Published online Oct 21, 2013. doi: 10.3748/wjg.v19.i39.6665
Revised: July 18, 2013
Accepted: August 13, 2013
Published online: October 21, 2013
Processing time: 87 Days and 22.8 Hours
AIM: To compare the effects of entecavir (ETV) and lamivudine (LAM) for the treatment of hepatitis B decompensated cirrhosis using a meta-analysis.
METHODS: We conducted a literature search for all eligible studies published prior to May 30, 2013 using PUBMED, MEDLINE, EMBASE, the China National Knowledge Infrastructure (CNKI), the VIP database, the Wanfang database and the Cochrane Controlled Trial Register. Randomized controlled trials (RCTs) comparing ETV with LAM for the treatment of hepatitis B decompensated cirrhosis were included. The data were analyzed with Review Manager Software 5.0.2. We used RR as an effect measure, and reported its 95%CI. The meta-analysis was performed using either a fixed-effect or random-effect model, based on the absence or presence of significant heterogeneity. Two reviewers assessed the risk of bias and extracted data independently and in duplicate. The analysis was executed using the main outcome parameters including hepatitis B virus (HBV) DNA undetectability, HBV DNA level, hepatitis B e antigen (HBeAg) seroconversion, alanine aminotransferase (ALT) level, albumin level, total bilirubin (TBIL) level, prothrombin time activity (PTA) level, Child-Turcotte-Pugh (CTP) score, mortality, drug-resistance, and adverse reactions. Meta-analysis of the included trials and subgroup analyses were conducted to examine the association between pre-specified characteristics and the therapeutic effects of the two agents.
RESULTS: Thirteen eligible trials (873 patients in total) were included and evaluated for methodological quality and heterogeneity. Of these studies, all had baseline comparability, 12 of them reported baseline values of the two treatment groups in detail. Following various treatment durations (12, 24, 36, 48 and > 48 wk), both ETV and LAM significantly reduced HBV DNA level, however, reductions were greater in the ETV group (MD = -0.66, 95%CI: -0.83-0.50, P < 0.00001), (MD = -0.93, 95%CI: -1.36-0.51, P < 0.0001), (MD = -1.4, 95%CI: -1.78-1.01, P < 0.00001), (MD = -1.18, 95%CI: -1.90-0.46, P = 0.001), (MD = -0.14, 95%CI: -0.17-0.11, P < 0.00001, respectively). At 12, 24 and 48 wk of treatment, ETV had a significant effect on the rate of HBV DNA undetectability (RR = 1.55, 95%CI: 1.22-1.99, P = 0.0004), (RR = 1.25, 95%CI: 1.13-1.38, P < 0.0001), (RR = 1.2, 95%CI: 1.10-1.32, P < 0.0001, respectively). Although HBeAg seroconversion in the ETV group was more pronounced than that in the LAM group at 24 wk (27.90% vs 26.19%) and 48 wk (31.52% vs 25.00%) of treatment, there was no statistically significant difference between them (RR = 1.49, 95%CI: 0.98-2.28, P = 0.07), (RR = 1.27, 95%CI: 0.98-1.65, P = 0.07, respectively). Following various treatment durations, both the ETV group and the LAM group showed significantly improved liver function (ALT, AIB, TBIL, PTA and CTP levels) and reduced mortality (ETV 6.37%, LAM 7.89%). The effects in the ETV group (0.33%) were statistically lower than those in the LAM group (14.33%) regarding the rate of drug-resistance (RR = 0.1, 95%CI: 0.04-0.24, P≤ 0.00001). In addition, no severe adverse reactions were observed in the two treatment groups.
CONCLUSION: ETV and LAM significantly improved liver function and reduced mortality. Both drugs produced similar serological responses, and were safe and well tolerated. However, ETV resulted in a better virological response and lower drug-resistance, but is more expensive.
Core tip: This meta-analysis was conducted to compare the effects of entecavir (ETV) and lamivudine (LAM) in the treatment of hepatitis B associated decompensated cirrhosis. The results suggested that ETV and LAM significantly improved liver function and reduced mortality. Both drugs produced similar serological responses, and were safe and well tolerated. However, LAM had higher drug-resistance and is therefore unsuitable for the long-term treatment of patients with hepatitis B decompensated cirrhosis. ETV can be used as the first-line drug for long-term treatment of patients with hepatitis B decompensated cirrhosis as it has stronger anti-viral activity and extremely low drug-resistance.
- Citation: Ye XG, Su QM. Effects of entecavir and lamivudine for hepatitis B decompensated cirrhosis: Meta-analysis. World J Gastroenterol 2013; 19(39): 6665-6678
- URL: https://www.wjgnet.com/1007-9327/full/v19/i39/6665.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i39.6665
The treatment of chronic hepatitis B (CHB) is a major healthcare problem affecting over 350 million people worldwide[1]. Approximately 25%-40% of infected patients will develop various life-threatening conditions such as liver failure, liver cirrhosis (LC) and hepatocellular carcinoma (HCC). The 5-year survival rate is 84% in patients with compensated cirrhosis, but decreases to 14%-35% in individuals with decompensated cirrhosis[2]. Antiviral therapy is now considered to be the most important measure to prevent further development of this disease. Rapid and effective antiviral therapy can not only improve liver function and clinical symptoms as well as postpone progression to LC, but can also reverse the process of LC, prolong survival time and improve the quality of life. Some researchers have shown that mortality due to hepatitis B virus (HBV) and LC was positively correlated[3,4]. As interferon is prohibited for the treatment of decompensated cirrhosis, nucleosides or nucleoside analogues have become the primary drugs for antiviral therapy. Entecavir (ETV) is currently the strongest nucleoside analogue and the first-line drug for hepatitis B. It has the advantages of low drug-resistance and high safety, thus it is suitable for long-term use. However, due to its higher cost, the long-term use of ETV results in heavy financial pressures for patients with hepatitis B decompensated cirrhosis and their families. Lamivudine (LAM) is a moderate strength nucleoside analogue, and has high resistance following long-term use, which leads to treatment failure. However, due to its lower cost, LAM has a pharmacoeconomic advantage. Although there have many studies conducted on the efficacy of ETV compared with LAM for the treatment of patients with hepatitis B decompensated cirrhosis, there are few systematic reviews on this topic[5]. The roles of the two drugs in hepatitis B decompensated cirrhosis are not yet completely clear. Therefore, we conducted a meta-analysis of randomized controlled trials (RCTs) using the Cochrane methodology and explored the efficacy of ETV compared with LAM in patients with hepatitis B decompensated cirrhosis.
We searched PUBMED, MEDLINE, EMBASE, CNKI (China National Knowledge Infrastructure), the VIP database, the Wanfang database and the Cochrane Controlled Trial Register for the relevant studies published up to May 30, 2013. The following keywords were used for the search: “hepatitis B”, “decompensated cirrhosis”, “entecavir”, “lamivudine”, and “RCTs”. The reference lists of eligible studies were also searched. The language of the trials was not limited.
The following inclusion criteria were used: (1) RCTs; (2) Articles studying hepatitis B decompensated cirrhosis patients, who were included in Chinese articles according to the diagnostic standards of the China guidelines for HBV management (2005)[6], in foreign articles diagnosis was based on clinical, biochemical, radiological and histological responses, and a Child-Turcotte-Pugh (CTP) score ≥ 7; (3) Studies comparing the treatment methods of ETV (0.5 mg/d) and LAM (100 mg/d). Both groups were given symptomatic treatment and conventional treatment; and (4) The main outcome parameters included the rate of HBV DNA undetectability, HBV DNA level, hepatitis B e antigen (HBeAg) seroconversion, alanine aminotransferase (ALT) levels, albumin (ALB) levels, total bilirubin (TBIL) levels, prothrombin time activity (PTA) levels, CTP score, mortality, drug-resistance, and adverse reactions.
The following exclusion criteria were used: (1) Non-RCTs; (2) Insufficient analytical information regarding treatment schedule, follow-up, and outcomes; (3) Receiving interferon, nucleosides or nucleotides for CHB within 6 mo of the trial; (4) Coinfection with hepatitis A, C, D, E virus, cytomegalovirus, or HIV; and (5) Patients with liver failure, HCC, and liver-related complications caused by alcoholism, autoimmune disease, and cholestasis.
Data extraction was assessed independently by two reviewers (Song LY and Zhang SR). Discrepancies among reviewers were resolved by discussions between the reviewers or by a third person (Ou-Yang RJ). Basic information obtained from each eligible trial included the study design (randomization, allocation concealment, blinding method, description of withdrawals and dropouts), patient characteristics, numbers in each group, related study results and treatment duration. Data were reviewed to eliminate duplicate reports of the same trial.
We used Review Manager Software 5.0.2 (Cochrane Collaboration, Oxford, United Kingdom) to carry out data analysis. We used RR as an effect measure for dichotomous data, mean difference (MD) as an effect measure for continuous data, and reported their 95%CI. The meta-analysis was performed using a fixed-effect or random-effect model, based on the absence or presence of significant heterogeneity.
Statistical heterogeneity between trials was evaluated by χ2 and I2) analysis. The fixed-effect method was used in the absence of statistically significant heterogeneity (P≥ 0.1), the random-effect method was used when the heterogeneity test was statistically significant (P < 0.1). A value of P < 0.05 was regarded as statistically significant. We used subgroup analyses to examine the association of pre-specified characteristics (treatment duration) with treatment effect, sensitivity analysis was used to estimate the stability of the results, and funnel plots were used to assess publication bias if more than five trials were included[7].
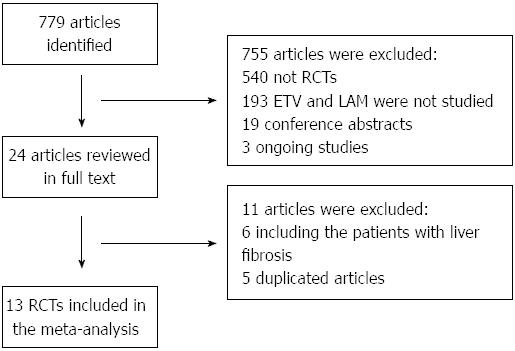
The process of identifying the included trials is presented in Figure 1. We initially identified 779 abstracts, and after evaluating the full texts, we included 13 trials (12 in Chinese and 1 in English) based on the pre-specified criteria. A total of 873 patients were included in the study: 423 treated with ETV and 450 treated with LAM. Table 1 shows the characteristics of the 13 trials. Of these studies, all showed baseline comparability, 12 of them reported the baseline values of the two groups in detail, 1 only referred to the two groups as having no significant differences in gender, age and duration[12]. One described the method of randomization in detail[9], 9 referred to randomization, but did not describe the method of randomization in detail[8,11-15,18-20]. None of the trials referred to allocation concealment and blinding method. Six described the reasons for withdrawals and dropouts[8,12-14,16]. Quality assessment of the trials was performed with Jadad scores that ranged between 1 and 5[21]. Based on these scores, 10 trials were of high quality (≥ 3 scores)[8-12,14,16,18-20], and 3 trials were of inferior quality (< 3 scores)[13,15,17].
| Trial | Sample size (n) | mean age (yr) | Regimen | Duration (wk) | Observation time (wk) | Outcome parameters | Jadad scores | |||
| ETV | LAM | ETV | LAM | ETV | LAM | |||||
| Feng et al[8] | 22 | 25 | - | - | 0.5 mg/d | 100 mg/d | 48 | 4, 12, 24, 36, 48 | ACDGI | 3 |
| Yang et al[9] | 30 | 30 | 47.5 ± 9.7 | 0.5 mg/d | 100 mg/d | 48 | 4, 8, 12, 24, 48 | ABCI | 3 | |
| Shen[10] | 40 | 40 | 46.5 | 48.5 | 0.5 mg/d | 100 mg/d | 48 | 48 | BDEFIJ | 3 |
| Huang et al[11] | 22 | 22 | 48.2 | 47.5 | 0.5 mg/d | 100 mg/d | 52 | 52 | BDEFGHIJ | 3 |
| Chen et al[12] | 23 | 24 | 48.5 | 0.5 mg/d | 100 mg/d | 48 | 24, 48 | ACJ | 3 | |
| Li et al[13] | 40 | 40 | 51.0 | 0.5 mg/d | 100 mg/d | 48 | 12, 24, 48 | ACDEFHI | 2 | |
| Shao et al[14] | 29 | 28 | 43.1 ± 10.262 | 44.11 ± 10.322 | 0.5 mg/d | 100 mg/d | 96 | 12, 24, 36, 48, 60, 72, 84, 96 | AHIJ | 3 |
| Kong[15] | 24 | 24 | 47.5 | 0.5 mg/d | 100 mg/d | 48 | 48 | BDEFGH | 2 | |
| Hyun et al[16] | 45 | 41 | 54 ± 9.4 | 53.7 ± 12.1 | 0.5 mg/d | 100 mg/d | 48 | 12, 24, 36, 48 | ABCHIJ | 3 |
| Wang et al[17] | 66 | 64 | 52.3 ± 15.8 | 50.8 ± 15.4 | 0.5 mg/d | 100 mg/d | 48 | 12, 24, 36, 48 | ABCGHI | 2 |
| Yang et al[18] | 32 | 42 | 47.8 ± 10.2 | 0.5 mg/d | 100 mg/d | 48 | 12, 24, 36, 48 | ACDEFI | 3 | |
| Zhou et al[19] | 40 | 40 | 46 ± 14 | 0.5 mg/d | 100 mg/d | 48 | 12, 24, 48 | BDH | 3 | |
| Liu et al[20] | 30 | 30 | 46.04 ± 10.79 | 45.75 ± 10.26 | 0.5 mg/d | 100 mg/d | 48 | 48 | B | 3 |
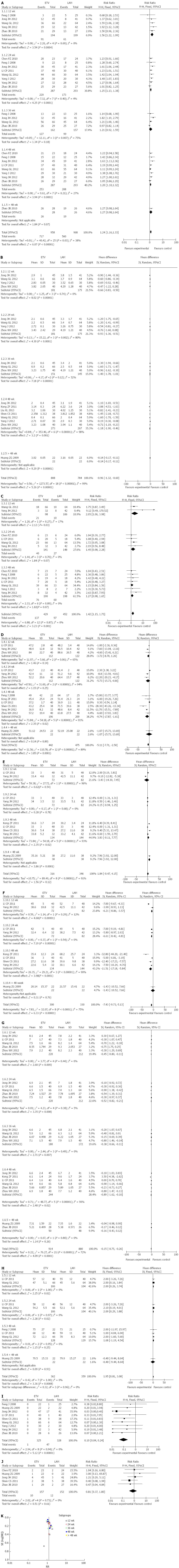
In this analysis, 8 trials reported rates of HBV DNA undetectability. According to χ2 and I2 analyses, heterogeneity was observed (χ2 = 40.42, P = 0.03, I2 = 38%); therefore, we used the random-effect method to analyze the data. At 12, 24 and 48 wk of treatment, the rate of HBV DNA undetectability was higher in the ETV group than in the LAM group, and the difference between the two groups was statistically significant [(RR = 1.55, 95%CI: 1.22-1.99, P = 0.0004), (RR = 1.25, 95%CI: 1.13-1.38, P < 0.0001), (RR = 1.2, 95%CI: 1.10-1.32, P < 0.0001, respectively)], while at 36 and > 48 wk, the rate of HBV DNA undetectability between the two groups was similar, and no statistically significant differences were observed [(RR = 1.21, 95%CI: 0.92-1.59, P = 0.18), (RR = 1.27, 95%CI: 0.98-1.64, P = 0.07), respectively] (Figure 2A).
In this analysis, 8 trials reported HBV DNA levels. According to χ2 and I2 analyses, heterogeneity was observed (χ2 = 1274.13, P < 0.00001, I2 = 99%); therefore, we used the random-effect method to analyze the data. At 12, 24, 36, 48, and > 48 wk, HBV DNA levels were lower in the ETV group than in the LAM group, and the difference between the two groups was statistically significant [(MD = -0.66, 95%CI: -0.83-0.50, P < 0.00001), (MD = -0.93, 95%CI: 1.36-0.51, P < 0.0001), (MD = -1.4, 95%CI: -1.78-1.01, P < 0.00001), (MD = -1.18, 95%CI: -1.90-0.46, P = 0.001), (MD = -0.14, 95%CI: -0.17-0.11, P < 0.00001), respectively] (Figure 2B).
In this analysis, 7 trials reported the rate of HBeAg seroconversion. According to χ2 and I2 analyses, heterogeneity was not observed (χ2 = 6.88, P = 0.87, I2 = 0%); therefore, we used the fixed-effect method to analyze the data. At 12 wk, the rate of HBeAg seroconversion was higher in the ETV group than in the LAM group, and the difference between the two groups was statistically significant (RR = 2.05, 95%CI: 1.06-3.98, P = 0.03), while at 24 and 48 wk, the rate of HBeAg seroconversion in the two groups was similar, and no statistically significant differences were observed. [(RR = 1.49, 95%CI: 0.98-2.28, P = 0.07), (RR = 1.27, 95%CI: 0.98-1.65, P = 0.07), respectively] (Figure 2C).
In this analysis, 7 trials reported ALT levels. According to χ2 and I2 analysis, heterogeneity was observed (χ2 = 110.78, P < 0.00001, I2 = 89%); therefore, we used the random-effect method to analyze the data. At 48 wk, ALT levels were lower in the ETV group than in the LAM group, and the difference was statistically significant (MD = -9.74, 95%CI: -17.87-1.61, P = 0.02), while at 12, 24 and > 48 wk, ALT levels in the two groups were similar, and no statistically significant differences were observed [(MD = -3.72, 95%CI: -8.7-1.26, P = 0.14), (MD = -5.73, 95%CI: -15.52-4.06, P = 0.25), (MD = -1.07, 95%CI: -15.73-13.59, P = 0.89), respectively] (Figure 2D).
In this analysis, 5 trials reported ALB levels. According to χ2 and I2 analysis, heterogeneity was observed (χ2 = 89.49, P < 0.00001, I2 = 91%); therefore, we used the random-effect method to analyze the data. At > 48 wk, ALB levels were higher in the ETV group than in the LAM group, and the difference was statistically significant (MD = 1.84, 95%CI: -0.47-4.15, P = 0.0001), while at 12, 24 and 48 wk, ALB levels in the two groups were similar, and no statistically significant differences were observed [(MD = -3.43, 95%CI: -14.3-7.45, P = 0.54), (MD = 0.15, 95%CI: -0.94-1.25, P = 0.78), (MD = 3.83, 95%CI: -0.11-7.77, P = 0.06), respectively] (Figure 2E).
In this analysis, 5 trials reported TBIL levels. According to χ2 and I2 analysis, heterogeneity was observed (χ2 = 29.21, P≤ 0.00001, I2 = 90%); therefore, we used the random-effect method to analyze the data. At 12, 24 and 48 wk, TBIL levels were lower in the ETV group than in the LAM group, and the difference was statistically significant [(MD = -6.21, 95%CI: -8.86-3.57, P < 0.00001), (MD = -6.61, 95%CI: -8.42-4.81, P < 0.00001), (MD = -11.51, 95%CI: -17.18-5.84, P < 0.0001), respectively], while at > 48 wk, TBIL levels in the two groups were similar, and no statistically significant difference was observed (MD = -1.43, 95%CI: -10.52-7.66, P = 0.76) (Figure 2F).
In this analysis, 4 trials reported PTA levels. According to χ2and I2 analysis, heterogeneity was observed (χ2 = 0.42, P = 1.0, I2 = 0%); therefore, we used the fixed-effect method to analyze the data. At 12 and 24 wk, PTA levels were higher in the ETV group than in the LAM group, and the differences were statistically significant [(MD = 2, 95%CI: 0.26-3.74, P = 0.02), (MD = 2.09, 95%CI: 0.29-3.88, P = 0.02), respectively], while at 48 and > 48 wk, PTA levels in the two groups were similar, and no statistically significant differences were observed [(MD = 1.68, 95%CI: -1.19-4.54, P = 0.25), (MD = -0.40, 95%CI: -9.44-8.64, P = 0.93), respectively] (Figure 2H).
In this analysis, 7 trials reported the CTP score. According to χ2 and I2 analysis, heterogeneity was observed (χ2 = 76.27, P < 0.00001, I2 = 72%); therefore, we used the random-effect method to analyze the data. At 12, 24, 36 and 48 wk, the CTP score was lower in the ETV group than in the LAM group, and the differences were statistically significant [(MD = -0.45, 95%CI: -0.80-0.11, P = 0.009), (MD = -0.52, 95%CI: -0.82-0.21, P = 0.0008), (MD = -0.38, 95%CI: -0.66-0.11, P = 0.007), (MD = -0.89, 95%CI: -1.62-0.16, P = 0.02), respectively], while at > 48 wk, the CTP score in the two groups was similar, and no statistically significant difference was observed (MD = -0.16, 95%CI: -0.43-0.12, P = 0.26) (Figure 2G).
In this analysis, 9 trials reported the rate of drug-resistance. According to χ2 and I2 analysis, heterogeneity was not observed (χ2 = 2.94, P = 0.94, I2 = 0%); therefore, we used the fixed-effect method to analyze the data. At the end of treatment, the rate of drug-resistance was lower in the ETV group (0.33%) than in the LAM group (14.33%), and the difference was statistically significant (RR = 0.1, 95%CI: 0.04-0.24, P≤ 0.00001) (Figure 2I).
In this analysis, 12 trials reported adverse reactions. The difference in adverse reactions between the two groups was not obvious. Patients in the two treatment groups did not experience severe adverse reactions, and common adverse reactions included headache, fatigue, nausea, diarrhea, hypersomnia, and insomnia. Five trials reported mortality. According to χ2 and I2 analysis, heterogeneity was not observed (χ2 = 1.37, P = 0.85, I2 = 0%); therefore, we used the fixed-effect method to analyze the data. At the end of treatment, the mortality rate in the two groups (ETV 6.37% vs LAM 7.89%) was similar, and no statistically significant difference was observed (RR = 0.81, 95%CI: 0.37-1.80, P = 0.61) (Figure 2J).
We examined publication bias using a funnel plot. The results showed that the plot was funnel shaped which suggested the absence of significant publication bias (Figure 2K).
Sensitivity analysis were performed by excluding certain studies. For example, when considering the rate of HBV DNA undetectability, using the fixed-effect model instead of the random-effect model, 3 inferior quality trials were removed. The ORs of all sensitivity analyses were larger than 1 and statistically significant (P < 0.05), suggesting that the results of the meta-analysis were stable (Table 2).
Nucleoside/nucleotide analogues (NUCs) are the only antiviral agents recommended for patients with hepatitis B decompensated cirrhosis[6]. As the first NUC used in the treatment of CHB, LAM has been widely used in he treatment of hepatitis B cirrhosis. A number of researchers have shown that LAM can effectively suppress HBV DNA replication and significantly improve liver function in patients with hepatitis B decompensated cirrhosis[22,23]. However, a critical weak point of LAM therapy is the frequent occurrence of resistant mutations and high drug-resistance in HBV[24]. As liver function in patients with LC is poor and progression of the disease is fast, the selection of appropriate drugs in the later period of treatment is difficult. ETV is a new cyclopentyl guanosine nucleoside analogue which is efficiently phosphorylated to its active triphosphate form by host cellular kinases. It blocks HBV replication by inhibiting HBV polymerase, the DNA strand via reverse transcription elongation, and DNA-dependent plus-strand DNA synthesis[25]. ETV has the advantage of a higher rate of HBV DNA suppression, low drug-resistance and high safety, especially in LAM-resistant CHB patients[26]. Therefore, some researchers have attempted to use ETV for the treatment of hepatitis B decompensated cirrhosis[27,28]. However, it is more expensive than other nucleoside analogues.
In the present study, we included RCTs comparing ETV with LAM in patients with hepatitis B decompensated cirrhosis. We conducted a meta-analysis on virological, serological, biochemical reactions, drug-resistance, mortality and adverse reactions in the included trials to examine the association between pre-specified characteristics (treatment duration) and treatment effect.
HBV DNA level is a primary prognostic marker and risk factor for patients with hepatitis B decompensated cirrhosis[29]. The early and sustained suppression of HBV DNA replication is associated with improved long-term virological, serological and biochemical response rates. Rapid and effective suppression of HBV DNA replication can reduce the incidence of LC, HCC and drug-resistance[30,31]. The results of our meta-analysis showed that following various treatment durations (12, 24, 36, 48 and > 48 wk), HBV DNA levels were lower in the ETV group than in the LAM group, and the difference between the two groups was statistically significant. At 12, 24 and 48 wk of treatment, ETV showed a significant effect on the rate of HBV DNA undetectability compared with LAM. These results showed that ETV was not only more effective than LAM in the early stages of treatment, but also had a continuous advantage after treatment. This suggests that ETV had a more rapid and effective anti-viral activity in patients with hepatitis B decompensated cirrhosis than LAM. Although HBeAg seroconversion in the ETV group was more pronounced than in the LAM group at 24 wk (27.9% vs 26.19%) and 48 wk (31.52% vs 25%) of treatment, these differences were not statistically significant.
Following various treatment durations, both ETV and LAM significantly reduced ALT, TBIL and CTP levels and increased ALB and PTA levels. These results indicated that both drugs significantly improved liver function.
ETV has a high genetic barrier to resistance[32]. The results in Figure 2D show that the rate of drug-resistance was higher in the LAM group (17.12%) than in the ETV group (0.44%), and this difference was statistically significant. ETV has lower drug-resistance, and is thus more suitable for the treatment of patients with hepatitis B decompensated cirrhosis than LAM.
The results in Figure 2G show that the rate of mortality in the two treatment groups was similar (ETV 6.37% vs LAM 7.89%), and no statistically significant difference was observed. No severe adverse reactions were observed in the two treatment groups. These results suggest that both ETV and LAM significantly reduced mortality, with excellent safety and tolerability.
Our study had several limitations. First, the number of included trials was small, and some outcome parameters of treatment duration included only 1 trial. Second, the quality of some of the included trials was not high (details on the method of randomization, allocation concealment, blinding method, or the reasons for withdrawals and dropouts were not included). Therefore, future studies should assess high-quality, well-designed, multi-center RCTs with larger sample sizes.
In conclusion, both ETV and LAM have powerful anti-viral activity, with a low incidence of adverse reactions. These drugs also improved liver function and reduced mortality. Therefore, the positive effects of ETV and LAM in patients with hepatitis B decompensated cirrhosis were confirmed. Due to lower cost, LAM has a pharmacoeconomic advantage before 48 wk of treatment. However, LAM has higher drug-resistance, and is thus unsuitable for the long-term treatment of patients with hepatitis B decompensated cirrhosis. ETV can be used as the first-line drug for long-term treatment of patients with hepatitis B decompensated cirrhosis due to its greater anti-viral activity and extremely low drug-resistance.
We are grateful to Song LY, Zhang SR and Ou-Yang RJ for the data extraction.
The treatment of chronic hepatitis B is a major healthcare problem affecting over 350 million people worldwide. Approximately 25%-40% of infected patients will develop various life-threatening conditions such as liver failure, liver cirrhosis and hepatocellular carcinoma. Recent studies have shown that entecavir (ETV) and lamivudine (LAM) are powerful nucleoside analogues in the treatment of hepatitis B decompensated cirrhosis. However, there were few systematic reviews on this topic.
Lamivudine effectively suppressed hepatitis B virus (HBV) DNA replication and significantly improved liver function in patients with hepatitis B decompensated cirrhosis. However, a critical weak point of lamivudine therapy is the frequent occurrence of resistant mutations and high drug-resistance in HBV. ETV is a new cyclopentyl guanosine nucleoside analogue. It has the advantage of a higher rate of HBV DNA suppression, low drug-resistance and high safety. However, it is expensive and long-term use of ETV would result in heavy financial pressures for patients with hepatitis B decompensated cirrhosis and their families.
There are few systematic reviews on the efficacy of ETV and LAM in the treatment of hepatitis B decompensated cirrhosis. The authors conducted a meta-analysis of randomized controlled trials using the Cochrane methodology and explored the efficacy of ETV and LAM for the treatment of hepatitis B decompensated cirrhosis.
Due to its lower cost, LAM has a pharmacoeconomic advantage before 48 wk of treatment. However, LAM has higher drug-resistance, and is thus unsuitable for the long-term treatment of patients with hepatitis B decompensated cirrhosis. ETV can be used as the first-line drug for the long-term treatment of patients with hepatitis B decompensated cirrhosis due to of its greater anti-viral activity and extremely low drug-resistance.
HBV DNA undetectability: undetectable levels of HBV DNA (HBV DNA levels < 1000 copies/mL), determined by quantitative polymerase chain reaction. Hepatitis B e antigen (HBeAg) seroconversion: HBeAg loss (HBeAg levels < 1.0 S/CO) and the presence of anti-HBeAg, determined by microparticle enzyme immunoassay or enzyme-linked immunosorbent assay. CTP score: employs five clinical measures of liver disease [total bilirubin, albumin, Prothrombin time, Ascites, Hepatic encephalopathy]. Each measure is scored 1-3, class A to C by total scores (A:5-6, B:7-9, C:10-15), with C indicating the most severe liver disease.
This is a good meta-analysis comparing the ETV and LAM in treatment of hepatitis B associated decompensated cirrhosis. Based on their analyses, the authors conclude that both ETV and LAM can significantly improve the liver function and reduce mortality for patients with hepatitis B decompensated cirrhosis. However, ETV has a better virological response and lower drug-resistance, which can be used as the first-line drug for long-term treatment of hepatitis B decompensated cirrhosis. The analysis was carefully performed, and the results were clearly presented and summarized.
P- Reviewers Gao C, Shi KQ, Shi ZJ, Zhao P S- Editor Wen LL L- Editor A E- Editor Ma S
| 1. | Chi ZC. Antiviral therapy of chronic hepatitis B with liver cirrhosis. Zhongxiyi Jiehe Ganbing Zazhi. 2006;16:129-132. |
| 2. | Schiff E, Simsek H, Lee WM, Chao YC, Sette H, Janssen HL, Han SH, Goodman Z, Yang J, Brett-Smith H. Efficacy and safety of entecavir in patients with chronic hepatitis B and advanced hepatic fibrosis or cirrhosis. Am J Gastroenterol. 2008;103:2776-2783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Realdi G, Fattovich G, Hadziyannis S, Schalm SW, Almasio P, Sanchez-Tapias J, Christensen E, Giustina G, Noventa F. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 206] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Liaw YF, Sheen IS, Chen TJ, Chu CM, Pao CC. Incidence, determinants and significance of delayed clearance of serum HBsAg in chronic hepatitis B virus infection: a prospective study. Hepatology. 1991;13:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 178] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Huang Y, Wu H, Wu S, Fu D, Ma Y, Shen X. A meta-analysis of nucleos(t)ide analogues in patients with decompensated cirrhosis due to hepatitis B. Dig Dis Sci. 2013;58:815-823. [PubMed] |
| 6. | Chinese Society of Infctious Diseases, Parasitic Epidemiology and Hepatology. China guidelines for HBV management. Zhonghua Ganranbing Zazhi. 2005;23:421-431. |
| 7. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12879] [Article Influence: 444.1] [Reference Citation Analysis (1)] |
| 8. | Feng J, Dou F, Su J, Li P, Lun YX. Effect of antiretroviral therapy to hepatitis B cirrhosis. Zhongguo Shiyong Yixue. 2008;3:13-14. |
| 9. | Yang J, Fan HZ. Virologic response of multiple nucleoside analogues therapy to patients with hepatitis B decompensated cirrhosis. Linchuang Luntan. 2012;14:83-84. |
| 10. | Shen CS. Effect of entecavir for treatment of 40 patients with active hepatitis B decompensated cirrhosis. Linchuang Guancha. 2011;30:376-377. |
| 11. | Huang ZG, Liu J, Xu YP. Entecavir therapy in patients with decompensated HBV-related cirrhosis. Chuanranbing Xinxi. 2009;22:112-114. |
| 12. | Chen FZ, Li GQ, Zhang L, Liu HY. Clinical effect of entecavir in patients with decompensated hepatitis B cirrhosis. Linchuang Gandanbing Zazhi. 2010;26:608-612. |
| 13. | Li CP, Deng LN, Hou HB, Wang QL. Effect of entecavir in patients with hepatitis B cirrhosis. Yinanbing Zazhi. 2011;10:211-212. |
| 14. | Shao JB, Xu J, Zhu DL, Dai WW. Clinical Observation of Entecavir in the Treatment of Decompensated Cirrhotic Patient with HBV for 96 Weeks. Zhongguo Yiyao Zhinan. 2010;8:11-13. |
| 15. | Kong ZF. Efficacy analysis of entecavir in patients with hepatitis B cirrhosis. Jiankang Bidu Zazhi. 2011;9:78. |
| 16. | Hyun JJ, Seo YS, Yoon E, Kim TH, Kim DJ, Kang HS, Jung ES, Kim JH, An H, Kim JH. Comparison of the efficacies of lamivudine versus entecavir in patients with hepatitis B virus-related decompensated cirrhosis. Liver Int. 2012;32:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Wang GL, Wen JB, Wen P, Qiu P, Gong M, Han M. Cost-effectiveness Analysis of 4 Pharmacotherapeutic Schemes for Decompensated Hepatic Cirrhosis Secondary to Chronic Hepatitis B. Yaowu Jingjixue. 2012;23:3169-3171. |
| 18. | Yang JH, Zheng S, You LY, Tang YM, Liu H. Therapeutic effects of 48-week entecavir treatment in patients with decompensated cirrhosis caused by hepatits B virus. Shiyong Ganzangbing Zazhi. 2012;15:407-410. |
| 19. | Zhou WX, Lu GL, Liu X. Clinical efficacy ananlysis of nucleoside analogs in the treatment of hepatitis B virus related decompensated cirrhosis. Zhongguo Shenghua Yaowu Zazhi. 2012;33:170-172. |
| 20. | Liu XL, Zhu JF, Li WL, Xu RR, Wu MS. Clinical analysis of nucleosis drugs used in treatment of liver cirrhosis after hepatitis B. Dangdai Yixue. 2013;19:100-101. |
| 21. | Wang JL, Wang B. Clinical epidemiology: Clinical scientific research and design, measure and evaluation. Shanghai: Shanghai Kexue Jishu Chubanshe 2009; . |
| 22. | Chien RN, Lin CH, Liaw YF. The effect of lamivudine therapy in hepatic decompensation during acute exacerbation of chronic hepatitis B. J Hepatol. 2003;38:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Tsubota A, Arase Y, Suzuki Y, Suzuki F, Sezaki H, Hosaka T, Akuta N, Someya T, Kobayashi M, Saitoh S. Lamivudine monotherapy for spontaneous severe acute exacerbation of chronic hepatitis B. J Gastroenterol Hepatol. 2005;20:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 493] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 25. | Osborn M. Safety and efficacy of entecavir for the treatment of chronic hepatitis B. Infect Drug Resist. 2011;4:55-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Shim JH, Lee HC, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Efficacy of entecavir in treatment-naïve patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2010;52:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 28. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 772] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 29. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 30. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2363] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 31. | Keeffe EB, Zeuzem S, Koff RS, Dieterich DT, Esteban-Mur R, Gane EJ, Jacobson IM, Lim SG, Naoumov N, Marcellin P. Report of an international workshop: Roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007;5:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 32. | Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, Boron-Kaczmarska A, Martin P, Goodman Z, Colonno R. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |