Published online Oct 21, 2013. doi: 10.3748/wjg.v19.i39.6651
Revised: September 2, 2013
Accepted: September 16, 2013
Published online: October 21, 2013
Processing time: 117 Days and 1.1 Hours
AIM: To investigate the use of multi-b-value diffusion-weighted imaging in diagnosing pancreatic cancer.
METHODS: We retrospectively analyzed 33 cases of pancreatic cancer and 12 cases of benign pancreatic tumors at the Second Affiliated Hospital of Kunming Medical University from December 2008 to January 2011. The demographic characteristics, clinical presentation, routine magnetic resonance imaging and diffusion weighted imaging (DWI) features with different b values were reviewed. Continuous data were expressed as mean ± SD. Comparisons between pancreatic cancer and benign pancreatic tumors were performed using the Student’s t test. A probability of P < 0.05 was considered statistically significant.
RESULTS: Thirty-three patients with pancreatic cancer were identified. The mean age at diagnosis was 60 ± 5.6 years. The male: female ratio was 21:12. Twenty cases were confirmed by surgical resection and 13 by biopsy of metastases. T1 weighted images demonstrated a pancreatic head mass in 16 patients, a pancreatic body mass in 10 cases, and a pancreatic tail mass with pancreatic atrophy in 7 cases. Eight patients had hepatic metastases, 13 had invasion or envelopment of mesenteric vessels, 4 had bone metastases, and 8 had lymph node metastases. DWI demonstrated an irregular intense mass with unclear margins. Necrotic tissue demonstrated an uneven low signal. A b of 1100 s/mm2 was associated with a high intensity signal with poor anatomical delineation. A b of 700 s/mm2 was associated with apparent diffusion coefficients (ADCs) that were useful in distinguishing benign and malignant pancreatic tumors (P < 0.05). b values of 50, 350, 400, 450 and 1100 s/mm2 were associated with ADCs that did not differentiate the two tumors.
CONCLUSION: Low b value images demonstrated superior anatomical details when compared to high b value images. Tumor tissue definition was high and contrast with the surrounding tissues was good. DWI was useful in diagnosing pancreatic cancer.
Core tip: In this study, we retrospectively analyzed the conventional magnetic resonance imaging and diffusion weighted imaging (DWI) characteristics of 33 cases of pancreatic cancer using different b values, and assessed the value of the DWI examination in differentiating pancreatic cancer from benign pancreatic tumors.
-
Citation: Hao JG, Wang JP, Gu YL, Lu ML. Importance of
b value in diffusion weighted imaging for the diagnosis of pancreatic cancer. World J Gastroenterol 2013; 19(39): 6651-6655 - URL: https://www.wjgnet.com/1007-9327/full/v19/i39/6651.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i39.6651
Pancreatic cancer is the fourth leading cause of cancer-related deaths[1] and accounts for 80% to 90% of exocrine gland malignant tumors[2]. Most patients present without symptoms and have a median survival of approximately 6 months. There is an urgent need for early diagnosis and accurate assessment of this disease. Magnetic resonance imaging (MRI) is a sensitive and specific imaging modality. MRI has been used to assess tumor macroscopic morphology, microscopic metabolism, and functional status[3,4]. Diffusion weighted imaging (DWI) is an imaging technique that is sensitive to water diffusion in living tissues. DWI was originally used to diagnose acute stroke[5,6]. DWI has also been used to diagnose liver, kidney, breast, prostate and uterine disease. DWI is frequently used to diagnose pancreatic diseases[7-10]. We retrospectively analyzed the conventional MRI and DWI characteristics of 33 patients with pancreatic cancer and 12 with benign pancreatic tumors to evaluate the value of DWI.
Thirty-three patients with pancreatic cancer were hospitalized at the Second Affiliated Hospital of Kunming Medical University between December 2008 and January 2011. Twenty patients had their diagnosis confirmed by pathological examination of the resected specimen and 13 by biopsy of metastases. There were 21 male and 12 female patients with an average age of 60 ± 5.6 years. Sixteen patients had a mass in the pancreatic head, 10 in the pancreatic body, and 7 in the pancreatic tail. Clinical symptoms included abdominal pain, abdominal discomfort, jaundice, abdominal mass, significant weight loss and loss of appetite. Control cases with benign pancreatic tumors were confirmed by histopathology.
Imaging was performed using a Siemens Sonata 1.5 T superconducting scanner with a body phased-array surface coil. A T1WI-FLASH sequence (repetition time, TR 124 ms and echo time, TE 2.47 ms) and T2WI-HASTE sequence (TR 1000 ms and TE 93 ms) were used with 18-24 layers, a thickness of 4-8 mm, spacing between 0 and 1.6 mm, and a field of view (FOV) of 240-280 mm × 300-380 mm. The matrix was 320 × 256. Scan time was 13-18 s.
DWI scanning was performed using a SE-EPI sequence (TR 4000 ms, TE 85-95 ms, Matrix 128 × 128, FOV 230 mm × 230 mm, thickness 5 mm, spacing 0.5 mm) with fat suppression, flow compensation and chemical shift saturation. The b value (apparent diffusion coefficient) was varied (50, 350, 400, 450, 700 and 1100 s/mm2) to capture images. Slice selection was performed using frequency encoding and phase encoding in 3 directions. Images were processed using MR software. Scan time was 13-18 s.
The original DWI scanning data and automatically generated apparent diffusion coefficients (ADC) were transferred to the workstation. The value of the ADC was measured from the ADC image of each region of interest (ROI). Solid tumor ROIs were not less than half of the lesion and located in the center of the mass. Areas of necrosis, the main pancreatic duct, vascular branches and chemical shift artifacts were avoided. Three ADCs were measured from each ROI and averaged.
All statistical analyses were performed using SPSS, version 17.0 for Windows. Continuous data were expressed as mean ± SD. The differences in ADC values of pancreatic cancer and benign pancreatic tumors were evaluated using the Student’s t test. All reported P values were two-sided. P < 0.05 was considered statistically significant.
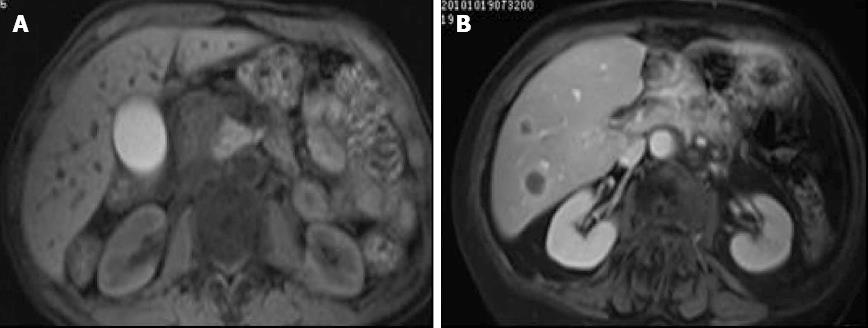
Sixteen patients had a pancreatic head mass, with a local or diffuse low intensity signal (Figure 1A). 10 had a pancreatic body mass with a low intensity signal (Figure 1B) and 7 patients had a pancreatic tail mass with pancreatic atrophy. Eight patients had liver metastases, 13 demonstrated invasion into or enveloping local mesenteric vessels, 4 had bone metastases and 8 had lymph node metastases.
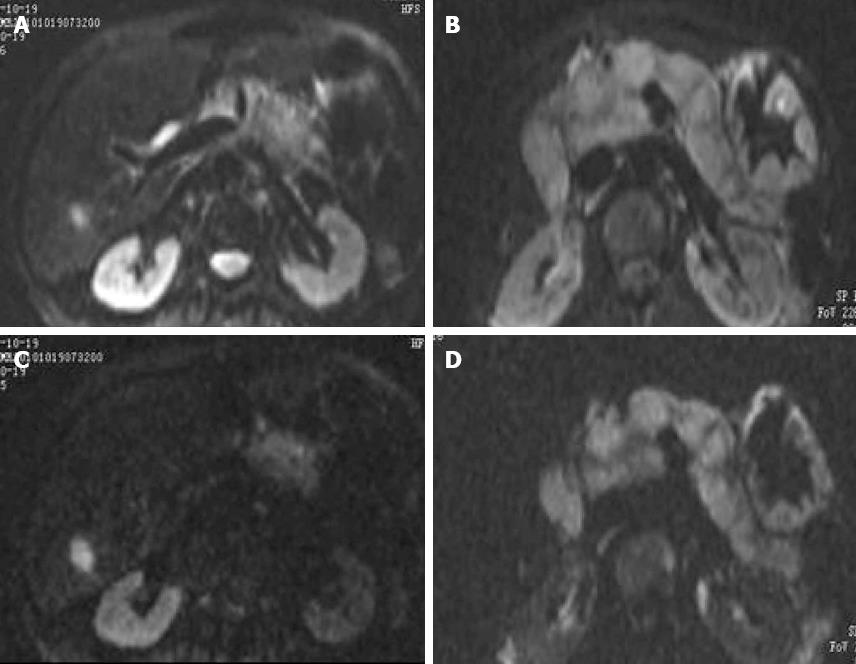
DWI demonstrated an uneven intense signal with margins that were not clearly delineated. The central necrotic tissue had an irregular low intensity signal. A low b value image provided better anatomical detail than a high b value image. Tumor tissue definition was high, and there was sharp contrast with the surrounding tissue (Figure 2). A b of 1100 s/mm2 was associated with a high value signal and poor definition of anatomic structures. A b of 700 s/mm2 was associated with benign pancreatic tumors and pancreatic cancer ADCs that were significantly different (P < 0.05). The two tumor types had similar ADCs when b values of 50, 350, 400, 450 and 1100 s/mm2 were used for imaging (P > 0.05) (Table 1).
Pancreatic cancer is one of the most common malignant tumors of the pancreas, accounting for approximately 75%-90% of tumors. It is the most common gastrointestinal malignant tumor[10]. The retroperitoneal location and lack of symptoms prevents early detection. Pancreatic cancers have a poor prognosis, with a five year survival of only 1%-3%[11]. Patients are generally male and 40-70 years of age. Only a minority of patients are candidates for surgery at the time of diagnosis[12].
The majority of pancreatic cancers are adenocarcinomas. Ductal adenocarcinomas account for 85%-90% of pancreatic carcinomas and originate in the ductal epithelium. Ductal adenocarcinomas are avascular solid tumors that are locally invasive. About 70% of pancreatic cancers are located in the pancreatic head, neck and uncinate process, 20% are located in the body of the pancreas, and 5%-10% are located in the tail of the pancreas.
Abdominal imaging is used to diagnose pancreatic tumors, distinguish benign and malignant pancreatic tumors, and evaluate the resectability of pancreatic cancers before surgery[13-15]. Endoscopic ultrasound (EUS) with zone sonography technology has been used in the diagnosis of pancreatic disease[16]. The sensitivity of EUS fine needle aspiration for pancreatic adenocarcinoma[17] in early studies was more than 85%. Further studies are needed. Egorov et al[18] demonstrated the utility of combined CT and EUS in the detection of arterial involvement by pancreatic cancer. Previous studies[19] have shown that DWI performed significantly better than multidetector-row CT in the detection of liver metastases in patients with pancreatic tumors. PET has also been useful as a diagnostic and predictive tool, but its efficacy in the staging of pancreatic cancer is not known[20]. A meta-analysis of pancreatic imaging[21] suggested that DWI was a potentially useful modality for differentiating malignant from benign pancreatic lesions. There are few studies on the effect of b value on DWI in the diagnosis of pancreatic cancer. Normal pancreatic tissue contains more water than pancreatic cancer, resulting in a high T1 weighted signal. Tumor liquefaction, necrosis and hemorrhage are associated with an irregular low intensity signal. T2 weighted images are mainly used to evaluate fluid composition, pancreatic duct dilation, and pseudocyst formation. These images are not specific for pancreatic cancer, eliciting low and high intensity signal. DWI is a noninvasive magnetic resonance imaging method, which can detect the irregular random movement of water molecules[22]. DWI can provide spatial information and evaluate the exchange rate of water molecules in tissues. ADCs have been used to describe and measure the activity of water molecules.
b values of 50 and 350 s/mm2 were associated with clear DWIs, but the ADC value was not precise. With small b values, the proportion of diffusion is small and blood perfusion had a greater impact on DWI. While T2 was associated with an intense signal, DWI did not show a good margin between tumors and the surrounding tissue[23-25]. These factors affect the quality of DWI and the measurement of ADC.
DWI and ADCs with small b values were not useful in diagnosing pancreatic tumors. A b value of 1100 s/mm2 was not useful in generating ADCs that could differentiate benign and malignant pancreatic tumors. This may be due to a decline in image quality seen with high b values. A b value of 700 s/mm2 was useful in generating ADCs that could differentiate the two tumor types. The amount of tumor fibrosis, necrosis, cell proliferation, and changes in the nuclear/cytoplasmic ratio and membranous structure restricted the movement of water molecules in pancreatic cancers, decreasing the ADC values[26].
The small sample size of this study increases the possibility of a type 2 error. Randomized controlled trials are needed to verify the utility of specific b values to aid in the differential diagnosis of pancreatic cancer.
In conclusion, low b value imaging demonstrated anatomical details that were superior to high b value images. Tumor tissue definition was high and contrast with the surrounding tissue was good. DWI was useful in diagnosing pancreatic cancer.
Pancreatic cancer is the fourth leading cause of cancer-related deaths. Early detection, early diagnosis, and suitable treatment play an important role in extending patient survival. Diffusion weighted imaging (DWI) is a technically feasible measure to differentiate malignant from benign pancreatic lesions.
DWI is a magnetic resonance imaging technique that can be used to evaluate liver, kidney, breast, prostate and uterine tissue, and is especially useful in evaluating the upper abdomen.
The authors retrospectively analyzed the DWI characteristics of 33 cases of pancreatic cancer using multiple b values in order to identify the optimal b value for differentiating malignant from benign pancreatic lesions.
The authors found that low b values provided superior anatomical details, a quality image, good tumor tissue definition, and good contrast with the surrounding tissue.
DWI is an imaging technique sensitive to water molecule diffusion. It can non-invasively evaluate diffusion processes inside living cells.
This is an interesting study with great promise. DWI appears useful in diagnosing pancreatic cancer.
P- Reviewers Chowdhury P, Du YQ, Hori T S- Editor Gou SX L- Editor Webster JR E- Editor Ma S
| 1. | Zagouri F, Sergentanis TN, Chrysikos D, Zografos CG, Papadimitriou CA, Dimopoulos MA, Filipits M, Bartsch R. Molecularly targeted therapies in metastatic pancreatic cancer: a systematic review. Pancreas. 2013;42:760-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Chow LC, Bammer R, Moseley ME, Sommer FG. Single breath-hold diffusion-weighted imaging of the abdomen. J Magn Reson Imaging. 2003;18:377-382. [PubMed] |
| 4. | Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505. [PubMed] |
| 5. | Thoeny HC, De Keyzer F, Oyen RH, Peeters RR. Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology. 2005;235:911-917. [PubMed] |
| 6. | Buckley BT, Wainwright A, Meagher T, Briley D. Audit of a policy of magnetic resonance imaging with diffusion-weighted imaging as first-line neuroimaging for in-patients with clinically suspected acute stroke. Clin Radiol. 2003;58:234-237. [PubMed] |
| 7. | Shinya S, Sasaki T, Nakagawa Y, Guiquing Z, Yamamoto F, Yamashita Y. Usefulness of diffusion-weighted imaging (DWI) for the detection of pancreatic cancer: 4 case reports. Hepatogastroenterology. 2008;55:282-285. [PubMed] |
| 8. | Shinya S, Sasaki T, Nakagawa Y, Guiquing Z, Yamamoto F, Yamashita Y. The efficacy of diffusion-weighted imaging for the detection and evaluation of acute pancreatitis. Hepatogastroenterology. 2009;56:1407-1410. [PubMed] |
| 9. | Yasui O, Sato M. Combined imaging with multi-detector row computed tomography and diffusion-weighted imaging in the detection of pancreatic cancer. Tohoku J Exp Med. 2011;224:195-199. [PubMed] |
| 10. | Rosenkrantz AB, Oei M, Babb JS, Niver BE, Taouli B. Diffusion-weighted imaging of the abdomen at 3.0 Tesla: image quality and apparent diffusion coefficient reproducibility compared with 1.5 Tesla. J Magn Reson Imaging. 2011;33:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Wiggermann P, Grützmann R, Weissenböck A, Kamusella P, Dittert DD, Stroszczynski C. Apparent diffusion coefficient measurements of the pancreas, pancreas carcinoma, and mass-forming focal pancreatitis. Acta Radiol. 2012;53:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Truty MJ, Thomas RM, Katz MH, Vauthey JN, Crane C, Varadhachary GR, Wolff RA, Abbruzzese JL, Lee JE, Fleming JB. Multimodality therapy offers a chance for cure in patients with pancreatic adenocarcinoma deemed unresectable at first operative exploration. J Am Coll Surg. 2012;215:41-51; discussion 51-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Schima W, Ba-Ssalamah A, Kölblinger C, Kulinna-Cosentini C, Puespoek A, Götzinger P. Pancreatic adenocarcinoma. Eur Radiol. 2007;17:638-649. [PubMed] |
| 14. | Yu MH, Lee JY, Kim MA, Kim SH, Lee JM, Han JK, Choi BI. MR imaging features of small solid pseudopapillary tumors: retrospective differentiation from other small solid pancreatic tumors. AJR Am J Roentgenol. 2010;195:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Kartalis N, Lindholm TL, Aspelin P, Permert J, Albiin N. Diffusion-weighted magnetic resonance imaging of pancreas tumours. Eur Radiol. 2009;19:1981-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Hirooka Y, Itoh A, Kawashima H, Ohno E, Itoh Y, Nakamura Y, Hiramatsu T, Sugimoto H, Sumi H, Hayashi D. Feasibility of newly developed endoscopic ultrasound with zone sonography technology for diagnosis of pancreatic diseases. Gut Liver. 2013;7:486-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Brugge WR. Endoscopic approach to the diagnosis and treatment of pancreatic disease. Curr Opin Gastroenterol. 2013;29:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Egorov VI, Petrov RV, Solodinina EN, Karmazanovsky GG, Starostina NS, Kuruschkina NA. Computed tomography-based diagnostics might be insufficient in the determination of pancreatic cancer unresectability. World J Gastrointest Surg. 2013;5:83-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Holzapfel K, Reiser-Erkan C, Fingerle AA, Erkan M, Eiber MJ, Rummeny EJ, Friess H, Kleeff J, Gaa J. Comparison of diffusion-weighted MR imaging and multidetector-row CT in the detection of liver metastases in patients operated for pancreatic cancer. Abdom Imaging. 2011;36:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Wang Z, Chen JQ, Liu JL, Qin XG, Huang Y. FDG-PET in diagnosis, staging and prognosis of pancreatic carcinoma: a meta-analysis. World J Gastroenterol. 2013;19:4808-4817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 21. | Wu LM, Xu JR, Hua J, Gu HY, Zhang XF, Lu Q, Hu JN. Value of diffusion-weighted imaging for the discrimination of pancreatic lesions: a meta-analysis. Eur J Gastroenterol Hepatol. 2012;24:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Yoshikawa T, Kawamitsu H, Mitchell DG, Ohno Y, Ku Y, Seo Y, Fujii M, Sugimura K. ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol. 2006;187:1521-1530. [PubMed] |
| 23. | Rösch T, Schusdziarra V, Born P, Bautz W, Baumgartner M, Ulm K, Lorenz R, Allescher HD, Gerhardt P, Siewert JR. Modern imaging methods versus clinical assessment in the evaluation of hospital in-patients with suspected pancreatic disease. Am J Gastroenterol. 2000;95:2261-2270. [PubMed] |
| 24. | Dixon WT. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging: a modest proposal with tremendous potential. Radiology. 1988;168:566-567. [PubMed] |
| 25. | Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998;170:397-402. [PubMed] |
| 26. | Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393-398. [PubMed] |