Published online Oct 28, 2013. doi: 10.3748/wjg.v19.i40.6774
Revised: August 14, 2013
Accepted: August 17, 2013
Published online: October 28, 2013
Processing time: 139 Days and 12.4 Hours
Restorative proctocolectomy is the most common surgical option for patients with familial adenomatous polyposis (FAP). However, adenomas may develop in the ileal pouch mucosa over time, and even carcinoma in the pouch has been reported. We therefore reviewed the prevalence, nature, and treatment of adenomas and carcinoma that develop after proctocolectomy in the ileal pouch mucosa in patients with FAP. In 25 reports that were reviewed, the incidence of adenomas in the ileal pouch varied from 6.7% to 73.9%. Several potential factors that favor the development of pouch polyposis have been investigated, but many remain controversial. Nevertheless, it seems certain that the age of the pouch is important. The risk appears to be 7% to 16% after 5 years, 35% to 42% after 10 years, and 75% after 15 years. On the other hand, only 21 cases of ileal pouch carcinoma have been recorded in the literature to date. The diagnosis of pouch carcinoma was made between 3 to 20 years (median, 10 years) after pouch construction. Although the risk of malignant transformation in ileal pouches is probably low, it is not negligible, and the long-term risk cannot presently be well quantified. Regular endoscopic surveillance, especially using chromoendoscopy, is recommended.
Core tip: To eliminate the risk of colorectal cancer, the majority of patients with familial adenomatous polyposis (FAP) are treated with restorative proctocolectomy and an ileal pouch-anal anastomosis. However, as these patients are followed-up for longer intervals, it has gradually become recognized that adenomas and adenocarcinomas may develop in the ileal pouch. If the standard-of-care surgery for FAP patients does not eliminate all cancer risk, surgical and follow-up strategies may need to be altered. In this review, we summarize the data from the published English literature regarding the incidence of adenomas and carcinomas in the ileal pouch after proctocolectomy in FAP patients.
- Citation: Tajika M, Niwa Y, Bhatia V, Tanaka T, Ishihara M, Yamao K. Risk of ileal pouch neoplasms in patients with familial adenomatous polyposis. World J Gastroenterol 2013; 19(40): 6774-6783
- URL: https://www.wjgnet.com/1007-9327/full/v19/i40/6774.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i40.6774
Familial adenomatous polyposis (FAP) is an inherited, autosomal-dominant disease caused by a germline mutation of the adenomatous polyposis coli gene (APC)[1]. The phenotype is characterized by the development of hundreds of colorectal adenomas, leading to a 100% lifetime risk of colorectal cancer[2]. For this reason, a prophylactic colectomy is recommended for patients with FAP to prevent the development of colorectal cancer. The main surgical strategy in patients with FAP is restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA)[3-6]. As originally described by Parks et al[7], IPAA included an anal mucosectomy to eliminate the risk of malignancy in the remaining anorectal mucosa. However, many surgeons now prefer to preserve the anal transition zone (ATZ) during the double-stapled IPAA technique because of its simplicity and better functional outcome[8-10]. The trade-off is a risk of neoplasia developing in the retained ATZ mucosa[8], with a 10%-15% incidence of adenoma[8,11-13]. Another widely accepted surgical procedure is colectomy with ileorectal anastomosis (IRA), performed when there are few polyps in the rectum. The major advantage of IRA is preservation of the rectal innervation, with subsequent better quality of life. However, continuing endoscopic surveillance for adenomas in the rectum is necessary, and there is a 13%-25% cumulative risk of rectal cancer after 15-25 years despite surveillance[14-16]. On the other hand, it has been thought that IPAA theoretically eliminates the risk of colorectal cancer and adenomas, and perhaps the need for further lower gastrointestinal surveillance. However, there are recent reports of adenomas or carcinomas developing not only in the residual rectal mucosa or anastomosis after IRA, but also in the ileal pouch mucosa after IPAA[17-39]. In addition, there are several reports of cancers arising from the ileal pouch mucosa, as opposed to the anastomotic site, in patients with FAP[31,36,37,40-47].
The aim of this review is to describe the prevalence, nature, and treatment of adenomas and carcinoma developing in the ileal pouch mucosa after proctocolectomy in patients with FAP.
The basic premise underlying the popularity of IPAA in patients with FAP is that it results in a significantly lower risk of rectal cancer than IRA. Although this is likely to be true, the risk of pouch polyposis and pouch cancer has not been recognized so far. After the advent of pouch surgery, several reports of adenomatous polyps in the pouches of FAP patients have appeared in the literature. In 1982, Beart et al[17] first described a FAP patient with continent ileostomy, in whom a large sessile tubulovillous adenoma and multiple smaller adenomatous polyps developed. Since then, there have been at least 25 reports of ileal pouch adenomas developing in these patients (Table 1). We reviewed only those studies that clearly described adenomas appearing within the ileal pouch mucosa. These included 8 case reports, 7 retrospective studies, and 10 prospective studies. The incidence of adenomas in the ileal pouch varied from 6.7% to 73.9%. This variation in the prevalence of ileal pouch adenomatosis could be due to differences in the way endoscopy was performed in the various studies. Adequacy of bowel preparation, type of instrument employed, use of chromoendoscopy, and other miscellaneous factors influence the detection rates of pouch polyps. Good bowel preparation and a flexible video colonoscope are essential in identifying pouch polyps because most ileal adenomas are flat or sessile, measuring only 1 mm to 3 mm in diameter, and having a morphology different from large bowel adenomas[36]. Therefore, a careful examination of the entire pouch, anal canal, and pouch-anal anastomosis should be performed. Although Polese et al[25] reported the prevalence of ileal pouch adenomas as 6.7%, these investigators used a rigid sigmoidoscope with enema preparation, without chromoendoscopy. These technical factors may explain the low prevalence of ileal pouch adenomas in the study by Polese et al.
| Ref. | Study | Year | Operation | n | Type of pouch | Findings | % of adenoma | Time to neoplasia |
| Beart et al[17] | Case | 1982 | Kock | 1 | Kock pouch | Tubulovillous Adenomas | 6 yr | |
| Wolfstein et al[18] | Case | 1982 | IAA | 2 | Soave | Adenomas in 2 pts | 3 and 7 yr | |
| Shepherd et al[19] | Retro | 1987 | IPAA | 12 | N | Tubular Adenoma in 2 pts | 16.7 | |
| Stryker et al[20] | Case | 1987 | Brooke ileostomy | 1 | Kock pouch | Tubular Adenomas | 12 yr | |
| Nugent et al[16] | Retro | 1993 | IPAA | 38 | N | Tubular Adenoma in 5 pts | 13.2 | 4 yr (1-7) |
| Bertoni[21] | Pros | 1995 | IAA | 3 | N | Tubular Adenoma in 2 pts | 66.7 | 53.7-75.4 mo |
| Wu et al[22] | Pros | 1998 | IPAA | 26 | S/J-pouch 9/16, 1 N | Tubular Adenoma in 11 pts | 42.3 | 1-14 yr |
| Valle et al[23] | Case | 2001 | IPAA | 5 | N | Adenomas | 20 | 5 yr |
| Thompson-Fawcett et al[24] | Pros | 2001 | Kock 5, IPAA 28 | 33 | N | Adenoma in 14 pts (with microadenoma 20 pts) | 42.4 (60.6) | 7 yr (1-19) |
| Parc et al[4] | Pros | 2001 | IPAA | 85 | J-pouch | 30 pts (28 grossly visible, 2 microadenoma) | 35.3 | Cumulative risk of 7%, 35%, and 75% at 5, 10, and 15 yr |
| Polese et al[25] | Retro | 2002 | IPAA | 46 | S/W/J-pouch 2/1/43, Kock 4 | Adenoma in 2 of 30 pts | 6.7 | risk of Adenoma after 8 yr; 20%, (9-11 yr) |
| Beveridge et al[26] | Case | 2004 | IPAA | 2 | J-pouch 1, Kock 1 | 2 large VA, VA | 4-10 yr | |
| Vrouenraets et al[27] | Case | 2004 | IPAA | 1 | J-pouch | Adenomas with focal severe dysplastic change | 6 yr | |
| Groves et al[28] | Pros | 2005 | Kock 4, IPAA 56 | 60 | W/J-pouch 13/43, Kock 4 | Mild dysplasia 23, more advanced 11 | 56.7 | 6 yr (1-17) |
| Nilubol et al[29] | Pros | 2007 | IPAA | 10 | N | Tubular Adenoma in 1of 9 pts | 11.1 | 11.3 yr |
| Moussata et al[30] | Retro | 2008 | IPAA | 23 | N | Low-grade 16, high-grade 1 | 73.9 | 4.76 yr (1-14) |
| Friederich et al[31] | Pros | 2008 | IPAA | 212 | N | Adenoma 74 pts, AAP 25 pts | 46.7 | Cumulative risk of 16% and 42.2% at 5 and 10 yr |
| Schulz et al[32] | Pros | 2008 | IPAA | 35 | N | Low-grade 8 | 22.8 | mean of 5 yr |
| Tajika et al[33] | Retro | 2009 | Kock 8, IPAA 16 | 24 | J-pouch | 16 pts, (advanced 1, carcinoma 2) | 66.7 | Cumulative risk of 13%, 43%, and 72% at 5, 10, and 20 yr |
| Kang et al[34] | Case | 2010 | Kock | 2 | Kock pouch | 2 (the largest one is 15mm in size) | ||
| Banasiewicz et al[35] | Pros | 2011 | IPAA | 165 | J-pouch | Low-grade 13, high-grade 8, neoplasia 5 | 15.8 | Cumulative risk of 50%, Low-grade at 15 yr, high-grade at 17.5 yr, neoplasia 18.5 yr |
| Tonelli et al[36] | Pros | 2012 | IPAA | 69 | S/J-pouch 25/29, SIMM 15 | Adenoma 25 pts, carcinoma 2 pts | 39.1 | Cumulative risk of 28.5% at 5 yr |
| Makni et al[37] | Case | 2012 | IPAA | 1 | J-pouch | Adenoma and carcinoma | 10 yr | |
| Wasmuth et al[38] | Retro | 2013 | IPAA | 61 | N | 14 pts | 23 | Estimated cumulative rate of first Adenoma diagnosed was 38% |
| Pommaret[39] | Retro | 2013 | IPAA | 118 | J-pouch | 57 pts (12 advanced Adenomas) | 48.3 | 15 yr |
The only meaningful estimates of adenoma incidence have come from prospective studies, with all 10 prospective studies showing that the incidence of pouch adenomas increases with the follow-up duration[4,21,22,24,28,29,31,32,35,36]. The risk appears to be 7% to 16% after 5 years, 35% to 42% after 10 years, and 75% after 15 years. The great majority of pouch polyps described in the literature are small, tubular adenomas with mild atypia. The incidence of adenoma with advanced pathology (size > 1 cm, villous pattern, or moderate-to-severe dysplasia) is less. According to Banasiewicz et al[35], the estimated frequency of low-grade dysplasia 4 years after IPAA surgery is 4%, and increases to 50% after 15 years. The same percentage of patients, but with high-grade dysplasia, develop high-grade dysplasia 2.5 years later, that is to say, 17.5 years after IPAA, and with neoplasia 18.5 years after the above procedure. The full impact of pouch polyposis will not be fully understood until most pouch patients reach a follow-up duration of 20 to 40 years[48].
At present it does not seem possible to predict who is at risk for developing polyps in the pouch. Some studies show that there is no apparent high-risk phenotype for the development of ileal polyps[22,24,30,33]. However, other studies describe risk factors that favor the development of pouch polyposis. Parc et al[49] observed that patients with pouch polyps are younger, and have a longer follow-up period since IPAA, than patients without pouch polyps. A more aggressive disease requiring earlier surgery could explain these features. However, Groves et al[28] analyzed the risk factors by using logistic regression, and found that age was a more significant predictor of pouch adenomas than the follow-up period, sex, or type of primary or secondary procedures. Groves et al[28] reported that, in their experience, all patients older than 60 years will develop polyps in the pouch. Tonelli et al[36] reported that an age of more than 50 years was associated with pouch adenomas, but not sex or elapsed time since restorative proctocolectomy.
Several studies found that the severity of duodenal polyposis was related to the presence of pouch adenomatous polyps. In a multivariate analysis of 118 FAP patients who had undergone surgery, Pommaret et al[39] discovered that the presence of advanced duodenal adenomas was an independent risk factor for the development of pouch adenomas, in addition to follow-up duration. Tonelli et al[36] found that patients affected by pouch adenomas had high polyp counts (> 1000) at colectomy, as well as duodenal adenomas.
Several potential factors that favor the development of pouch polyposis have been investigated; a number of them remain controversial, although it seems certain that the age of the pouch is important.
The mucosa of the ileal pouch may be subjected to not only the tumorigenic consequences of APC gene mutations[50], but also to luminal factors due to fecal stasis, which may also exert an important effect. Fecal stasis, such as occurs in a reconstructed pouch, may promote neoplastic changes in the ileal mucosa. Several authors have implicated colonic metaplasia of the ileal mucosa as a precursor for the development of ileal adenomas[19,51,52], and even carcinomas in the surgically constructed pouches of patients with FAP[53-55]. Colonic metaplasia was frequently recognized even in earlier descriptions of the changes observed in the ileal pouch mucosa. Some authors have considered colonic metaplasia as an adaptive response of the ileal pouch to its role as a neorectum[18,19,56,57]. Further investigations have shown that colonic transformation is only partial. Small-bowel brush border disaccharidase activity is preserved, as is the ability to absorb vitamin B12, D-xylose, phenylalanine, and bile acids[52,58-60]. The mucosal changes described as colonic metaplasia are likely a response to chronic inflammation caused by changes in the luminal contents due to stasis. In FAP, these changes may, at least in theory, favor the development of adenomas in a region of the gut where they are not usually observed. There is an increase in the concentration of luminal short chain fatty acids to levels that are seen in the colon[61], an increase in anaerobic bacterial counts with a more colonic type flora[62,63], and increased deconjugation and dehydroxylation of bile acids by the anaerobic bacteria[64]. In particular, deoxycholic acid and lithocholic acid, which are known carcinogens, have concentrations several times higher in an ileal pouch than in an end ileostomy[65]. On the other hand, reduction of glutathione S-transferase (GST) detoxification activity in the pouch compared with the afferent ileal loop after IPAA may promote tumorigenesis[64].
FAP develops due to a dominant autosomal mutation of the APC gene in more than 80% of patients[65]. Recently, it has been discovered that a biallelic mutation of the MUTYH gene might exist in 5% of patients with colorectal polyposis and 20% of FAP patients, with no APC mutation found[66]. Many researchers have investigated APC gene mutations in pouch patients with FAP, although none has demonstrated obvious genotype-phenotype correlations that would predict the development of pouch adenomas[28,30,36,49]. Hence, the available evidence suggests that systematic surveillance of all patients who undergo IPAA is necessary. Targeted surveillance of a defined subgroup of patients is currently not feasible.
There are different pouch configurations. Parks et al[7] originally devised a triple-limb S-shaped pouch. This pouch was relatively complicated to construct, and suffered from kinking of the efferent limb if it was left too long[67]. Alternative designs have included the high-capacity W-pouch, the H-pouch and the J-pouch. The majority of surgeons now favor the J-pouch due to ease of construction, economical use of the terminal ileum, and reliable emptying[68]. Functional results are equal to those of other reservoir designs[69-71]. The pouch is formed from the terminal 40 cm of ileum, using several applications of a linear-cutting stapler to join the anti-mesenteric borders of two 20-cm ileal limbs.
Several authors have shown that there is no apparent relationship between the development of pouch polyps and the type of ileal pouch construction[36] or the suture used (hand-sewn or stapled)[25,28,36]. However, other authors have reported that patients with a stapled IPAA are at a significantly increased risk of developing adenomas at the anastomotic site: 1.5% to 20.9% vs 27% to 66% for hand-sewn and stapled anastomoses, respectively[9,11,22,36,72,73]. These data suggest that a hand-sewn IPAA may be a preferable strategy for decreasing the occurrence of adenomas at the anastomotic ileo-anal site. Recently, Wasmuth et al[38] evaluated the differences between adenoma formation at the anastomotic site and in the ileal pouch after IPAA, with or without mucosectomy. These investigators found that an occurrence of adenomas at the anastomotic site was significantly reduced after mucosectomy. However, there was no difference in the occurrence of ileal pouch adenomas between patients who underwent mucosectomy and those who retained a rectal mucosal remnant (8/39 vs 6/22; P = 0.57)[38].
In patients with ulcerative colitis, concern about the risk of neoplasia in ileal pouches was raised after observing a combination of histologic changes in the ileal mucosa of the pouch, including villous atrophy, inflammation and metaplasia[74-76]. These transformations in the ileal pouches are likely caused by the chronic inflammatory state. The inflammatory process in pouchitis may lead to dysplasia[47] and loss of heterozygosity, consistent with precancerous lesions of the colon[77]. Thus, a dysplasia-to-neoplasia progression can occur in ileal pouches and can lead to cancer of the pouch. The cumulative risk of pouchitis is up to 50% in patients with ulcerative colitis[78-81], with most patients experiencing at least one episode of pouchitis during the first ten years after surgical pouch construction. In contrast, the pouchitis rate is below 25% in patients with FAP[4-6,29,33,35]. Banasiewicz et al[35] analyzed the frequency and progression of dysplasia and inflammation in the intestinal pouch of FAP patients after restorative proctocolectomy. Although these authors diagnosed pouchitis in 20.6% of patients after restorative proctocolectomy, no relationship was found between pouchitis and pouch dysplasia in FAP patients.
Although it is currently recognized that adenomas may develop in the ileal pouch, the risk of adenomas occurring in the afferent ileal loop above the pouch is unclear. The incidence of adenomas above the IPAA pouch was rarely recognized previously, and it seemed to be low, with reported figures ranging from 4% to 16%[22,28,33,82]. The majority of pre-pouch ileal adenomas have measured 4 mm or smaller. Pommaret et al[39] reported that only nine (6.5%) of 118 patients had afferent ileal loop adenomas after an IPAA. The only independent predictive factor for the occurrence of afferent ileal loop adenoma was found to be the presence of pouch adenomas (OR: 2.16; 95%CI: 0.17-26.98; P = 0.007). Pommaret et al[39] concluded that because afferent ileum loop adenomas are rare and have an unclear pathologic significance, there is no justification for their systematic search, particularly among patients without any duodenal or pouch adenomas. In cases of extensive pouch polyposis with a significant cancer risk, this viewpoint could allow clinicians to consider resection of the adenomatous pouch, with construction of a new one using the afferent ileum.
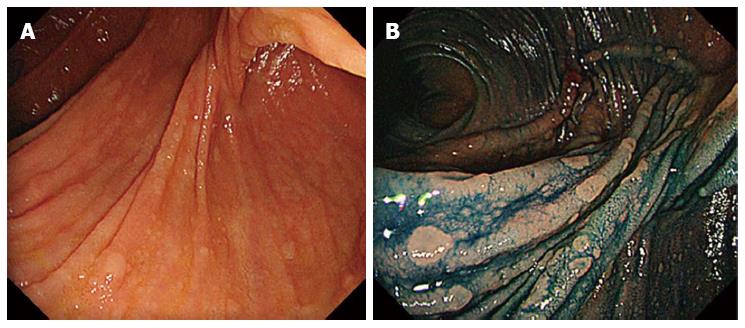
Saurin et al[83] described methods of surveillance and therapeutic indications in FAP patients following colectomy. Although there are no validated data in the literature, on the basis of expert opinion, endoscopic surveillance is performed at 6 mo, 1 year, and then every 2 years after surgery. In the presence of polyps with high-grade dysplasia, and/or polyps > 1 cm size, and/or presence of large polyp number (> 30), surveillance should be repeated every 6 mo[83]. Many authors have performed pouch endoscopy every 6-12 mo after surgery[30,33,35,36,38,84]. Optimum bowel preparation and the use of indigo carmine surface staining are necessary[31-33,35]. The main utility of chromoendoscopy is to highlight the small lymphoid lesions that are characteristic of the terminal ileum in FAP patients, and to distinguish flat polyps (Figure 1). In the experience of the Dutch Registry[31], indigo carmine chromoendoscopy significantly increases the detection rates of adenomas < 5 mm in size. Investigators with the Dutch Registry found that 75.7% of FAP patients harbored adenomas in the pouch at a median follow-up duration of 8 years after IPAA[31]. Because some patients have a stricture at the anal anastomosis, a pediatric colonoscope or gastroscope may sometimes be required[35].
According to Saurin et al[83], no systematic endoscopic treatment of adenomas of the ileal pouch or afferent loop can be recommended. For large adenomas (> 1 cm), or in patients with high-grade dysplasia, endoscopic resection must be considered; however, a skilled team is needed because of the thin ileal mucosa[83]. Our current strategy in patients with IPAA is regular follow-up starting at 1 year after surgery, and then every year thereafter[33]. If adenomas are observed in the pouch, we recommend endoscopic resection or argon plasma coagulation where feasible, and then follow-up every 6 mo. Other reports[30,35,36,84] also describe polypectomy of large polyps, and ablation by fulguration or electrocoagulation for small lesions. In patients having extensive pouch polyposis with no possibility of endoscopic treatment, together with invasive cancer, pouch excision and terminal ileostomy has to be considered[40,83].
Although there have been some reports suggesting the efficacy of nonsteroidal anti-inflammatory drugs (NSAIDs) in suppressing the development of ileal pouch adenomas[32,48,85], this has not been systematically studied[34].
The progression from a dysplastic lesion in the ileal pouch to invasive carcinoma appears to be rare, occurring in no more than 1% of patients with ileal pouches[31]. However, in the past few years, several cases of carcinoma have been observed after IPAA, although the majority of them occurred at the level of the anal canal[8,9,13,27,38,86-91]. Patients with either hand-sewn or stapled IPAA are at risk for developing a carcinoma in any residual rectal mucosa that harbors dysplasia or is prone to dysplasia. To date, only 21 cases of ileal pouch carcinoma have been recorded in the literature (Table 2). No relationship has been found between the occurrence of pouch carcinoma and the shape (J or S) of the pouch, or the type (hand-sewn or stapled) of IPAA[36]. These pouch cancers have clearly appeared in the ileal pouch, and not in the ATZ. The time elapsed between pouch construction and diagnosis of pouch carcinoma has been between 3 and 20 years (median, 10 years).
| Ref. | Year | Sex | Operation | Type of pouch | Staging of initial surgery | Age of pouch (yr) | Shape | Size (mm) | Staging of pouch cancer | No. of pouch polyps | Time to cancer (yr) | Outcome | Interval since last endoscopy (yr) |
| Bassuini et al[40] | 1996 | M | IPAA | /handsewn | No cancer | 28 | Large polypoid | N | T3,N+ | N | 3 | N | No follow-up |
| Palkar et al[41] | 1997 | F | IPAA | J-pouch handsewn | No cancer | 39 | Large polypoid | 40 × 35 | T4N0 | Exist | 4.7 | Alive | 0.3 |
| Kim et al[42] | 1997 | N | N | N | N | N | N | N | N | N | N | N | N |
| Cherki et al[43] | 2003 | F | IPAA | J-pouch handsewn | TisN0M0 | 35 | N | N | T3N1M1 | N | 3.5 | Died | 0.5 |
| Linehan et al[44] | 2007 | M | IPAA | /double stapled | Dukes A | 30 | N | N | T3N0 | N | 9 | Alive | No follow-up |
| Friederich et al[31] | 2008 | M | IPAA | /handsewn | No cancer | 21.3 | N | N | Dukes C | 0 | 14 | N | 4.4 |
| M | IPAA | /stapled | No cancer | 26.7 | N | N | Dukes B | 0 | 10 | N | 2.1 | ||
| M | IPAA | /handsewn | No cancer | 16 | N | N | Dukes B | N | 16 | N | No follow-up | ||
| F | IPAA | /stapled | No cancer | 29.6 | N | N | Dukes B | exist | 6 | N | 0.6 | ||
| Tajika et al[45] | 2009 | F | IPAA | J-pouch/handsewn | TisN0M0 | 46 | Type 2 | 30 × 25 | T4N2M0 | 0 | 8.6 | Died 3Y | 0.75 |
| M | Kock | Kock/handsewn | No cancer | 48 | Type 1 | 40 × 35 | T3N0M0 | 10 < | 20 | Died by U | No follow-up | ||
| Ault et al[46] | 2009 | M | IPAA | S-pouch/handsewn | Four cancer | 61 | ND | 20-30 | T2N1 | N | 11 | Died by U | 6 |
| F | IPAA | ND | No cancer | 40 | Type 1 | N | N | N | 13 | meta | No follow-up | ||
| Lee et al[47] | 2009 | F | IPAA | J-pouch/ | T2N0 | 56 | Type 2 | 30 × 25 | T3N2 | 0 | 7 | meta 2Y | 4 |
| Banasiewicz et al[35] | 2011 | N | IPAA | J-pouch/ | N | N | N | N | N | N | N | N | N |
| N | IPAA | J-pouch/ | N | N | N | N | N | N | N | N | N | ||
| N | IPAA | J-pouch/ | N | N | N | N | N | N | N | N | N | ||
| N | IPAA | J-pouch/ | N | N | N | N | N | N | N | N | N | ||
| Tonelli et al[36] | 2012 | M | IPAA | S-pouch/handsewn | No cancer | 26 | Type 2 | 20 < | T3N0M0 | ND | 3 | Died 6 mo | 1 |
| F | IPAA | S-pouch/handsewn | TisN0M0 | 47 | IIa + IIc | N | T2N0M0 | 0 | 11 | Alive at 56 mo | 0.5 | ||
| Makni et al[37] | 2012 | F | IPAA | J-pouch/ | No cancer | 26 | N | 20 | N | Many | 10 | Died 1Y+ | 0.66 |
It is noteworthy that in at least seven patients, the development of advanced cancer was detected within a very short interval-within 1 year since last pouch endoscopy[25,36]. Furthermore, in four of the patients, ileal polyps were not found during endoscopic follow-up until the development of pouch carcinoma. It seems that neoplasia that appears in the ileal pouch may not always follow the classic adenoma-carcinoma sequence. We strongly recommend a strict surveillance program for FAP patients, including annual flexible colonoscopy, irrespective of the phenotype and genotype.
In a review of 12 studies containing 1002 patients with FAP (53.4% IPAA, 46.6% IRA), Aziz et al[92] showed that bowel frequency, nocturnal defecation, and incontinence rates were significantly less in IRA patients, although fecal urgency was less among IPAA patients. There was no significant difference between IPAA and IRA in terms of sexual dysfunction, dietary restrictions, or postoperative complications. In their review, Aziz et al[92] could not identify any malignancies in IPAA patients, and rectal cancer was a diagnosis only in the IRA patients (5.5%). At the present time, IPAA anastomosis is recommended by a majority of surgical teams as the preferred option for FAP patients[4-6,10,92,93]. Although cancer formation after IPAA in patients with FAP may be rare, it is of concern that several of the recently reported patients had an advanced stage at diagnosis, with poor outcome.
Recent reports of a high frequency of adenomas after IPAA, together with favorable functional outcomes in patients who underwent IRA, may lead to reconsideration of the latter surgical option for some FAP patients, especially when quality of life and fertility criteria are taken into account[30]. In clinical practice, the results of preoperative evaluation of the rectal stump using indigo carmine chromoendoscopy are a major deciding factor. A limited number (< 10-20) of rectal polyps without any cancer would lead to preservation of the rectal stump in a majority of patients[94,95]. However, with the availability of better endoscopic instruments and resection techniques, and the possibility of enhanced post-operative surveillance, > 20 rectal polyps and/or non-invasive cancers can now also be managed endoscopically. So, there is a possibility that the criteria for IRA will expand in the near future. Of course, based on clinical and genetic data, a stepwise surgical strategy with a primary IRA followed at a later age by a secondary proctectomy and IPAA could be proposed[5].
An ongoing multicenter study in Japan is being conducted by Ishikawa et al[96] under the title “Intervention trial for colorectal cancer prevention by endoscopic polypectomy in patients with familial adenomatous polyposis” (UMIN000009365). The aim of this study is to evaluate the usefulness and safety of thorough endoscopic polypectomy in FAP patients who have (or had) ≥ 100 colonic adenomas and who refuse surgery, as well as post-operative patients who have (or had) ≥ 100 colonic adenomas and who have ≥ 10 cm of remnant colon.
The development of adenomas with high-grade dysplasia and carcinoma in the ileal pouch is an important issue because the choice between IPAA and IRA is based mainly on the expected low risk of cancer development after the former surgery. Although the risk of malignant transformation in ileal pouches is probably low, it is not negligible, and the long-term risk cannot presently be well quantified. IPAA will not prevent cancer development in the terminal remnant intestine, and patients who undergo IPAA require regular follow-up similar to patients who receive IRA. A detailed analysis of the phenotypes and mutations in the APC gene of patients with FAP may allow tailoring of the surgical options in the future.
P- Reviewers Izbicki JR, Leedham SJ S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1920] [Cited by in RCA: 1863] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 2. | Bussey HJ, Veale AM, Morson BC. Genetics of gastrointestinal polyposis. Gastroenterology. 1978;74:1325-1330. [PubMed] |
| 3. | Jagelman DG. Choice of operation in familial adenomatous polyposis. World J Surg. 1991;15:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Parc YR, Olschwang S, Desaint B, Schmitt G, Parc RG, Tiret E. Familial adenomatous polyposis: prevalence of adenomas in the ileal pouch after restorative proctocolectomy. Ann Surg. 2001;233:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Kartheuser AH, Parc R, Penna CP, Tiret E, Frileux P, Hannoun L, Nordlinger B, Loygue J. Ileal pouch-anal anastomosis as the first choice operation in patients with familial adenomatous polyposis: a ten-year experience. Surgery. 1996;119:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Nyam DC, Brillant PT, Dozois RR, Kelly KA, Pemberton JH, Wolff BG. Ileal pouch-anal canal anastomosis for familial adenomatous polyposis: early and late results. Ann Surg. 1997;226:514-519; discussion 514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978;2:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 913] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 8. | Remzi FH, Church JM, Bast J, Lavery IC, Strong SA, Hull TL, Harris GJ, Delaney CP, O’Riordain MG, McGannon EA. Mucosectomy vs. stapled ileal pouch-anal anastomosis in patients with familial adenomatous polyposis: functional outcome and neoplasia control. Dis Colon Rectum. 2001;44:1590-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Ozdemir Y, Kalady MF, Aytac E, Kiran RP, Erem HH, Church JM, Remzi FH. Anal transitional zone neoplasia in patients with familial adenomatous polyposis after restorative proctocolectomy and IPAA: incidence, management, and oncologic and functional outcomes. Dis Colon Rectum. 2013;56:808-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Fazio VW, Kiran RP, Remzi FH, Coffey JC, Heneghan HM, Kirat HT, Manilich E, Shen B, Martin ST. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 526] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 11. | van Duijvendijk P, Vasen HF, Bertario L, Bülow S, Kuijpers JH, Schouten WR, Guillem JG, Taat CW, Slors JF. Cumulative risk of developing polyps or malignancy at the ileal pouch-anal anastomosis in patients with familial adenomatous polyposis. J Gastrointest Surg. 1999;3:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | von Roon AC, Tekkis PP, Clark SK, Heriot AG, Lovegrove RE, Truvolo S, Nicholls RJ, Phillips RK. The impact of technical factors on outcome of restorative proctocolectomy for familial adenomatous polyposis. Dis Colon Rectum. 2007;50:952-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | von Roon AC, Will OC, Man RF, Neale KF, Phillips RK, Nicholls RJ, Clark SK, Tekkis PP. Mucosectomy with handsewn anastomosis reduces the risk of adenoma formation in the anorectal segment after restorative proctocolectomy for familial adenomatous polyposis. Ann Surg. 2011;253:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Bess MA, Adson MA, Elveback LR, Moertel CG. Rectal cancer following colectomy for polyposis. Arch Surg. 1980;115:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | De Cosse JJ, Bülow S, Neale K, Järvinen H, Alm T, Hultcrantz R, Moesgaard F, Costello C. Rectal cancer risk in patients treated for familial adenomatous polyposis. The Leeds Castle Polyposis Group. Br J Surg. 1992;79:1372-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Nugent KP, Phillips RK. Rectal cancer risk in older patients with familial adenomatous polyposis and an ileorectal anastomosis: a cause for concern. Br J Surg. 1992;79:1204-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 100] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Beart RW, Fleming CR, Banks PM. Tubulovillous adenomas in a continent ileostomy after proctocolectomy for familial polyposis. Dig Dis Sci. 1982;27:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Wolfstein IH, Bat L, Neumann G. Regeneration of rectal mucosa and recurrent polyposis coli after total colectomy and ileoanal anastomosis. Arch Surg. 1982;117:1241-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Shepherd NA, Jass JR, Duval I, Moskowitz RL, Nicholls RJ, Morson BC. Restorative proctocolectomy with ileal reservoir: pathological and histochemical study of mucosal biopsy specimens. J Clin Pathol. 1987;40:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 245] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Stryker SJ, Carney JA, Dozois RR. Multiple adenomatous polyps arising in a continent reservoir ileostomy. Int J Colorectal Dis. 1987;2:43-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Bertoni G, Sassatelli R, Nigrisoli E, Tansini P, Roncucci L, Ponz de Leon M, Bedogni G. First observation of microadenomas in the ileal mucosa of patients with familial adenomatous polyposis and colectomies. Gastroenterology. 1995;109:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Wu JS, McGannon EA, Church JM. Incidence of neoplastic polyps in the ileal pouch of patients with familial adenomatous polyposis after restorative proctocolectomy. Dis Colon Rectum. 1998;41:552-556; discussion 552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Valle RD, de’Angelis GL. Pouch adenomas after ileal pouch-anal anastomosis for familial adenomatous polyposis. Dis Colon Rectum. 2001;44:456-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Thompson-Fawcett MW, Marcus VA, Redston M, Cohen Z, Mcleod RS. Adenomatous polyps develop commonly in the ileal pouch of patients with familial adenomatous polyposis. Dis Colon Rectum. 2001;44:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Polese L, Keighley MR. Adenomas at resection margins do not influence the long-term development of pouch polyps after restorative proctocolectomy for familial adenomatous polyposis. Am J Surg. 2003;186:32-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Beveridge IG, Swain DJ, Groves CJ, Saunders BP, Windsor AC, Talbot IC, Nicholls RJ, Phillips RK. Large villous adenomas arising in ileal pouches in familial adenomatous polyposis: report of two cases. Dis Colon Rectum. 2004;47:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Vrouenraets BC, Van Duijvendijk P, Bemelman WA, Offerhaus GJ, Slors JF. Adenocarcinoma in the anal canal after ileal pouch-anal anastomosis for familial adenomatous polyposis using a double-stapled technique: report of two cases. Dis Colon Rectum. 2004;47:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Groves CJ, Beveridge lG, Swain DJ, Saunders BP, Talbot IC, Nicholls RJ, Phillips RK. Prevalence and morphology of pouch and ileal adenomas in familial adenomatous polyposis. Dis Colon Rectum. 2005;48:816-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Nilubol N, Scherl E, Bub DS, Gorfine SR, Marion J, Harris MT, Kornbluth A, Lichtiger S, Rubin P, George J. Mucosal dysplasia in ileal pelvic pouches after restorative proctocolectomy. Dis Colon Rectum. 2007;50:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Moussata D, Nancey S, Lapalus MG, Prost B, Chavaillon A, Bernard G, Ponchon T, Saurin JC. Frequency and severity of ileal adenomas in familial adenomatous polyposis after colectomy. Endoscopy. 2008;40:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Friederich P, de Jong AE, Mathus-Vliegen LM, Dekker E, Krieken HH, Dees J, Nagengast FM, Vasen HF. Risk of developing adenomas and carcinomas in the ileal pouch in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Schulz AC, Bojarski C, Buhr HJ, Kroesen AJ. Occurrence of adenomas in the pouch and small intestine of FAP patients after proctocolectomy with ileoanal pouch construction. Int J Colorectal Dis. 2008;23:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Tajika M, Nakamura T, Nakahara O, Kawai H, Komori K, Hirai T, Kato T, Bhatia V, Baba H, Yamao K. Prevalence of adenomas and carcinomas in the ileal pouch after proctocolectomy in patients with familial adenomatous polyposis. J Gastrointest Surg. 2009;13:1266-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Kang JM, Byeon JS, Park JH, Ahn JY, Ko OB, Myung SJ, Yang SK, Kim JH. [Two cases of multiple adenomas in the ileal pouch after total proctocolectomy in patients with familial adenomatous polyposis]. Korean J Gastroenterol. 2010;56:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Banasiewicz T, Marciniak R, Kaczmarek E, Krokowicz P, Paszkowski J, Lozynska-Nelke A, Gronek P, Plawski A, Drews M. The prognosis of clinical course and the analysis of the frequency of the inflammation and dysplasia in the intestinal J-pouch at the patients after restorative proctocolectomy due to FAP. Int J Colorectal Dis. 2011;26:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Tonelli F, Ficari F, Bargellini T, Valanzano R. Ileal pouch adenomas and carcinomas after restorative proctocolectomy for familial adenomatous polyposis. Dis Colon Rectum. 2012;55:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Makni A, Chebbi F, Rebai W, Ayadi S, Fekih M, Jouini M, Kacem M, Ben Safta Z. Adenocarcinoma arising in the ‘J’ pouch after total proctocolectomy for familial polyposis coli. Tunis Med. 2012;90:80-81. [PubMed] |
| 38. | Wasmuth HH, Tranø G, Myrvold HE, Aabakken L, Bakka A. Adenoma formation and malignancy after restorative proctocolectomy with or without mucosectomy in patients with familial adenomatous polyposis. Dis Colon Rectum. 2013;56:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Pommaret E, Vienne A, Lefevre JH, Sogni P, Florent C, Desaint B, Parc Y. Prevalence and risk factors for adenomas in the ileal pouch and the afferent loop after restorative proctocolectomy for patients with familial adenomatous polyposis. Surg Endosc. 2013;27:3816-3822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Bassuini MM, Billings PJ. Carcinoma in an ileoanal pouch after restorative proctocolectomy for familial adenomatous polyposis. Br J Surg. 1996;83:506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Palkar VM, deSouza LJ, Jagannath P, Naresh KN. Adenocarcinoma arising in “J” pouch after total proctocolectomy for familial polyposis coli. Indian J Cancer. 1997;34:16-19. [PubMed] |
| 42. | Kim HR, Kim DY, Kim YJ. Carcinoma in an ileal pouch after proctocolectomy, with ileal pouch-anal [abstract]. J Korean Soc Coloproctol. 1997;13:623-628. |
| 43. | Cherki S, Glehen O, Moutardier V, François Y, Gilly FN, Vignal J. Pouch adenocarcinoma after restorative proctocolectomy for familial adenomatous polyposis. Colorectal Dis. 2003;5:592-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Linehan G, Cahill RA, Kalimuthu SN, O’Connell F, Redmond HP, Kirwan WO. Adenocarcinoma arising in the ileoanal pouch after restorative proctocolectomy for familial adenomatous polyposis. Int J Colorectal Dis. 2008;23:329-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Tajika M, Nakamura T, Bhatia V, Komori K, Kato T, Yamao K. Ileal pouch adenocarcinoma after proctocolectomy for familial adenomatous polyposis. Int J Colorectal Dis. 2009;24:1487-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Ault GT, Nunoo-Mensah JW, Johnson L, Vukasin P, Kaiser A, Beart RW. Adenocarcinoma arising in the middle of ileoanal pouches: report of five cases. Dis Colon Rectum. 2009;52:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Lee SH, Ahn BK, Chang HK, Baek SU. Adenocarcinoma in ileal pouch after proctocolectomy for familial adenomatous polyposis: report of a case. J Korean Med Sci. 2009;24:985-988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Church J, Simmang C. Practice parameters for the treatment of patients with dominantly inherited colorectal cancer (familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer). Dis Colon Rectum. 2003;46:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Parc Y, Piquard A, Dozois RR, Parc R, Tiret E. Long-term outcome of familial adenomatous polyposis patients after restorative coloproctectomy. Ann Surg. 2004;239:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Will OC, Robinson J, Günther T, Phillips RK, Clark SK, Tomlinson I. APC mutation spectrum in ileoanal pouch polyps resembles that of colorectal polyps. Br J Surg. 2008;95:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Corfield AP, Warren BF, Bartolo DC, Wagner SA, Clamp JR. Mucin changes in ileoanal pouches monitored by metabolic labelling and histochemistry. Br J Surg. 1992;79:1209-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | de Silva HJ, Millard PR, Kettlewell M, Mortensen NJ, Prince C, Jewell DP. Mucosal characteristics of pelvic ileal pouches. Gut. 1991;32:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Johnson JA, Talton DS, Poole GV. Adenocarcinoma of a Brooke ileostomy for adenomatous polyposis coli. Am J Gastroenterol. 1993;88:1122-1124. [PubMed] |
| 54. | Johnson CD, White H. Colonic metaplasia with colonic-type polyps on an ileostomy stoma in polyposis coli. Report of a case. Dis Colon Rectum. 1988;31:405-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Nakahara S, Itoh H, Iida M, Iwashita A, Ohsato K. Ileal adenomas in familial polyposis coli. Differences before and after colectomy. Dis Colon Rectum. 1985;28:875-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Go PM, Lens J, Bosman FT. Mucosal alterations in the reservoir of patients with Kock’s continent ileostomy. Scand J Gastroenterol. 1987;22:1076-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Lerch MM, Braun J, Harder M, Hofstădter F, Schumpelick V, Matern S. Postoperative adaptation of the small intestine after total colectomy and J-pouch-anal anastomosis. Dis Colon Rectum. 1989;32:600-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Bayat M, Brynskov J, Dige-Petersen H, Hippe E, Lønborg-Jensen H. Direct and quantitative vitamin B12 absorption measurement in patients with disorders in the distal part of the bowel. Comparison of stool spot test [SST] with whole body counting in patients with ileal pelvic reservoir, ileostomy or Crohn’s disease. Int J Colorectal Dis. 1994;9:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | de Silva HJ, Kettlewell MG, Mortensen NJ, Jewell DP. Review Acute inflammation in ileal pouches (pouchitis). Eur J Gastroenterol Hepatol. 1991;3:343-349. |
| 60. | Apel R, Cohen Z, Andrews CW, McLeod R, Steinhart H, Odze RD. Prospective evaluation of early morphological changes in pelvic ileal pouches. Gastroenterology. 1994;107:435-443. [PubMed] |
| 61. | Clausen MR, Tvede M, Mortensen PB. Short-chain fatty acids in pouch contents from patients with and without pouchitis after ileal pouch-anal anastomosis. Gastroenterology. 1992;103:1144-1153. [PubMed] |
| 62. | Nasmyth DG, Godwin PG, Dixon MF, Williams NS, Johnston D. Ileal ecology after pouch-anal anastomosis or ileostomy. A study of mucosal morphology, fecal bacteriology, fecal volatile fatty acids, and their interrelationship. Gastroenterology. 1989;96:817-824. [PubMed] |
| 63. | Natori H, Utsunomiya J, Yamamura T, Benno Y, Uchida K. Fecal and stomal bile acid composition after ileostomy or ileoanal anastomosis in patients with chronic ulcerative colitis and adenomatosis coli. Gastroenterology. 1992;102:1278-1288. [PubMed] |
| 64. | Friederich P, Berkhout M, Roelofs HM, van Goor H, van Krieken JH, Peters WH, Nagengast FM. Decreased levels of mucosal detoxification enzymes in the pouch of patients with familial adenomatous polyposis. Br J Surg. 2006;93:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | Moisio AL, Järvinen H, Peltomäki P. Genetic and clinical characterisation of familial adenomatous polyposis: a population based study. Gut. 2002;50:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Lefevre JH, Rodrigue CM, Mourra N, Bennis M, Flejou JF, Parc R, Tiret E, Gespach C, Parc YR. Implication of MYH in colorectal polyposis. Ann Surg. 2006;244:874-879; discussion 874-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Liljeqvist L, Lindquist K. A reconstructive operation on malfunctioning S-shaped pelvic reservoirs. Dis Colon Rectum. 1985;28:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Lewis WG, Miller AS, Williamson ME, Sagar PM, Holdsworth PJ, Axon AT, Johnston D. The perfect pelvic pouch--what makes the difference? Gut. 1995;37:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Utsunomiya J, Iwama T, Imajo M, Matsuo S, Sawai S, Yaegashi K, Hirayama R. Total colectomy, mucosal proctectomy, and ileoanal anastomosis. Dis Colon Rectum. 1980;23:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 457] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 70. | McHugh SM, Diamant NE, McLeod R, Cohen Z. S-pouches vs. J-pouches. A comparison of functional outcomes. Dis Colon Rectum. 1987;30:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Oresland T, Fasth S, Nordgren S, Hallgren T, Hultén L. A prospective randomized comparison of two different pelvic pouch designs. Scand J Gastroenterol. 1990;25:986-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Uff CR, Phillips RK. Effect of suture material on tumor cell adherence at sites of colonic injury. Br J Surg. 1993;80:1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 73. | Remzi FH, Fazio VW, Delaney CP, Preen M, Ormsby A, Bast J, O’Riordain MG, Strong SA, Church JM, Petras RE. Dysplasia of the anal transitional zone after ileal pouch-anal anastomosis: results of prospective evaluation after a minimum of ten years. Dis Colon Rectum. 2003;46:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 74. | Gullberg K, Ståhlberg D, Liljeqvist L, Tribukait B, Reinholt FP, Veress B, Löfberg R. Neoplastic transformation of the pelvic pouch mucosa in patients with ulcerative colitis. Gastroenterology. 1997;112:1487-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Veress B, Reinholt FP, Lindquist K, Löfberg R, Liljeqvist L. Long-term histomorphological surveillance of the pelvic ileal pouch: dysplasia develops in a subgroup of patients. Gastroenterology. 1995;109:1090-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 104] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Setti Carraro P, Talbot IC, Nicholls RJ. Longterm appraisal of the histological appearances of the ileal reservoir mucosa after restorative proctocolectomy for ulcerative colitis. Gut. 1994;35:1721-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Gullberg K, Lindforss U, Zetterquist H, Stålberg D, Reinholt FP, Veress B, Tribukait B, Olivecrona H, Löfberg R. Cancer risk assessment in long-standing pouchitis. DNA aberrations are rare in transformed neoplastic pelvic pouch mucosa. Int J Colorectal Dis. 2002;17:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Dozois RR, Kelly KA, Welling DR, Gordon H, Beart RW, Wolff BG, Pemberton JH, Ilstrup DM. Ileal pouch-anal anastomosis: comparison of results in familial adenomatous polyposis and chronic ulcerative colitis. Ann Surg. 1989;210:268-271; discussion 272-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 126] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, Schroeder TK. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 854] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 80. | Meagher AP, Farouk R, Dozois RR, Kelly KA, Pemberton JH. J ileal pouch-anal anastomosis for chronic ulcerative colitis: complications and long-term outcome in 1310 patients. Br J Surg. 1998;85:800-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 401] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 81. | Heuschen UA, Autschbach F, Allemeyer EH, Zöllinger AM, Heuschen G, Uehlein T, Herfarth C, Stern J. Long-term follow-up after ileoanal pouch procedure: algorithm for diagnosis, classification, and management of pouchitis. Dis Colon Rectum. 2001;44:487-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Will OC, Man RF, Phillips RK, Tomlinson IP, Clark SK. Familial adenomatous polyposis and the small bowel: a loco-regional review and current management strategies. Pathol Res Pract. 2008;204:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Saurin JC, Napoleon B, Gay G, Ponchon T, Arpurt JP, Boustiere C, Boyer J, Canard JM, Dalbies PA, Escourrou J. Endoscopic management of patients with familial adenomatous polyposis (FAP) following a colectomy. Endoscopy. 2005;37:499-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Church J. Ileoanal pouch neoplasia in familial adenomatous polyposis: an underestimated threat. Dis Colon Rectum. 2005;48:1708-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Church JM, Oakley JR, Wu JS. Pouch polyposis after ileal pouch-anal anastomosis for familial adenomatous polyposis: report of a case. Dis Colon Rectum. 1996;39:584-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | von Herbay A, Stern J, Herfarth C. Pouch-anal cancer after restorative proctocolectomy for familial adenomatous polyposis. Am J Surg Pathol. 1996;20:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Vuilleumier H, Halkic N, Ksontini R, Gillet M. Columnar cuff cancer after restorative proctocolectomy for familial adenomatous polyposis. Gut. 2000;47:732-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Brown SR, Donati D, Seow-Choen F. Rectal cancer after mucosectomy for ileoanal pouch in familial adenomatous polyposis: report of a case. Dis Colon Rectum. 2001;44:1714-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Ooi BS, Remzi FH, Gramlich T, Church JM, Preen M, Fazio VW. Anal transitional zone cancer after restorative proctocolectomy and ileoanal anastomosis in familial adenomatous polyposis: report of two cases. Dis Colon Rectum. 2003;46:1418-1423; discussion 1418-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Ulaş M, Neşşar G, Bostanoğlu A, Aydoğ G, Kayaalp C, Ozoğul Y, Seven C. Development of two cancers in the same patient after ileorectal and ileal pouch anal anastomosis for familial adenomatous polyposis. Med Princ Pract. 2006;15:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Booij KA, Mathus-Vliegen EM, Taminiau JA, Ten Kate FJ, Slors JF, Tabbers MM, Aronson DC. Evaluation of 28 years of surgical treatment of children and young adults with familial adenomatous polyposis. J Pediatr Surg. 2010;45:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Aziz O, Athanasiou T, Fazio VW, Nicholls RJ, Darzi AW, Church J, Phillips RK, Tekkis PP. Meta-analysis of observational studies of ileorectal versus ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg. 2006;93:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 93. | Ambroze WL, Dozois RR, Pemberton JH, Beart RW, Ilstrup DM. Familial adenomatous polyposis: results following ileal pouch-anal anastomosis and ileorectostomy. Dis Colon Rectum. 1992;35:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Tonelli F, Valanzano R, Monaci I, Mazzoni P, Anastasi A, Ficari F. Restorative proctocolectomy or rectum-preserving surgery in patients with familial adenomatous polyposis: results of a prospective study. World J Surg. 1997;21:653-658; discussion 659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Valanzano R, Ficari F, Curia MC, Aceto G, Veschi S, Cama A, Battista P, Tonelli F. Balance between endoscopic and genetic information in the choice of ileorectal anastomosis for familial adenomatous polyposis. J Surg Oncol. 2007;95:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Ishikawa H, Wakabayashi K, Suzuki S, Mutoh M, Hirata K, Nakamura T, Takeyama I, Kawano A, Gondo N, Abe T. Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: double-blind, randomized clinical trial. Cancer Med. 2013;2:50-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |