Published online Oct 21, 2013. doi: 10.3748/wjg.v19.i39.6579
Revised: April 2, 2013
Accepted: May 19, 2013
Published online: October 21, 2013
Processing time: 276 Days and 15.4 Hours
AIM: To use more representative sample size to evaluate whether computed tomography (CT) scan evidence of the concomitant presence of pneumatosis and portomesenteric venous gas is a predictor of transmural bowel necrosis.
METHODS: Data from 208 patients who were referred for a diagnosis of bowel ischemia were retrospectively reviewed. Only patients who underwent a surgical intervention following a diagnosis of bowel ischemia who also had a post-operative histological confirmation of such a diagnosis were included. Patients were split into two groups according to the presence of histological evidence of transmural bowel ischemia (case group) or partial bowel ischemia (control group). CT images were reviewed for findings of ischemia, including mural thickening, pneumatosis, bowel distension, portomesenteric venous gas and arterial or venous thrombi.
RESULTS: A total of 248 subjects who underwent surgery for bowel ischemia were identified. Among the 208 subjects enrolled in our study, transmural bowel necrosis was identified in 121 subjects (case group), and partial bowel necrosis was identified in 87 subjects (control group). Based on CT findings, including mural thickening, bowel distension, pneumatosis, pneumatosis plus portomesenteric venous gas and presence of thrombi or emboli, there were no significant differences between the case and control groups. The concomitant presence of pneumatosis and porto-mesenteric venous gas showed an odds ratio of 1.95 (95%CI: 0.491-7.775, P = 0.342) for the presence of transmural necrosis. The presence of pneumatosis plus porto-mesenteric venous gas exhibited good specificity (83%) but low sensitivity (17%) in the identification of transmural bowel infarction. Accordingly, the positive and negative predictive values were 60% and 17%, respectively.
CONCLUSION: Although pneumatosis plus porto-mesenteric venous gas is associated with bowel ischemia, we have demonstrated that their co-occurrence cannot be used as diagnostic signs of transmural necrosis.
Core tip: Although the finding of pneumatosis plus porto-mesenteric venous gas is useful in verifying transmural necrosis with a specificity of 83%, the very low sensitivity of 17% indicates that the diagnosis of transmural necrosis cannot be excluded based on a normal findings. Thus, our results appear to be encouraging by indicating that neither portomesenteric venous gas nor pneumatosis were pathognomonic of bowel transmural infarction.
- Citation: Milone M, Minno MNDD, Musella M, Maietta P, Iaccarino V, Barone G, Milone F. Computed tomography findings of pneumatosis and portomesenteric venous gas in acute bowel ischemia. World J Gastroenterol 2013; 19(39): 6579-6584
- URL: https://www.wjgnet.com/1007-9327/full/v19/i39/6579.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i39.6579
Acute bowel ischemia is an urgent, life-threatening vascular condition with a mortality rate of approximately 70%[1-3].
The slightest suspicion of mesenteric ischemia should be ruled out through imaging diagnostics[1]. In such a case, the diagnostic tool of choice is computed tomography (CT) angiography using an early arterial contrast medium bolus injection, followed by an evaluation of the primary axial layers and a multiplanar reconstruction of the imaging technique[1].
In recent years, CT has increasingly been used to evaluate patients with clinical signs and symptoms of ischemic bowel disease. Depending on the severity and the extent of disease, intestinal ischemia may be detectable by CT based on a spectrum of findings, including intestinal pneumatosis, bowel wall thickening, portomesenteric venous gas and arterial or venous thrombi[4-7].
Pneumatosis plus portomesenteric venous gas have been considered signs of advanced disease that usually indicate irreversible injury and transmural necrosis[6-8].
Although Wiesner et al[9] and Kernagis et al[6] found an association between transmural bowel infarction and the comorbid presence of pneumatosis and porto-mesenteric venous gas, these studies represented only reports and included up to seven patients.
The purpose of our study was to use a more representative sample size to evaluate whether CT scan evidence of the concomitant presence of pneumatosis and portomesenteric venous gas is a predictor of transmural bowel necrosis.
Data of patients referred during a 13-year period (from January 2000 to December 2012) to the University of Naples “Federico II” and to the “Fatebenefratelli” Hospital of Naples with diagnoses of bowel ischemia were retrospectively reviewed. Only patients who underwent a surgical intervention following a diagnosis of bowel ischemia and obtained a post-operative histological confirmation of the diagnosis were included.
The patients were split into two groups according to the presence of histological evidence of transmural bowel ischemia (case group) or partial bowel ischemia (control group). Medical records were reviewed to determine the demographic and clinical characteristics of the patients in both groups.
CT images were reviewed for findings that were consistent with the most common signs of ischemia that had been identified in previous literature[4-9], including mural thickening, pneumatosis, bowel distension, portomesenteric venous gas and arterial or venous thrombi.
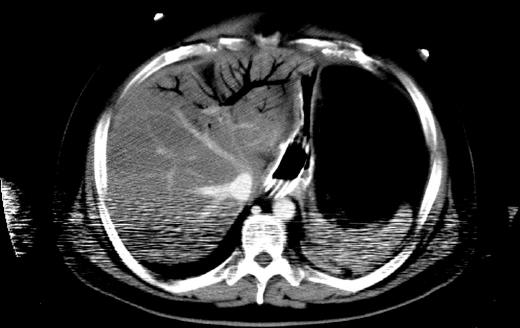
A measurement of 2-3 mm as the upper limit of normal thickness has been used[10], and air bubbles or continuous bands of air in the bowel wall were considered to be a sign of pneumatosis[9]. Gas in the mesenteric veins or gas in the intrahepatic branches of the portal vein were indicative of gas in the portomesenteric circulation (Figure 1)[9], and only the emboli or thrombi in the mesenteric arteries and veins that were clearly shown on contrast-enhanced CT images were assessed[11].
To minimise selection bias, all CT scans were analysed by an observer with 30 years of experience related to the interpretation of CT who was blinded to the surgical and pathological findings and to the eventual clinical outcomes for each patient included in the chart review.
Patients without a contrast-enhanced CT obtained with 150 mL of iodinate contrast media and 5- and 7-mm slice collimations (pitch, 1.3:1, 200-220 mAs), reconstructed with a soft-tissue algorithm, were excluded from the study.
Superficial damage of the bowel mucosa and submucosa was classified as partial bowel ischemia before the development of transmural infarction based on the histological examination[12].
The results were then correlated with the clinical and pathological data to determine the frequency with which pneumatosis plus portomesenteric venous gas was associated with irreversible transmural infarction.
Statistical analysis was performed with SPSS 16.0. The Yates corrected χ2 test was used to evaluate differences in categorical variables, and the independent samples t test was used to analyse continuous variables. To adjust for covariates and to make predictions, linear and logistic regression models were used. Statistical significance was accepted when the P value was less than 0.05.
A total of 248 subjects who underwent surgery for bowel ischemia were identified. Of these, 208 subjects were included in the analysis; 28 subjects were excluded because they lacked adequate CT images, and 12 subjects were excluded for lacking a properly conducted pathological differentiation of mural bowel necrosis.
Among the 208 subjects enrolled in our study, transmural bowel necrosis was identified in 121 subjects (case group), and partial bowel necrosis was identified in 87 subjects (control group).
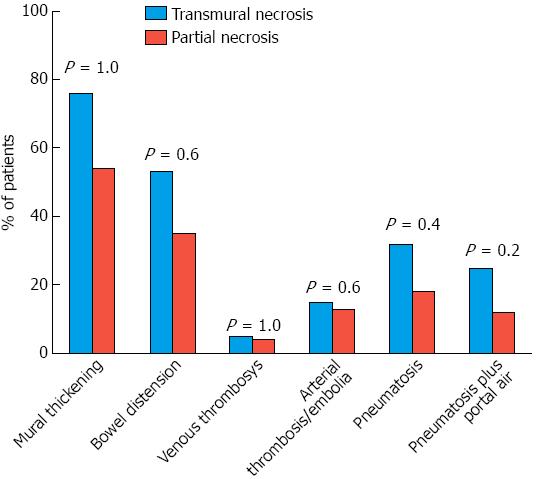
There were no significant demographic, clinical and laboratory differences between the case and control groups (Table 1). Based on a group comparison of CT findings of mural thickening, bowel distension, pneumatosis, pneumatosis plus portomesenteric venous gas and the presence of thrombi or emboli, no significant differences were observed between the case and control groups (Figure 2).
| Characteristics | Transmural necrosis group | Partial necrosis group | P value |
| Age (mean ± SD), yr | 66.6 ± 8.1 | 66.2 ± 5.9 | 0.7 |
| Sex (male) | 61 (50.8) | 43 (49.4) | 1.0 |
| Hypertension | 57 (47.1) | 43 (49.4) | 0.7 |
| Type 2 diabetes | 26 (21.5) | 20 (23.0) | 0.8 |
| Chronic pulmonary disease | 21 (17.3) | 13 (14.9) | 0.7 |
| Atrial fibrillation | 10 (8.3) | 14 (16.0) | 0.1 |
| Chronic renal failure | 7 (5.8) | 6 (6.9) | 0.7 |
| Coronary artery diseases | 15 (12.4) | 15 (17.2) | 0.4 |
| Pain | 121 (100.0) | 87 (100.0) | 1.0 |
| White blood cell count > 15000/mL | 75 (62.0) | 56 (64.4) | 0.7 |
| pH < 7.2 on blood gas analysis | 56 (46.3) | 36 (41.4) | 0.5 |
| Lactate in serum > 4 mmol/L | 69 (57.0) | 55 (63.2) | 0.3 |
A multivariate analysis indicated that after adjusting for major clinical and demographic patient characteristics, none of the CT findings were found to be predictive of transmural necrosis. Specifically, the concomitant presence of pneumatosis and porto-mesenteric venous gas showed an OR of 1.95 (95%CI: 0.491-7.775, P = 0.342) for the presence of transmural necrosis. Similarly, the presence of isolated pneumatosis (P = 0.840), of mural thickening (P = 0.974) and of thrombi (P = 0.483) did not predict the presence of transmural necrosis.
The presence of pneumatosis plus porto-mesenteric venous gas showed good specificity (83%) but low sensitivity (17%) in the identification of transmural bowel infarction. Accordingly, the positive and negative predictive values were 60% and 17%, respectively. Specifically, we registered 96 cases of transmural necrosis without pneumatosis plus porto-mesenteric venous gas (false negatives) and 12 cases of transmural necrosis with pneumatosis plus porto-mesenteric venous gas (false positives). In a ROC curve analysis, the evidence of pneumatosis plus porto-mesenteric venous gas was associated with an area under the curve of 0.50 (95%CI: 0.420-0.580) for predicting the presence of transmural bowel infarction.
To our knowledge, no previous group has evaluated a large series of patients to statistically analyse whether the presence of pneumatosis intestinalis and portomesenteric venous gas facilitates the prediction of ischemic bowel wall damage severity.
Acute intestinal ischemia is pathogenetically and histologically divided into four primary clinical categories, including acute mesenteric embolus, acute mesenteric thrombus, non-occlusive disease and mesenteric vein thrombosis[3,13-15].
Although acute intestinal ischemia is generally uncommon, as it accounts for < 1 in 1000 hospital admissions[16,17], it is a highly complex clinical problem faced during the daily routines of surgeons, gastroenterologists, and radiologists[4] due to the difficulty of providing a diagnosis.
The severity of bowel ischemia may range from mild and transient superficial damage of the bowel mucosa to life-threatening transmural bowel infarction[18,19].
Early diagnosis and treatment determine the overall outcome. As a result, increased awareness among physicians who have clinical patients with suspected mesenteric ischemia and a timely diagnostic workup and initiation of therapy are the keys to saving a patient’s life[4,20].
However, the absence of specific symptoms upon clinical examination may make an appropriate assessment more difficult[4,21]. Typically, the course of an ischemic condition is triphasic. During the initial stage (0-6 h), there is intense, acute abdominal pain that is often accompanied by shock and diarrhoea. These signs are normally followed by a phase with little evidence of symptoms, known as the silent phase (7-12 h), which is characterised by dull abdominal pain, intestinal paralysis, and a rapid deterioration of the general condition. During the final phase (12-24 h), manifest ileus and bacterial peritonitis with sepsis are evident, and multi-organ failure has begun. Satisfactory treatment results are only possible in the early stage (0-12 h)[1].
Necrosis is typically accompanied by increased serum lactate (> 4 mmol/L), increased CRP (> 10 mg/L), and leukocytosis (> 15000/mL). Furthermore, the blood gas analysis indicates an ischemic necrosis exhibiting acidosis with a pH of < 7.2 and a base excess of minus 7-8 mmol/L[1]. Despite these signs, no laboratory-chemical parameters currently exist that are specifically designed to confirm intestinal necrosis.
Currently, contrast-enhanced biphasic multidetector row helical CT of the abdomen is conducted as the primary imaging modality in many medical centres. This technique enables the imaging of the entire abdomen with high temporal and spatial resolution in the arterial and portal venous phases[4].
In addition to high sensitivity and specificity, multidetector CT provides the following advantages: it is a rapid and non-invasive technique that is available 24 h a day in most acute-care medical centres. Due to its high sensitivity, specificity, availability, and non-invasiveness, the multidetector CT is a suitable modality for the diagnosis of acute mesenteric ischemia. Furthermore, it is extremely helpful for triage patients who require subsequent conventional angiography, surgery, or clinical surveillance[4].
Aschoff et al[5] reported a sensitivity of 93% and a specificity of 100% of CT angiography for the diagnosis of acute mesenteric ischemia. Diagnosis was based on an analysis of both vascular occlusions and the consequences of tissue damage, such as intestinal pneumatosis, bowel wall thickening, portomesenteric venous gas, or solid organ infarction[4].
An association between pneumatosis intestinalis and portomesenteric venous gas is a strong indicator of the presence of mesenteric infarction or segmental ischemia, and it is therefore an indication for emergency exploratory surgery[9,22-26].
Data from the literature suggest that the combination of pneumatosis intestinalis and portal venous gas is associated with the presence of bowel ischemia in approximately 70% of all cases[22,27,28]. We extended these results by evaluating the association of this CT finding with the severity of mural necrosis.
Bowel pneumatosis is an imaging phenomenon that can represent the presence of gas in the bowel wall[22]. Hepatic portal venous gas is defined radiologically as tubular areas of decreased attenuation in the liver periphery[29,30].
Although the etiology of pneumatosis appears to be multifactorial, the exact causes are not known. Two primary theories have been proposed in the medical literature. A mechanical theory hypothesises that gas dissects into the bowel wall from either the intestinal lumen or the lungs, via the mediastinum, due to some mechanism that causes increased pressure (i.e., bowel obstruction or emphysema). In contrast, a bacterial theory proposes that gas-forming bacilli enter the submucosa through mucosal rents or increased mucosal permeability, thereby producing gas within the bowel wall. A combination of both theories is also plausible. Bacterial overgrowth in the gastrointestinal tract arising from a variety of causes can lead to excessive hydrogen gas production, bowel distension, and subsequently, the dissection of intraluminal hydrogen gas into the bowel wall[25]. In the case of ischemia, pneumatosis has been considered to be an ominous radiographic finding, particularly if it is associated with portomesenteric venous gas. In some articles, pneumatosis has been described as an advanced sign of ischemic bowel disease, usually indicating irreversible injury and transmural necrosis[6-8].
Wiesner et al[9] supports the hypothesis that patients with pneumatosis and portomesenteric venous gas are more likely to exhibit transmural infarction. Similarly, Kernagis et al[6] determined that patients with CT findings of pneumatosis and portomesenteric venous gas were more likely to have transmural infarction than those with pneumatosis alone.
However, these studies share several limitations, as they were retrospective investigations with small sample sizes (up to seven patients), which may have magnified the effects of selection bias on the study population.
Although pneumatosis plus porto-mesenteric vanous gas is associated with bowel ischemia, we have demonstrated that it cannot be used as a diagnostic sign of transmural necrosis. No statistical associations were identified between transmural infarction and the presence of pneumatosis plus porto-mesenteric venous gas, according to both univariate and multivariate analyses. Although the finding of concomitant pneumatosis plus porto-mesenteric venous gas is useful in verifying transmural necrosis with a specificity of 83%, the very low sensitivity of 17% indicates that the diagnosis of transmural necrosis cannot be excluded based on normal findings. Some limitations of our study warrant discussion. A major concern is the retrospective design of the present study, which has inherent limitations and biases. Moreover, we included only patients who underwent surgical intervention in the analyses. As a result, we are likely to have selected patients with more severe clinical presentations. Thus, further ad hoc-designed prospective studies with adequate sample sizes are needed to evaluate whether pneumatosis intestinalis and portomesenteric venous gas facilitates the prediction of ischemic bowel wall damage severity. However, our encouraging results appear to suggest that neither portomesenteric venous gas nor pneumatosis were pathognomonic of bowel trans-mural infarction.
Pneumatosis plus portomesenteric venous gas have been considered signs of advanced disease, usually indicating irreversible injury and transmural necrosis. Although some authors have identified an association between transmural bowel infarction and the presence of concomitant pneumatosis plus porto-mesenteric venous gas, these studies only represented reports that included up to seven patients.
Although a strong indicator of the presence of mesenteric infarction or segmental ischemia is the association of pneumatosis intestinalis with portomesenteric venous gas, their study demonstrates that these findings are not useful in assessing the presence of transmural necrosis.
The finding that pneumatosis plus porto-mesenteric venous gas is useful in verifying transmural necrosis with a specificity of 83%, but its very low sensitivity of 17% indicates that the diagnosis of transmural necrosis cannot be excluded based on normal findings.
Their study emphasises that pneumatosis and portomesenteric venous gas cannot be considered signs of transmural necrosis.
A superficial damage of bowel mucosa and submucosa was histologically assessed as partial bowel ischemia before the development of transmural infarction. Air bubbles or continuous bands of air in the bowel walls were considered to be signs of pneumatosis, whereas gas in the mesenteric veins or gas in the intrahepatic branches of the portal vein were indicative of gas in the portomesenteric circulation at the time of computed tomography (CT) examination.
This is an interesting study in which authors use more representative sample size to evaluate whether CT scan evidence of the concomitant presence of pneumatosis and portomesenteric venous gas is a predictor of transmural bowel necrosis. The results are interesting and suggest that although pneumatosis plus porto-mesenteric venous gas is associated with bowel ischemia, they have demonstrated that their co-occurrence cannot be used as diagnostic signs of transmural necrosis.
P- Reviewer Triantopoulou C S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | Debus ES, Müller-Hülsbeck S, Kölbel T, Larena-Avellaneda A. Intestinal ischemia. Int J Colorectal Dis. 2011;26:1087-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Czerny M, Trubel W, Claeys L, Scheuba C, Huk I, Prager M, Polterauer P. [Acute mesenteric ischemia]. Zentralbl Chir. 1997;122:538-544. [PubMed] |
| 3. | Debus ES, Luther B, Daum H, Larena-Avellaneda A. [Chronic intestinal ischemia]. Chirurg. 2009;80:473-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Renner P, Kienle K, Dahlke MH, Heiss P, Pfister K, Stroszczynski C, Piso P, Schlitt HJ. Intestinal ischemia: current treatment concepts. Langenbecks Arch Surg. 2011;396:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Aschoff AJ, Stuber G, Becker BW, Hoffmann MH, Schmitz BL, Schelzig H, Jaeckle T. Evaluation of acute mesenteric ischemia: accuracy of biphasic mesenteric multi-detector CT angiography. Abdom Imaging. 2009;34:345-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Kernagis LY, Levine MS, Jacobs JE. Pneumatosis intestinalis in patients with ischemia: correlation of CT findings with viability of the bowel. AJR Am J Roentgenol. 2003;180:733-736. [PubMed] |
| 7. | Kelvin FM, Korobkin M, Rauch RF, Rice RP, Silverman PM. Computed tomography of pneumatosis intestinalis. J Comput Assist Tomogr. 1984;8:276-280. [PubMed] |
| 8. | Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954-968. [PubMed] |
| 9. | Wiesner W, Mortelé KJ, Glickman JN, Ji H, Ros PR. Pneumatosis intestinalis and portomesenteric venous gas in intestinal ischemia: correlation of CT findings with severity of ischemia and clinical outcome. AJR Am J Roentgenol. 2001;177:1319-1323. [PubMed] |
| 10. | Macari M, Balthazar EJ. CT of bowel wall thickening: significance and pitfalls of interpretation. AJR Am J Roentgenol. 2001;176:1105-1116. [PubMed] |
| 11. | Furukawa A, Kanasaki S, Kono N, Wakamiya M, Tanaka T, Takahashi M, Murata K. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192:408-416. [PubMed] |
| 12. | Feurle GE, Haag B. Acute small bowel ischemia without transmural infarction. Z Gastroenterol. 1991;29:349-352. [PubMed] |
| 13. | Di Minno MN, Milone F, Milone M, Iaccarino V, Venetucci P, Lupoli R, Sosa Fernandez LM, Di Minno G. Endovascular Thrombolysis in Acute Mesenteric Vein Thrombosis: a 3-year follow-up with the rate of short and long-term sequaelae in 32 patients. Thromb Res. 2010;126:295-298. [PubMed] |
| 14. | Byard RW. Acute mesenteric ischaemia and unexpected death. J Forensic Leg Med. 2012;19:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Georgescu EF, Carstea D, Dumitrescu D, Teodorescu R, Carstea A. Ischemic colitis and large bowel infarction: a case report. World J Gastroenterol. 2012;18:5640-5644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Wyers MC. Acute mesenteric ischemia: diagnostic approach and surgical treatment. Semin Vasc Surg. 2010;23:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Schoots IG, Levi MM, Reekers JA, Lameris JS, van Gulik TM. Thrombolytic therapy for acute superior mesenteric artery occlusion. J Vasc Interv Radiol. 2005;16:317-329. [PubMed] |
| 18. | Lai WH, Hwang TL, Chen HW. Portomesenteric venous gas in acute bowel ischemia: report of a case. Surg Today. 2008;38:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Wiesner W, Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226:635-650. [PubMed] |
| 20. | Lobo Martínez E, Meroño Carvajosa E, Sacco O, Martínez Molina E. [Embolectomy in mesenteric ischemia]. Rev Esp Enferm Dig. 1993;83:351-354. [PubMed] |
| 21. | Heys SD, Brittenden J, Crofts TJ. Acute mesenteric ischaemia: the continuing difficulty in early diagnosis. Postgrad Med J. 1993;69:48-51. [PubMed] |
| 22. | Khalil PN, Huber-Wagner S, Ladurner R, Kleespies A, Siebeck M, Mutschler W, Hallfeldt K, Kanz KG. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res. 2009;14:231-239. [PubMed] |
| 24. | Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol. 2007;188:1604-1613. [PubMed] |
| 25. | Greenstein AJ, Nguyen SQ, Berlin A, Corona J, Lee J, Wong E, Factor SH, Divino CM. Pneumatosis intestinalis in adults: management, surgical indications, and risk factors for mortality. J Gastrointest Surg. 2007;11:1268-1274. [PubMed] |
| 26. | Susman N, Senturia HR. Gas embolization of the portal venous system. Am J Roentgenol Radium Ther Nucl Med. 1960;83:847-850. [PubMed] |
| 27. | Wiesner W, Mortelé KJ, Glickman JN, Ji H, Ros PR. Portal-venous gas unrelated to mesenteric ischemia. Eur Radiol. 2002;12:1432-1437. [PubMed] |
| 28. | Paran H, Epstein T, Gutman M, Shapiro Feinberg M, Zissin R. Mesenteric and portal vein gas: computerized tomography findings and clinical significance. Dig Surg. 2003;20:127-132. [PubMed] |
| 29. | Nelson AL, Millington TM, Sahani D, Chung RT, Bauer C, Hertl M, Warshaw AL, Conrad C. Hepatic portal venous gas: the ABCs of management. Arch Surg. 2009;144:575-581; discussion 581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Sebastià C, Quiroga S, Espin E, Boyé R, Alvarez-Castells A, Armengol M. Portomesenteric vein gas: pathologic mechanisms, CT findings, and prognosis. Radiographics. 2000;20:1213-1224; discussion 1224-1226;. [PubMed] |