pathological diagnosis and classification were performed according to the criteria of the World Health Organization[8,9].
Primary antibodies used in this study were as follows: mouse monoclonal antihuman DNA-PKcs (Abcam, Cambridge, United Kingdom) and rabbit polyclonal anti-human Ku 70/Ku 80 (Anbobio, San Francisco, CA). The anti-human DNA-PKcs antibody was diluted 1:400, and the anti-human Ku70/80 antibody was diluted 1:3500.
The paraffin sections were mounted on slides and dewaxed in xylene and sequentially dehydrated in 100%, 95% and 85% ethanol. The sections were stained using the PV-6000 Polymer Detection System (Zhongshan Goldenbridge, Beijing, PRC) staining protocol. The sections were then washed in PBS, and endogenous peroxidase was blocked using 3% H2O2. After the specimens were incubated with the primary antibody overnight at 4 °C, they were washed with PBS, followed by incubation with polymer helper for 30 min and poly peroxidase-anti-mouse or rabbit IgG for 30 min. After the sections were washed with PBS, they were incubated with 3,3-diaminobenzidin (DAB, Zhongshan Goldenbridge). The control sections incubated with PBS instead of the primary antibodies were used as negative controls. The sections were counterstained with hematoxylin.
The slides were examined under a light microscope. The cells that stained a yellow or brown color in the nucleus and/or cytoplasm were defined as positive. Five randomly selected fields per section were analyzed. In a randomly selected field from representative areas, the immunoreactive cells among 100 cells were assessed and quantified by percentage. Then, the average percentage of the five fields was used to assess the area of immunostaining (0 = 0%-5%; 1 = 6%-25%; 2 = 26%-50%; 3 = 51%-75%; 4 = 76%-100%). In addition, the intensity of immunostaining was also semi-quantitatively assessed (0 = negative, 1 = weak, 2 = moderate, 3 = intense). Then, the integrals of the ‘‘area × intensity’’ were calculated, by which the overall expression levels of the proteins in the section were defined as follows: negative (-): score 0-2; weakly positive (+): score 3-5; moderately positive (++): score 6-8; and strongly positive (+++): score 9-12. The positive rate was the sum of weakly, moderately and strongly positive staining. The assessment of the sections was performed blindly by two pathologists, and when two views were not consistent, the assessment was judged by a third person.
All of the data are presented as the mean ± SD or percentage. The χ2 test (SPSS v.16.0 for Windows; SPSS, Inc., Chicago, IL) was used to evaluate the difference in categorical variables, such as the positive rate between groups. The Mann-Whitney U test (SPSS v.16.0) was used to determine the differences in numerical variables between differently defined groups. Correlations were analyzed using Spearman’s rank correlation co-efficient (SPSS v.16.0). A P value of less than 0.05 was considered statistically significant.
Overall, the positive rates of both DNA-PKcs and Ku 70/80 were significantly increased in GC (χ2 = 133.04, P < 0.001 for DNA-PKcs and χ2 = 13.06, P < 0.01 for Ku) compared with NGM. In fact, there was hardly any detectable expression of DNA-PKcs in normal gastric mucosa, and the positive rate of DNA-PKcs protein expression in patients with NGM was 0% (0/34), whereas the rate in GC was 93.8% (137/146). The difference between the two groups is statistically significant. Additionally, the positive rate of Ku 70/80 was 79.4% (27/34) in NGM and 96.6% (141/146) in GC (Figure 1, Table 1).
| Group | n | DNA-PKcs | P value | Ku 70/80 | P value | ||||||
| - | + | ++ | +++ | - | + | ++ | +++ | ||||
| NGM | 34 | 34 | 0 | 0 | 0 | < 0.01 | 7 | 12 | 12 | 3 | < 0.01 |
| GC | 146 | 9 | 24 | 65 | 48 | 5 | 19 | 50 | 72 | ||
| H. pylori positive | |||||||||||
| NGM | 16 | 16 | 0 | 0 | 0 | < 0.01 | 2 | 4 | 8 | 2 | < 0.10 |
| GC | 57 | 3 | 6 | 26 | 22 | 1 | 4 | 19 | 33 | ||
| H. pylori negative | |||||||||||
| NGM | 18 | 18 | 0 | 0 | 0 | < 0.01 | 5 | 8 | 4 | 1 | < 0.01 |
| GC | 89 | 6 | 18 | 39 | 26 | 4 | 15 | 31 | 39 | ||
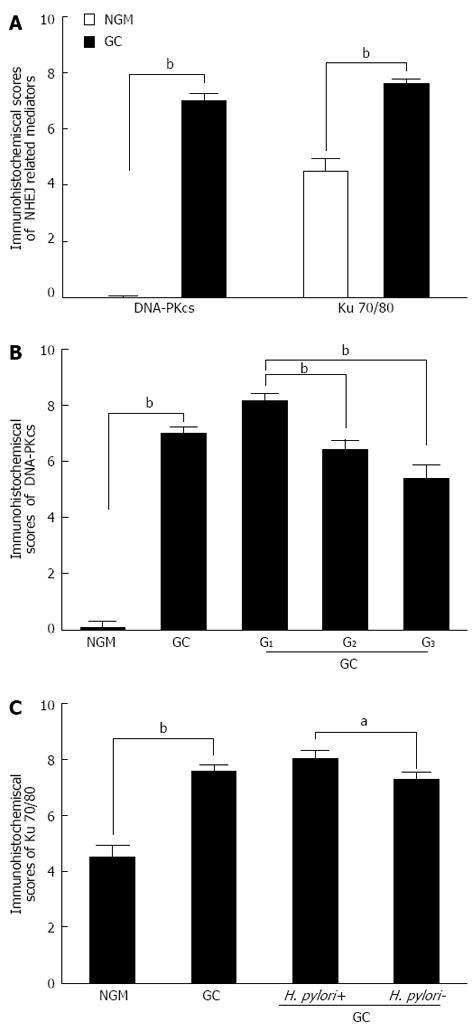
The average immunohistochemistry scores of the DNA-PKcs protein were significantly increased in GC (Mann-Whitney U = 39.00, P < 0.001), compared with NGM (Figure 2A, Table 1). In addition, there was a significant difference in the average immunohistochemistry scores of Ku 70/80 (Mann-Whitney U = 1117.00, P < 0.001) between GC and NGM (Figure 2A, Table 1).
Overall, DNA-PKcs expression was negatively correlated with differentiation degree (r = -0.447, P < 0.01) in patients with GC (Figure 2B, Table 2). Its expression increased from well differentiated (G1) tumor tissues to moderately differentiated (G2) tissues and from G1 to poorly differentiated (G3) tumor tissues, but there was no significant increase from G2 to G3 (Table 3). Ku 70/80 expression was negatively correlated with H. pylori infection (r = -0.168, P = 0.043) and clinical stage (r =-0.189, P = 0.022) in patients with GC (Figure 2C, Table 2). There was no correlation between DNA-PKcs expression and Ku 70/80 expression.
| Clinicopathological parameters | DNA-PKcs | Ku 70/80 | ||
| r | P value | r | P value | |
| Age (yr) | 0.557 | 0.049 | 0.083 | 0.317 |
| Sex | 0.073 | 0.382 | -0.052 | 0.531 |
| H. pylori infection | -0.139 | 0.102 | -0.168 | 0.043 |
| Lesion location | 0.131 | 0.115 | 0.107 | 0.200 |
| Histological type | 0.046 | 0.583 | 0.008 | 0.922 |
| Local Invasive | 0.042 | 0.615 | -0.052 | 0.534 |
| Differentiation degree | -0.447 | < 0.001 | 0.068 | 0.414 |
| Lymph node metastasis | 0.029 | 0.729 | 0.123 | 0.139 |
| Clinical stages | 0.010 | 0.902 | -0.189 | 0.022 |
| Clinicopathological parameters | n | DNA-PKcs | P value | Ku 70/80 | P value | ||||||
| - | + | ++ | +++ | - | + | ++ | +++ | ||||
| Age (yr) | 0.555 | 0.315 | |||||||||
| < 55 | 60 | 5 | 9 | 28 | 18 | 2 | 10 | 21 | 27 | ||
| ≥ 55 | 86 | 4 | 15 | 37 | 30 | 3 | 9 | 29 | 45 | ||
| Sex | 0.380 | 0.530 | |||||||||
| Male | 96 | 5 | 20 | 40 | 31 | 3 | 10 | 35 | 48 | ||
| Female | 50 | 4 | 4 | 25 | 17 | 2 | 9 | 15 | 24 | ||
| H. pylori infection | 0.116 | 0.044 | |||||||||
| Positive | 57 | 3 | 6 | 26 | 22 | 1 | 4 | 19 | 33 | ||
| Negative | 89 | 6 | 18 | 39 | 26 | 4 | 15 | 31 | 39 | ||
| Lesion location | 0.114 | 0.199 | |||||||||
| Antrum | 74 | 3 | 16 | 36 | 19 | 3 | 8 | 32 | 31 | ||
| Others | 72 | 6 | 8 | 29 | 29 | 2 | 11 | 18 | 41 | ||
| Histological type | |||||||||||
| Protrude1 | 15 | 3 | 1 | 6 | 5 | 0.8401-2 | 0 | 4 | 4 | 7 | 0.6231-2 |
| Ulcerative2 | 57 | 2 | 10 | 29 | 16 | 0.5861-3 | 0.5461-3 | ||||
| Infiltrative ulcer3 | 59 | 4 | 9 | 24 | 22 | 0.7921-4 | 0.9111-4 | ||||
| 0.9462-3 | 2 | 6 | 21 | 28 | 0.8942-3 | ||||||
| Diffuse infiltration4 | 15 | 0 | 4 | 6 | 5 | 0.9462-4 | 1 | 6 | 23 | 29 | 0.6552-4 |
| 0.7913-4 | 2 | 3 | 2 | 8 | 0.5913-4 | ||||||
| Local invasion | |||||||||||
| Mucosa and submucosa1 | 14 | 2 | 2 | 6 | 4 | 0.6421-2 | 0 | 1 | 5 | 8 | 0.5011-2 |
| Muscular layer2 | 21 | 1 | 4 | 9 | 7 | 0.5181-3 | 1 | 2 | 8 | 10 | 0.3951-3 |
| Serosa and subserosa3 | 111 | 6 | 18 | 50 | 37 | 0.9262-3 | 4 | 16 | 37 | 54 | 0.9542-3 |
| Differentiation degree | |||||||||||
| G11 | 64 | 3 | 3 | 22 | 36 | 0.0001-2 | 1 | 6 | 24 | 33 | 0.3211-2 |
| G22 | 57 | 4 | 13 | 29 | 11 | 0.0001-3 | 3 | 6 | 23 | 25 | 0.6181-3 |
| G33 | 25 | 2 | 8 | 14 | 1 | 0.1462-3 | 1 | 7 | 3 | 14 | 0.8662-3 |
| Lymph node metastasis | 0.728 | 0.318 | |||||||||
| Positive | 113 | 6 | 36 | 5 | 15 | 41 | 52 | ||||
| Negative | 33 | 3 | 12 | 0 | 4 | 9 | 20 | ||||
| Clinical stages | |||||||||||
| I1 | 13 | 2 | 4 | 0.5391-2 | 0 | 1 | 5 | 7 | 0.6731-2 | ||
| II2 | 46 | 2 | 14 | 0.5451-3 | 2 | 3 | 12 | 29 | 0.3821-3 | ||
| III3 | 34 | 1 | 12 | 0.6381-4 | 0 | 3 | 18 | 13 | 0.2431-4 | ||
| IV4 | 53 | 4 | 18 | 0.9832-3 | 3 | 12 | 15 | 23 | 0.0752-3 | ||
| 0.8322-4 | 0.0292-4 | ||||||||||
| 0.8203-4 | 0.5113-4 | ||||||||||
NHEJ is a key source of genomic rearrangements, which are typically found in cancer cells[10]. Presently, numerous reports have demonstrated that chromosome translocations are a common cause of malignancy, and oncogenic fusion genes have been found in many hematological and solid tumors[11-13]. The over-expression of DNA-PKcs and Ku 70/80 has been found in various human tumors, but the pathways that promote translocations in gastric cancer are still unclear[14-16]. It is well-established that H. pylori infection is a definite etiological factor in gastric carcinogenesis[17-19]. However, the mechanism through which H. pylori infection contributes to the development of gastric cancer has not been fully elucidated. Obst et al[20] reported that H. pylori infection could cause DNA damage in gastric epithelial cells. Because Toller et al[21] showed an increased expression of γ-H2AX in H. pylori-infected GES-1 cells and indicated that GC may be associated with DNA double strand breaks (DSBs), we expected to detect increased DNA-PKcs expression in GC to explain the error-prone repair pathway found in the gastric mucosa.
In this study, we found that the expression of DNA-PKcs and Ku 70/80 was increased in GC compared with NGM. Spearman analysis indicated a negative correlation between the expression of DNA-PKcs and tumor differentiation degrees. Moreover, Ku 70/80 was increased in H. pylori-positive GC patients compared with the negative group. Analysis of the data from 146 cases of GC did not show an obvious association between DNA-PKcs or Ku 70/80 expression levels and invasive lymph node metastasis or tumor histological type. We hypothesized that the enhanced DNA-PKcs and Ku 70/80 expression in gastric cancer may result from H. pylori-induced DNA damage in gastric epithelial cells. We initially analyzed the relationship between DNA-PKcs and Ku 70/80 protein expression and H. pylori infection status. As shown in the right panel of Figure 1C, the Ku 70/80 protein expression level was higher in H. pylori-positive GC tissues than in the H. pylori-negative tissue.
Numerous studies have indicated that genome instability can be induced by NHEJ, which may increase the risk of malignant transformation in cells[6]. In line with our results, Hu et al[22] reported that the expression of Ku70 proteins in precancerous lesions and GC tissue was significantly higher than that in normal gastric mucosal tissue. Moreover, the expression of Ku70 proteins in GC tissue was significantly higher than in precancerous lesions. Lee et al[23] verified that DNA-PKcs expression status was increased in consecutive cases of gastric cancer (450/564) by immunohistochemistry. Moreover, they found that DNA-PKcs expression was negative in the foveolar epithelium of normal gastric mucosal tissues but was positive in most H. pylori-associated gastritis, intestinal metaplasia and gastric adenoma tissues. Lim et al[24] found that Ku70 and Ku80 expression is mediated by constitutively activated NF-kappaB and constitutively expressed COX-2 in gastric cancer cells and indicated that Ku70 and Ku80 expression may be related to gastric cell proliferation and carcinogenesis. These data are consistent with our findings. Here, we show that exposure to H. pylori significantly upregulated Ku 70/80 expression but did not influence the expression of DNA-PKcs. We speculated that a lack of effectively activated DNA-PKcs might be responsible for the severe DNA damage and abnormal NHEJ repair activities due to H. pylori infection in gastric epithelial cells. In addition, environmental factors, such as H. pylori infection, stress and behavioral habits, may upset the balance between DNA-PKcs and Ku 70/80, and thus the damaged cells cannot launch the rapid and non-specific repair mechanism, leading to more serious and irreparable damage.
To our knowledge, this is the first study to clarify the two key promoters of the NHEJ pathway in human gastric carcinoma tissue, DNA-PKcs and Ku 70/80. The upregulated expression of DNA-PKcs and Ku 70/80 in GC tissues compared with NGM confirms that there is enhanced NHEJ in human gastric carcinoma. As NHEJ is an error-prone and non-specific repair mechanism and can be induced before homologous recombination (HR), its excessive activation is capable of regulating cell cycle arrest, cell apoptosis, chromosome recombination and genome instability[25,26]. Our finding that Ku 70/80 expression is positively correlated with H. pylori colonization suggests that this alteration may be highly relevant for pathological processes during H. pylori infection. Infection with H. pylori can serve as a unique model system to determine the role of Ku 70/80-mediated abnormal NHEJ repair activities in gastric carcinogenesis (Figure 3).
We found the following results: (1) gastric carcinogenesis is associated with enhanced non-homologous end joining repair; and (2) H. pylori mainly upregulates the expression of Ku 70/80 as opposed to DNA-PKcs to trigger NHEJ in gastric epithelial cells. Our data contribute to the complexity of the NHEJ function in GC. To illustrate the molecular mechanism underlying DNA-PK function in gastric carcinogenesis, further studies with larger samples in the various stages of gastric carcinoma are needed.
In conclusion, the present study provides new perspectives into the role of DNA-PKcs and Ku 70/80 in gastric carcinogenesis. Our data indicated that dysregulation of DNA-PK occurs frequently in GC and is associated with GC progression, which may be another important mechanism leading to abnormal repair activities in damaged gastric epithelial cells, especially the upregulated Ku 70/80 expression in H. pylori-related GC. In the current study, we found that both DNA-PKcs and Ku 70/80 were over-expressed in GC tissues. These results suggest that altered expression of DNA-PKcs and Ku 70/80 may function as a potential factor that may lead to genome instability in gastric carcinoma.
Gastric carcinoma (GC) is characterized by genome instability through severe DNA damage caused by various factors, including Helicobacter pylori (H. pylori) infection, heredity and living habits. The incidence of gastric carcinoma is currently rising rapidly throughout the world, especially in developing countries. The exact pathogenesis of this disease is still unknown. Abnormal expression of DNA-dependent protein kinase (DNA-PKcs) and Ku 70/80 is associated with tumor growth, invasion and metastasis and may also play a crucial role in error-prone DNA repair activities in gastric epithelial cells, thus leading to genome instability and gastric carcinogenesis.
DNA-PKcs and Ku 70/80 are crucial promoters of the non-homologous end joining (NHEJ) pathway. Accumulating evidence has shown that dysregulation of DNA-PKcs and Ku 70/80 is associated with pathological processes in various tumors. The expression changes and biological function of DNA-PKcs and Ku 70/80 in gastric carcinoma are still unclear. In this study, the authors demonstrate that the overexpression of DNA-PKcs and Ku 70/80 could be a potential mechanism for mediating error-prone DNA repair pathways in human gastric cancer tissues and verified that H. pylori infection may increase the expression of Ku 70/80, disrupting the standard activation of the NHEJ repair pathway, which is associated with gastric epithelial malignancies.
Recent reports have highlighted the importance of genome instability-related DNA repair promoters, including DNA-PKcs and Ku 70/80, in gastric carcinogenesis. Both DNA-PKcs and Ku 70/80 are over-expressed in gastric carcinoma compared to normal gastric mucosa. This is the first study to explore the change in expression of DNA-PKcs and Ku 70/80 in gastric carcinoma, to analyze the correlation between the expression of DNA-PKcs and Ku 70/80 and to investigate a series of clinical parameters to illustrate the potential pathological mechanism of carcinogenesis.
By verifying the changes of DNA-PKcs and Ku 70/80 and their relationship with gastric carcinoma, this study may provide new insights into the pathological mechanism of gastric cancer. Furthermore, the combination of DNA repair molecules and targeting drugs may offer a synergistic effect in the therapeutic treatment of gastric carcinoma.
The catalytic subunit of the DNA-PKcs and the Ku 70/Ku 80 hetero-dimer (Ku 70/80) are crucial promoters of the error-prone NHEJ pathway. First, DNA-PKcs receives a DNA damage signal and launches a damage response. Next, Ku 70/80 binds to the damaged DNA ends and then attracts DNA-PKcs to form DNA-PK, which can trigger NHEJ repair activities. Accumulating evidence has shown that dysregulation of DNA-PKcs and Ku 70/80 is associated with pathological processes in various tumors.
The authors examined the expression of DNA-PKcs and Ku 70/80 in gastric carcinoma. The study revealed that both DNA-PKcs and Ku 70/80 were increased in gastric carcinoma. The increase of DNA-PKcs expression was negatively correlated with differentiation degree, and Ku 70/80 expression was negatively correlated with H. pylori infection and the clinical stage of patients with GC. The results are interesting and may represent a new horizon for exploring the pathological mechanism of gastric carcinoma.
P- Reviewer Ricci V S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25540] [Article Influence: 1824.3] [Reference Citation Analysis (7)] |
| 2. | Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, La Vecchia C. Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Ann Oncol. 2013;24:2657-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Shrivastav M, Miller CA, De Haro LP, Durant ST, Chen BP, Chen DJ, Nickoloff JA. DNA-PKcs and ATM co-regulate DNA double-strand break repair. DNA Repair (Amst). 2009;8:920-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Lieber MR, Gu J, Lu H, Shimazaki N, Tsai AG. Nonhomologous DNA end joining (NHEJ) and chromosomal translocations in humans. Subcell Biochem. 2010;50:279-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Kloosterman WP, Tavakoli-Yaraki M, van Roosmalen MJ, van Binsbergen E, Renkens I, Duran K, Ballarati L, Vergult S, Giardino D, Hansson K. Constitutional chromothripsis rearrangements involve clustered double-stranded DNA breaks and nonhomologous repair mechanisms. Cell Rep. 2012;1:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Abe T, Ishiai M, Hosono Y, Yoshimura A, Tada S, Adachi N, Koyama H, Takata M, Takeda S, Enomoto T. KU70/80, DNA-PKcs, and Artemis are essential for the rapid induction of apoptosis after massive DSB formation. Cell Signal. 2008;20:1978-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Yang Z, Shu X, Chen L, Chen J, Xie Y, Lu NH. Expression of p53-MDM2 feedback loop related proteins in different gastric pathologies in relation to Helicobacter pylori infection: implications in gastric carcinogenesis. Clin Res Hepatol Gastroenterol. 2012;36:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Hamilton SRAL. Pathology and genetics of tumours of the digestive system. Lyon: IARCP 2000; . |
| 9. | Fléjou JF. [WHO Classification of digestive tumors: the fourth edition]. Ann Pathol. 2011;31:S27-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Bunting SF, Nussenzweig A. End-joining, translocations and cancer. Nat Rev Cancer. 2013;13:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 273] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 11. | Bee L, Fabris S, Cherubini R, Mognato M, Celotti L. The efficiency of homologous recombination and non-homologous end joining systems in repairing double-strand breaks during cell cycle progression. PLoS One. 2013;8:e69061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4401] [Cited by in RCA: 4382] [Article Influence: 273.9] [Reference Citation Analysis (0)] |
| 13. | Furusawa Y, Fujiwara Y, Hassan MA, Tabuchi Y, Morita A, Enomoto A, Kondo T. Inhibition of DNA-dependent protein kinase promotes ultrasound-induced cell death including apoptosis in human leukemia cells. Cancer Lett. 2012;322:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Gibaud A, Vogt N, Brison O, Debatisse M, Malfoy B. Characterization at nucleotide resolution of the homogeneously staining region sites of insertion in two cancer cell lines. Nucleic Acids Res. 2013;41:8210-8219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Alshareeda AT, Negm OH, Albarakati N, Green AR, Nolan C, Sultana R, Madhusudan S, Benhasouna A, Tighe P, Ellis IO. Clinicopathological significance of KU70/KU80, a key DNA damage repair protein in breast cancer. Breast Cancer Res Treat. 2013;139:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Kang MJ, Jung SM, Kim MJ, Bae JH, Kim HB, Kim JY, Park SJ, Song HS, Kim DW, Kang CD. DNA-dependent protein kinase is involved in heat shock protein-mediated accumulation of hypoxia-inducible factor-1alpha in hypoxic preconditioned HepG2 cells. FEBS J. 2008;275:5969-5981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 18. | Amaro HM, Barros R, Guedes AC, Sousa-Pinto I, Malcata FX. Microalgal compounds modulate carcinogenesis in the gastrointestinal tract. Trends Biotechnol. 2013;31:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Kato M, Asaka M. Recent development of gastric cancer prevention. Jpn J Clin Oncol. 2012;42:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Obst B, Wagner S, Sewing KF, Beil W. Helicobacter pylori causes DNA damage in gastric epithelial cells. Carcinogenesis. 2000;21:1111-1115. [PubMed] |
| 21. | Toller IM, Neelsen KJ, Steger M, Hartung ML, Hottiger MO, Stucki M, Kalali B, Gerhard M, Sartori AA, Lopes M. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci USA. 2011;108:14944-14949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Hu H, Zhang Y, Zou M, Yang S, Liang XQ. Expression of TRF1, TRF2, TIN2, TERT, KU70, and BRCA1 proteins is associated with telomere shortening and may contribute to multistage carcinogenesis of gastric cancer. J Cancer Res Clin Oncol. 2010;136:1407-1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Lee HS, Choe G, Park KU, Park do J, Yang HK, Lee BL, Kim WH. Altered expression of DNA-dependent protein kinase catalytic subunit (DNA-PKcs) during gastric carcinogenesis and its clinical implications on gastric cancer. Int J Oncol. 2007;31:859-866. [PubMed] |
| 24. | Lim JW, Kim H, Kim KH. Expression of Ku70 and Ku80 mediated by NF-kappa B and cyclooxygenase-2 is related to proliferation of human gastric cancer cells. J Biol Chem. 2002;277:46093-46100. [PubMed] |
| 25. | Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1257] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 26. | Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 906] [Cited by in RCA: 959] [Article Influence: 56.4] [Reference Citation Analysis (0)] |