Published online Oct 28, 2013. doi: 10.3748/wjg.v19.i40.6834
Revised: August 10, 2013
Accepted: September 3, 2013
Published online: October 28, 2013
Processing time: 147 Days and 15.6 Hours
AIM: To investigate the risk factors and characteristics of hepatocellular carcinoma (HCC) in the patients with drug-resistant chronic hepatitis B (CHB).
METHODS: A total of 432 patients with drug-resistant CHB were analyzed retrospectively from January 2004 to December 2012. The patients were divided into two groups: the HCC group (n = 57) and the non-HCC group (n = 375). Two groups compared using logistic regression for various patients and viral characteristics in order to identify associated risk factors for HCC. Secondarily, patient and tumor characteristics of HCC patients with naïve CHB (N group, n = 117) were compared to the HCC group (R group, n = 57) to identify any difference in HCC characteristics between them.
RESULTS: A significant difference was found for age, platelet count, alpha-fetoprotein (AFP), positivity of HBeAg, seroconversion rate of HBeAg, virologic response, the Child-Pugh score, presence of rtM204I, and the duration of antiviral treatment in non-HCC and HCC group. Cirrhosis, age (> 50 years), HBeAg (+), virologic non-responder status, and rtM204I mutants were independent risk factors for the development of HCC. The R group had lower serum C-reactive protein (CRP) and AFP levels, earlier stage tumors, and a shorter mean tumor surveillance period than the N group. However, the total follow-up duration was not significantly different between the two groups.
CONCLUSION: 13.2% of patients with drug-resistant CHB developed HCC. Age, cirrhosis, YIDD status, HBeAg status, and virologic response are associated with risk of HCC. Patients with drug-resistant CHB and these clinical factors may benefit from closer HCC surveillance.
Core tip: There are few studies on hepatocarcinogenesis in patients with drug-resistant chronic hepatitis B (CHB) or on the characteristics of tumors arising from drug-resistant CHB. In the present study, the cumulative incidence rate of hepatocellular carcinoma (HCC) in patients with drug-resistant CHB was 4.6%, 6.9%, 8.87%, and 11.8% at the end of 1, 2, 3, and 5 years, respectively. Additionally, cirrhosis, age > 50 years, HBeAg (+), YIDD mutations, and a virologic non-responder status were independent risk factors for the development of HCC in CHB patients with drug resistance. Furthermore, there was a trend of poorer survival in patients with HCC arising from resistant CHB than in patients with HCC arising from naive CHB.
- Citation: Jun CH, Hong HJ, Chung MW, Park SY, Cho SB, Park CH, Joo YE, Kim HS, Choi SK, Rew JS. Risk factors for hepatocellular carcinoma in patients with drug-resistant chronic hepatitis B. World J Gastroenterol 2013; 19(40): 6834-6841
- URL: https://www.wjgnet.com/1007-9327/full/v19/i40/6834.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i40.6834
Chronic hepatitis B (CHB) is an important cause of morbidity and mortality worldwide[1,2]. The main goals of therapy for CHB patients are to prevent disease progression and to avoid the development of liver failure and hepatocellular carcinoma (HCC)[3].
The introduction of oral nucleos(t)ide analogue (NA) therapy during the last two decades has revolutionized the CHB treatment[4,5].
Although nucleoside/nucleotide analogues (NAs) are very effective at inhibiting HBV reverse transcriptase, the long-term use of NAs leads to the development of drug resistance. The risk of developing lamivudine (LAM) resistance is 14%-32% in the first year and up to 70% by the fifth year. For LAM resistance, the signature rtM204V/I and rtL180M mutations occur in more than 70% of CHB patients[6,7]. The rtM204V/I, a mutation located at the catalytic YMDD motif[8-10], and the rtL180M mutations serve as compensatory mutations[10]. The rtA181T mutation has also been reported in a substantial proportion of LAM-resistant patients[9]. The risk of developing adefovir (ADV) resistance is 2%-3% and 28%-29% by the second and fifth year of monotherapy treatment in naïve patients, respectively[11,12]. The major ADV-resistant mutants were rtN236T and rtA181T/V[13]. Resistance to entecavir (ETV) is rare when being used to treat naïve patients (1.5% by the fifth year)[5]. However, in the presence of the rtM204I/V mutation, ETV resistance can occur if the rtI169T, rtT184A/F/G/I/L/S, rtS202G/I, or rtM250V mutation also exist[14,15].
It has been demonstrated that in patients with compensated cirrhosis, LAM therapy significantly reduces the risk of liver failure and HCC[16]. However, in patients who have developed LAM resistance, this beneficial effect is drastically compromised[17]. One study reported that old age, male gender, family history of HCC, HBeAg positivity, genotype C, and increased levels of ALT, HBV DNA and HBsAg were risk factors for the development of HCC in chronic hepatitis B patients[18]. However, there are few studies on hepatocarcinogenesis in patients with drug-resistant CHB.
Therefore, we determined the risk factors for the development of HCC in patients with drug-resistant CHB. We also compared the tumor characteristics between HCC patients with drug-resistant CHB and HCC patients with CHB who were treated with antivirals.
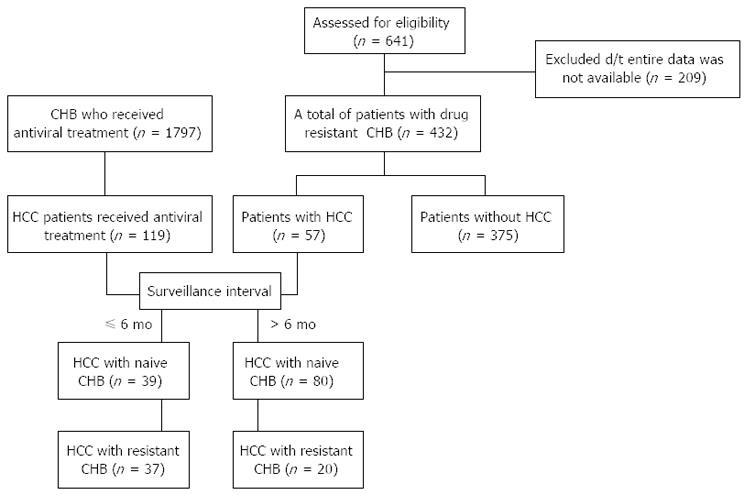
Six hundred and forty-one patients with a documented CHB mutation who had experienced a viral breakthrough at our institution (Chonnam National University Hospital, Gwangju, Korea) between January 2004 and December 2012 were selected for this retrospective study. The 209 patients who were excluded those whose entire set of laboratory data were not available at the end of the follow-up period, those who were co-infected with HIV, hepatitis C or hepatitis D, those who were chronic alcohol drinkers, those who had poor medication compliance and those who had been diagnosed with HCC before the CHB mutation occurred. In total, the data of 432 CHB patients (most of them, genotype C, we did not check the genotype of all patients (n = 90/432), but 100% of the 90 patients whose genotypes were checked were genotype C) with drug resistance were finally evaluated (Figure 1). To determine the risk factors for the development of HCC in patients with drug-resistant CHB, the patients were divided into two groups according to the occurrence of HCC (57 patients with HCC vs 375 patients without HCC).
To compare the tumor characteristics between the HCC patients with drug-resistant CHB (R group) and the HCC patients with CHB treated with antivirals, we selected 119 HCC patients who had received first line antiviral treatment without evidence of drug resistance (N group) during the same period (Figure 1).
The diagnosis of HCC was based on the following guidelines proposed by the Korea Liver Cancer Study Group and the National Cancer Center[19]: (1) nodules > 2 cm in diameter with a typical pattern of HCC in one imaging study or AFP levels > 200 ng/mL; and (2) nodules between 1 and 2 cm in diameter with a coincidental typical vascular pattern in two imaging studies. If these criteria were not met, biopsies were performed. Clinical staging was based on the modified UICC tumor-node-metastasis classification and the Cancer of the Liver Italian Program (CLIP) scores[19,20].
Clinicoradiological and virological variables and the occurrence of HCC were compared between the two groups. The clinicoradiological variables were age, sex, the Child-Pugh score, blood chemistry, AFP level, and the duration of the antiviral treatment. The virological variables were positivity of HBeAg, seroconversion of HBeAg, HBV DNA level, virologic response rate, and mutant type. Informed consent was obtained from all patients about the nature and purpose of the treatment modalities.
The hepatitis B e antigen (HBeAg) and antibodies to HBeAg (anti-HBe) were determined by commercially available radioimmunoassay systems (Abbott Laboratories, Abbott Park, IL, United States). The serum HBV DNA was quantified by the TaqMan® real time polymerase chain reaction (PCR) system (Applied Biosystems, Foster city, CA, United States). The lower limit of detection was 100 copies/mL.
Mutations were identified at the baseline using restriction fragment mass polymorphism (RFMP) analysis.
All patients were examined for HCC by abdominal ultra-sonography (US) and/or abdominal computed tomography (CT) ± serum alpha-fetoprotein (AFP) examination every 3-12 mo. If HCC was suspected, additional procedures, such as CT, magnetic resonance imaging (MRI), angiography, and US guided biopsy, were performed as necessary to confirm the diagnosis.
A primary non-response was defined as a less than 1 log copy/mL decrease in the HBV DNA level from the baseline at 3 mo of treatment. A partial virologic response was defined as a ≥ 1 log copy/mL decline in the HBV DNA level from the baseline, but with a detectable load, at week 24. A complete virologic response was defined as an undetectable HBV DNA level by a sensitive PCR assay[21].
The R group was defined as patients with HCC arising from drug-resistant CHB, and the N group was defined as patients with HCC arising from CHB receiving first line antiviral treatment without evidence of drug resistance.
Subgroup analysis according to tumor surveillance interval, the R’ group was defined as patients with HCC arising from drug-resistant CHB who underwent frequent tumor surveillance (≤ 6 mo), and the N’ group was defined as patients with HCC arising from CHB treated with antivirals who underwent frequent tumor surveillance (≤ 6 mo).
The parametrical data were expressed as the means ± SD when a normal distribution was assumed and were expressed as a median (range) when a normal distribution was not assumed to be present. The group comparisons were performed using the Pearson’s χ2 test and Mann-Whitney U-test.
Logistic regression analysis was performed to evaluate the factors for the development of HCC. Factors that were significant in the univariate analysis were entered into a stepwise multivariate analysis to find the most significant risk factors. The hazard function data were estimated using the Kaplan-Meier curve and compared using the log rank test. A P-value of less than 0.05 was considered to be statistically significant. We performed statistical analysis using SPSS 20.0 (SPSS Inc., Chicago, United States).
The median follow-up duration was 49.3 mo (range: 0-137 mo). The mean age of the patients was 48.48 (range 16-81) years. The mean duration of the antiviral treatment was 30.55 mo (4-120 mo). There were 309 men (71.5%) and 123 women (28.5%) included in the study. Additionally, 127 patients (29.4%) had cirrhosis; 350 patients (81%) were positive for HBeAg; 118 patients (27.3%) were rtM204I-positive; 54 patients (12.5%) were rtM204I/rtL180 M-positive; 39 patients (9.0%) were rtM204I+V/rtL180 M-positive; 125 patients (28.9%) were rtM204 V/rtL180 M-positive; 25 patients (5.8%) were rtA181T-positive; 2 patients (0.4%) were rtA181T/V-positive; 12 patients (2.7%) were rtN236T-positive; and 57 patients (13.2%) were positive for other mutations.
The clinical characteristics of the two groups (HCC-positive group vs HCC-negative group) are shown in Table 1. There were no significant differences between the two groups for gender, initial HBV DNA level, serum aspartate aminotransferase (AST) level, alanine aminotransferase (ALT) level, or total bilirubin or albumin. A significant difference was found for age, platelet count, AFP, positivity of HBeAg, seroconversion rate of HBeAg, virologic response, the Child-Pugh score, presence of rtM204I, and the duration of antiviral treatment.
| Variables | Patients with HCC (n = 57) | Patients without HCC (n = 375) | P-value |
| Gender (male) | 42 (73.7) | 267 (71.2) | 0.755 |
| Age (mean, yr) | 57.44 ± 8.35 | 47.03 ± 12.25 | < 0.001 |
| Presence of cirrhosis | 42 (73.7) | 85 (22.7) | < 0.001 |
| HBeAg positivity | 36 (63.2) | 314 (83.7) | 0.003 |
| Seroconversion of HBeAg | 3 (5.3) | 78 (20.8) | 0.022 |
| Initial HBV DNA (< 2000 IU) | 2 (3.5) | 27 (7.2) | 0.61 |
| Initial HBV DNA (> 20000 IU) | 46 (80.7) | 314 (83.7) | 0.162 |
| Complete virologic response | 11 (19.3) | 135 (36) | 0.02 |
| Partial virologic response | 31 (54.4) | 271 (72.3) | 0.012 |
| Platelet count (103/mm3) (median, range) | 103 (35-380) | 164.5 (34-330) | < 0.001 |
| AST U/L (median, range) | 79.5 (22-707) | 63 (14-1494) | 0.126 |
| ALT U/L (median, range) | 86.5 (11-590) | 82 (9-2280) | 0.770 |
| AFP IU/mL (median, range) | 7.58 (1.54-1890) | 2.8 (0.65-980) | < 0.001 |
| Child-Pugh score | 5.51 ± 0.85 | 5.12 ± 0.71 | 0.002 |
| Viral mutation | |||
| rtM204I | 25 (43.9) | 93 (24.8) | 0.004 |
| rtM204I/rtL180M | 4 (7) | 50 (13.3) | 0.098 |
| rtM204V/rtL180M | 14 (24.6) | 111 (29.6) | 0.492 |
| rtM204V + I/rtL180M | 3 (5.3) | 36 (9.6) | 0.451 |
| rtA181T | 3 (5.3) | 22 (5.9) | 1 |
| rtA181T/V | 1 (1.8) | 1 (0.26) | 0.224 |
| Duration of antiviral treatment (mo) | 24.31 ± 15.28 | 31.43 ± 19.43 | 0.004 |
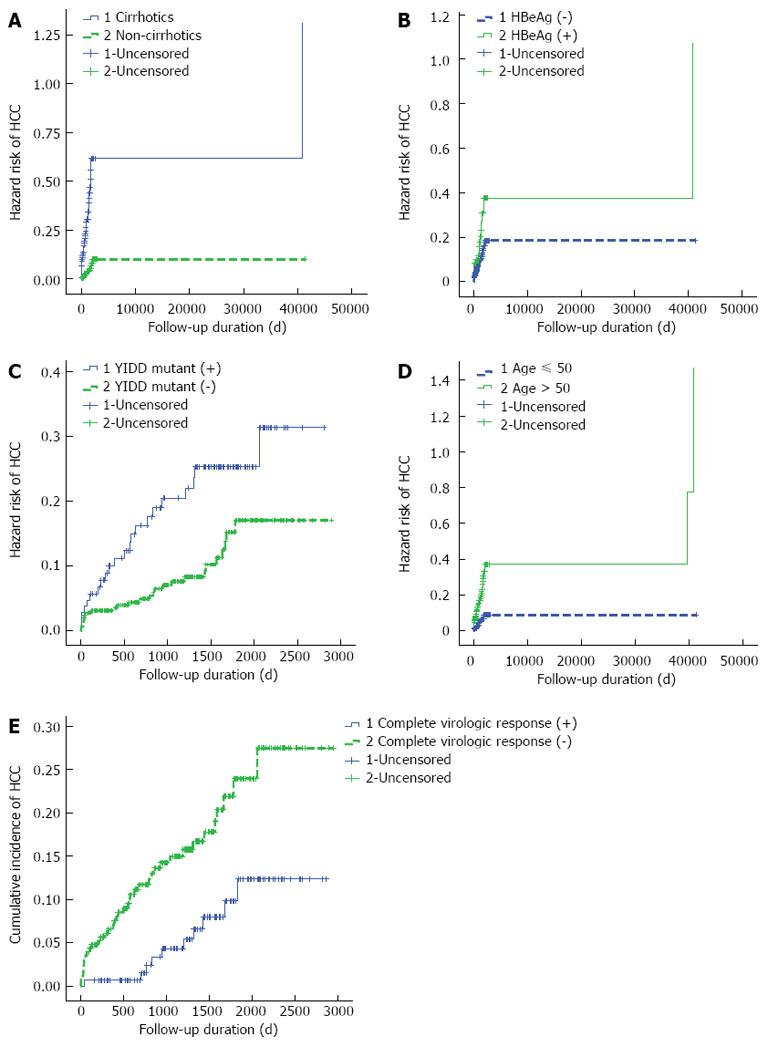
The univariate analysis showed that cirrhosis, age > 50 years, partial virologic response, complete virologic response, the rtM204I (YIDD) mutation, HBeAg (+), platelet count, the Child-Pugh score, and the duration of antiviral treatment were associated with the development of HCC. The multivariate analysis showed that cirrhosis, age >50 years, negative complete virologic response, HBeAg (+), and the rtM204I (YIDD) mutation were independent risk factors for the development of HCC (Table 2).
| Variables | HR | 95%CI | P-value |
| Presence of cirrhosis | 8.196 | 3.623-18.518 | < 0.01 |
| Age > 50 yr | 3.426 | 1.445-8.123 | < 0.01 |
| Complete virologic response | 0.164 | 0.054-0.276 | < 0.01 |
| Positivity of HBeAg | 2.893 | 1.143-7.327 | < 0.05 |
| Presence of rtM204I mutation | 3.412 | 1.54-6.440 | < 0.01 |
The cumulative hepatocarcinogenesis rate in patients with drug-resistant CHB was 4.6%, 6.9%, 8.87%, and 11.8% at the end of 1, 2, 3, and 5 years, respectively.
Kaplan-Meier analysis for the incidence rate of HCC in patients with drug-resistant CHB stratified by the patient and viral factors identified in multivariate analysis is shown in Figure 2.
The cumulative occurrence rate of HCC in cirrhotic patients with drug-resistant CHB was 3.93%, 5.55%, 6.71%, and 9.02% at the end of 1, 2, 3 and 5 years, respectively. The cumulative occurrence rate of HCC in HBeAg (+) patients was 3.0%, 5.09%, 6.01%, and 7.87% at the end of 1, 2, 3 and 5 years, respectively. The cumulative occurrence rate of HCC in rtM204I (YIDD) mutant patients was 2.3%, 3.0%, 4.16%, and 6.01% at the end of 1, 2, 3 and 5 years, respectively. The cumulative occurrence rate of HCC in patients greater than 50 years of age was 6.9%, 11.5%, 20.8%, and 23.1% at the end of 1, 2, 3 and 5 years, respectively.
The cumulative occurrence rate of HCC in virologic non-responder patients was 2.3%, 4.6%, 11.5%, and 23.1% at the end of 1, 2, 3 and 5 years, respectively.
To compare the tumor characteristics between HCC patients with drug-resistant CHB (R group) and HCC patients with CHB treated with antivirals, we selected 119 HCC patients with CHB who received antiviral treatment (N group). The clinical characteristics of the two groups (R group vs N group) are shown in Table 3. There were no significant differences between the two groups for sex, age, presence of cirrhosis, tumor type, serum ALT, the Child-Pugh score, or the total follow-up duration. A significant difference was found for portal vein thrombosis, tumor stage (CLIP score, modified UICC), distant metastasis, platelet count, serum AST, C-reactive protein (CRP), AFP, and the mean tumor surveillance interval. The R group had lower serum CRP and AFP levels and earlier stage tumors than the N group. The total follow-up duration was not significantly different between the two groups.
| Variables | R group (n = 57) | N group (n = 119) | P-value |
| Gender (male) | 42 (73.7) | 100 (84) | 0.158 |
| Age (mean, yr) | 57.44 ± 8.35 | 55.37 ± 10.61 | 0.161 |
| Presence of cirrhosis | 55 (96.5) | 117 (98.3) | 1 |
| Portal vein thrombosis | 8 (14) | 43 (36.1) | 0.004 |
| Vascular invasion | 20 (35.1) | 47 (39.5) | 0.623 |
| Multi-nodular tumor type | 21 (36.8) | 55 (46.2) | 0.259 |
| CLIP score | 0.75 ± 0.85 | 1.67 ± 1.43 | < 0.001 |
| Modified UICC stage (< IVA) | 50 (87.7) | 73 (61.3) | < 0.001 |
| Modified UICC stage (I/II/III/IVA/IVB) | 13/18/19/6/1 | 18/34/21/28/18 | |
| LN involvement | 1 (2.1) | 14 (11.7) | 0.071 |
| Distant metastasis | 0 (0) | 13 (10.9) | 0.019 |
| AST U/L (median, range) | 48 (20-415) | 61 (16-481) | 0.022 |
| ALT U/L (median, range) | 39 (8-532) | 44 (3-203) | 0.616 |
| Platelet count (103/mm3), (median, range) | 103 (23-380) | 126 (24-426) | 0.009 |
| CRP (mg/dL) (median, range) | 0.34 ± 0.37 | 1.71 ± 2.75 | < 0.001 |
| AFP IU/mL (median, range) | 48 (2-40591) | 107 (2-50000) | 0.003 |
| Child-Pugh score | 5.51 ± 0.85 | 5.91 ± 1.32 | 0.344 |
| Duration of anti-viral Tx. (mo) | 20 (0-72) | 6 (1-276) | 0.001 |
| Mean tumor surveillance period (mo) | 6.35 ± 1.69 | 8.71 ± 2.86 | < 0.001 |
| Total follow-up duration (d) | 842.51 ± 702.57 | 801.74 ± 713.24 | 0.721 |
The R group had tumors of an earlier stage and a lower AFP than the N group because of frequent tumor surveillance. Therefore, we performed a subgroup analysis to exclude the frequent tumor surveillance effect on tumor prognosis. Patients were divided into two groups (> 6 mo vs≤ 6 mo) according to the interval of their surveillance.
Thirty-seven patients with drug-resistant CHB (R’ group) and 39 patients with naïve CHB who received antiviral treatment (N’ group) (surveillance interval ≤6 mo) were selected. The clinical characteristics of the two groups (R’ group vs N’ group) are shown in Table 4. There were no significant differences between the two groups for most variables analyzed. A significant difference was found in the serum CRP and AFP levels, the duration of the antiviral treatment, and the total follow-up duration. The R’ group had lower serum CRP and AFP levels than the N’ group at a similar stage. However, the total follow-up duration was shorter in the R’ group than the N’ group.
| Variables | R’ group (n = 37) | N’ group (n = 39) | P-value |
| Gender (male) | 27 (73) | 31 (79.5) | 0.594 |
| Age (mean, yr) | 55.24 ± 7.94 | 56.08 ± 10.3 | 0.695 |
| Presence of cirrhosis | 36 (97.3) | 39 (100) | 1 |
| Portal vein thrombosis | 5 (13.5) | 8 (20.5) | 0.546 |
| Vascular invasion | 11 (29.7) | 8 (20.5) | 0.431 |
| Multi-nodular tumor type | 27 (73) | 27 (69.2) | 0.803 |
| CLIP score | 0.59 ± 0.72 | 0.97 ± 0.98 | 0.061 |
| Modified UICC stage (< IVA) | 34 (91.9) | 35 (89.7) | 1.0 |
| Modified UICC stage (I/II/III/IVA/IVB) | 11/11/12/3/0 | 11/15/9/2/2 | |
| LN involvement | 0 (0) | 2 (5.1) | 0.496 |
| Distant metastasis | 0 (0) | 1 (2.6) | 1 |
| AST U/L (median, range) | 48 (20-141) | 52 (16-481) | 0.283 |
| ALT U/L (median, range) | 38 (8-199) | 44 (11-150) | 0.14 |
| Platelet count (103/mm3) (median, range) | 101 (23-232) | 131 (24-260) | 0.059 |
| CRP (mg/dL) (median, range) | 0.3 (0-2) | 0.5 (0-16) | 0.029 |
| AFP IU/mL (median, range) | 27 (2-737) | 52 (3-16644) | 0.039 |
| Child-Pugh score | 5.51 ± 0.85 | 5.91 ± 1.32 | 0.982 |
| Duration of anti-viral Tx | 21.5 (8-72) | 7 (1-60) | < 0.001 |
| Total follow-up duration | 791.95 ± 643.06 | 1114.23 ± 646.84 | 0.033 |
In addition, 20 patients with drug-resistant CHB and 80 patients with CHB who received antiviral treatment (surveillance interval > 6 mo) were selected. There were no significant differences between the two groups for most of the variables analyzed, including tumor stage, serum CRP and AFP levels, and total follow-up duration.
Antiviral therapy was shown to significantly reduce the risk of liver failure and HCC in patients with compensated cirrhosis and CHB[16]. However, the development of LAM-resistance significantly compromises the effectiveness of this strategy[17]. Until now, there have been few studies about hepatocarcinogenesis in patients with drug-resistant CHB.
Data regarding the characteristics of HCC that arise in patients with drug-resistant CHB are also lacking.
Many countries rely on LAM as first-line antiviral therapy for CHB, and over time many patients will develop drug resistance.
Therefore, it is imperative that clinical and virologic factors associated with the development of HCC in this group of patients be delineated.
In our study, we demonstrated that the cumulative incidence rate of HCC in patients with drug-resistant CHB was 4.6%, 6.9%, 8.87%, and 11.8% at the end of 1, 2, 3, and 5 years, respectively, and that cirrhosis, age > 50 years, HBeAg (+), the rtM204I (YIDD) mutation, and virologic non-responder status were independent risk factors for the development of HCC in CHB patients with drug resistance.
Previous studies primarily identified the rate of hepatocarcinogenesis with varying results. Akuta et al[22] reported a cumulative hepatocarcinogenesis rate in LAM resistant hepatitis B genotype C patients of 2.2%, 5.9%, and 8.1% at the end of 1, 2, and 3 years, respectively, and they indicated that these hepatocarcinogenesis rates were similar to those in cirrhosis patients who had not received antiviral therapy (namely, the high-risk group for HCC development)[23]. Hosaka et al[24] reported that HCC developed in 18 of the 247 (7.3%) patients who had received adefovir add-on lamivudine during a 5-year period. Consistent with the results of other studies, the cumulative incidence rate of HCC in patients with drug-resistant CHB in our study was 4.6%, 6.9%, 8.87%, and 11.8% at the end of 1, 2, 3, and 5 years, respectively; however, our study had a larger number of cases than the other two studies.
Regarding the risk factors that influence the development of HCC, Hosaka et al[24] reported that AST > 70 IU/L, the rtM204I (YIDD) mutation, age > 50 years and cirrhosis were independent risk factors for the development of HCC. Additionally, Yeh et al[9] reported that the rtA181T mutation, age > 50 years, and liver cirrhosis were significantly associated with the occurrence of HCC. In another study[22], the cumulative HBV DNA non-detectable rate and ALT normalization rate were not significantly different with regards to the development of HCC. Our study showed, distinctly from other studies, that cirrhosis, age > 50 years, a negative complete virologic response, the rtM204I (YIDD) mutation, and HBeAg (+) were independent risk factors for the development of HCC. In our study, the rtA181T mutation and HBV DNA level at the time of the documentation of the drug mutation were not associated with the occurrence of HCC. Consistent with other studies on naïve CHB patients[18], HBeAg (+) and a negative complete virologic response were associated with the development of HCC.
HCC arising from drug-resistant CHB (R group) had lower serum CRP and AFP levels and earlier stage tumors than HCC arising from naïve CHB (N group). Although the R group had an earlier stage tumors and lower serum CRP and AFP levels, the total follow-up duration was not significantly different between the two groups. We think these differences may be attributed to more frequent tumor surveillance in the R group. The R group may have had the same survival rates even if the R group had better prognostic factors, such as low tumor stage and low AFP and CRP levels compared to the N group. Therefore, we performed a subgroup analysis to exclude the frequent tumor surveillance effect on the tumor prognosis. The subgroup analysis showed that the patients with HCC arising from drug-resistant CHB who underwent frequent tumor surveillance (≤ 6 mo) (R’ group) had lower serum CRP and AFP levels than patients with HCC arising from CHB treated with antiviral treatment who underwent frequent tumor surveillance (≤ 6 mo) (N’ group) at the same stage. However, the total follow-up duration was shorter in the R’ group than the N’ group, paradoxically. Recent studies reported that an increased serum CRP level is associated with poor prognosis (tumor recurrence after a surgical resection, large tumor size, and poorly defined tumor type) of patients with HCC[25,26]. Additionally, serum AFP is well known as a prognostic factor for patients with HCC[27,28]. These finding suggest that HCC patients with naïve CHB may have a better prognosis than HCC patients with resistant CHB, irrespective of the tumor stage or serum CRP or AFP levels in the frequent tumor surveillance group. That is, patients with HCC arising from resistant CHB may have poorer survival than patients with HCC arising from naïve CHB. These findings may be explained by the R group having poorer liver function because of incomplete viral suppression during follow-up. In fact, hepatic failure was the main cause of death in the R group (data not shown). To us, this is the most striking finding.
However, in the HCC surveillance group, in which the surveillance exceeded 6 mo, there were no significant differences between the two subgroups for most of the variables analyzed, including tumor stage, serum CRP and AFP levels, and total follow-up duration.
The first limitation of our study is that it was a retrospective single center study. Therefore, our results may not be generalizable to other patient populations.
The second limitation is a single data point for various viral markers (e.g., viral DNA) and laboratory values is another limitation since these factors are not static over time. Third, the relationship between risk factors, such as nucleotide substitution and the development of HCC, could not be presented. Fourth, we did not check the genotype of all patients (n = 90/432), but 100% of the 90 patients whose genotypes were checked were genotype C. In South Korea, it is well known that the genotype of CHB patients is almost always genotype C.
Larger, prospective studies will be needed to confirm these findings.
In summary, frequent HCC surveillance may identify early HCC in these high-risk patients, and patients with HCC arising from resistant CHB may have poor survival, irrespective of tumor stage or serum CRP or AFP levels.
In patients with compensated cirrhosis, antiviral therapy significantly reduces the risk of liver failure and hepatocellular carcinoma (HCC). However, in patients who have developed drug resistance, this beneficial effect is drastically compromised. However, there are few studies on hepatocarcinogenesis in patients with drug-resistant chronic hepatitis B (CHB) or on the characteristics of tumors arising from drug-resistant CHB.
Hosaka et al reported that aspartate aminotransferase > 70 IU/L, the rtM204I (YIDD) mutation, age > 50 years and cirrhosis were independent risk factors for the development of HCC. Yeh et al reported that the rtA181T mutation, age > 50 years, and liver cirrhosis were significantly associated with the occurrence of HCC.
Cirrhosis, age > 50 years, HBeAg (+), YIDD mutants, and virologic non-responder status were independent risk factors for the development of HCC in CHB patients with drug resistance, and there was a trend of poorer survival in patients with HCC arising from resistant CHB than in patients with HCC arising from naive CHB.
Frequent HCC surveillance may identify HCC early in these high-risk patients.
This is a large (n = 432) monocenter study aimed at evaluating the relationship between drug-resistant HBV chronic infection and the development of HCC. It is well written.
P- Reviewers Lai Q, Zhang XL S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346:1682-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 337] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 3. | Conjeevaram HS, Lok AS. Management of chronic hepatitis B. J Hepatol. 2003;38 Suppl 1:S90-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Wright TL. Introduction to chronic hepatitis B infection. Am J Gastroenterol. 2006;101 Suppl 1:S1-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Zoulim F. Antiviral therapy of chronic hepatitis B. Antiviral Res. 2006;71:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 589] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 7. | Holomán J, Glasa J. EASL clinical practice guidelines. J Hepatol. 2009;51:821-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Lau DT, Doo E, Park Y, Kleiner DE, Schmid P, Kuhns MC, Hoofnagle JH. Lamivudine for chronic delta hepatitis. Hepatology. 1999;30:546-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Yeh CT, Chien RN, Chu CM, Liaw YF. Clearance of the original hepatitis B virus YMDD-motif mutants with emergence of distinct lamivudine-resistant mutants during prolonged lamivudine therapy. Hepatology. 2000;31:1318-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 183] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Ono SK, Kato N, Shiratori Y, Kato J, Goto T, Schinazi RF, Carrilho FJ, Omata M. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J Clin Invest. 2001;107:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Angus P, Vaughan R, Xiong S, Yang H, Delaney W, Gibbs C, Brosgart C, Colledge D, Edwards R, Ayres A. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology. 2003;125:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 422] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 12. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 681] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 13. | Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1019] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 14. | Colonno RJ, Rose R, Baldick CJ, Levine S, Pokornowski K, Yu CF, Walsh A, Fang J, Hsu M, Mazzucco C. Entecavir resistance is rare in nucleoside naïve patients with hepatitis B. Hepatology. 2006;44:1656-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, Boron-Kaczmarska A, Martin P, Goodman Z, Colonno R. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 17. | Elefsiniotis I, Buti M, Jardi R, Vezali E, Esteban R. Clinical outcome of lamivudine-resistant chronic hepatitis B patients with compensated cirrhosis under adefovir salvage treatment. Importance of HCC surveillance. Eur J Intern Med. 2009;20:478-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, Lu SN, You SL, Wang LY, Chen CJ. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology. 2013;58:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 19. | Park JW. [Practice guideline for diagnosis and treatment of hepatocellular carcinoma]. Korean J Hepatol. 2004;10:88-98. [PubMed] |
| 20. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 21. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1155] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 22. | Akuta N, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Arase Y. Virological response and hepatocarcinogenesis in lamivudine-resistant hepatitis B virus genotype C patients treated with lamivudine plus adefovir dipivoxil. Intervirology. 2008;51:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Kobayashi M, Ikeda K, Hosaka T, Sezaki H, Someya T, Akuta N, Suzuki F, Suzuki Y, Saitoh S, Arase Y. Natural history of compensated cirrhosis in the Child-Pugh class A compared between 490 patients with hepatitis C and 167 with B virus infections. J Med Virol. 2006;78:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Hosaka T, Suzuki F, Kobayashi M, Hirakawa M, Kawamura Y, Yastuji H, Sezaki H, Akuta N, Suzuki Y, Saitoh S. Development of HCC in patients receiving adefovir dipivoxil for lamivudine-resistant hepatitis B virus mutants. Hepatol Res. 2010;40:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Jun CH, Ki HS, Lee KH, Park KJ, Park SY, Cho SB, Park CH, Joo YE, Kim HS, Choi SK. Impact of serum C-reactive protein level on the prognosis of patients with hepatocellular carcinoma undergoing TACE. Clin Mol Hepatol. 2013;19:70-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Zheng Z, Zhou L, Gao S, Yang Z, Yao J, Zheng S. Prognostic role of C-reactive protein in hepatocellular carcinoma: a systematic review and meta-analysis. Int J Med Sci. 2013;10:653-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, Lam KC, Chan AT, Mok TS, Yeo W. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 28. | Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, Ibrahim SM, Sato KT, Baker T, Miller FH. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27:5734-5742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |