Published online Oct 14, 2013. doi: 10.3748/wjg.v19.i38.6453
Revised: August 12, 2013
Accepted: August 20, 2013
Published online: October 14, 2013
Processing time: 117 Days and 0.9 Hours
AIM: To investigate whether nonalcoholic fatty liver disease (NAFLD) affects coronary artery disease (CAD) and identify candidate mediators.
METHODS: Patients who underwent coronary angiography were consecutively recruited. The patients were classified into four groups by coronary artery stenosis: A, insignificant; B, one-vessel disease; C, two-vessel disease; and D, three-vessel disease. Abdominal ultrasonography was performed to determine the presence of a fatty liver and categorize by grade: 0, no evidence; 1, mild; 2, moderate; and 3, severe. We measured not only known CAD risk factors, but also serum insulin, HOMA-index, adiponectin, interleukin-6, tumor necrosis factor-α and high-sensitivity C-reactive protein levels.
RESULTS: Of the 134 patients who met the inclusion criteria, 82 (61.2%) had ultrasonographically diagnosed NAFLD. Among the 46 patients with CAD, 37 (80.4%) had evidence of a fatty liver. The two groups (A vs B-D) were significantly different in terms of age, total cholesterol, triglycerides, low-density lipoprotein levels and fatty liver. Coronary artery stenosis was strongly associated with fatty liver in a grade-dependent manner (P = 0.025). In binary logistic regression, NAFLD was a significant independent predictor of CAD (P = 0.03, OR = 1.685; 95%CI: 1.051-2.702). Among the candidate mediators, the serum adiponectin level showed a trend toward lowering based on CAD progression (P = 0.071).
CONCLUSION: NAFLD is an independent risk factor for CAD in a grade-dependent manner. Moreover, adiponectin might be related to the pathogenesis of NAFLD.
Core tip: This article shows that angiographically proven coronary artery stenosis is strongly associated with nonalcoholic fatty liver disease (NAFLD) in a grade-dependent manner. Although many recent studies used coronary artery calcification score, carotid artery intima-media thickness, or carotid artery plaque measurements as surrogate markers for coronary artery disease (CAD), we evaluated the interaction between fatty liver and cardiovascular outcomes using coronary angiograms in a prospective case-controlled study. Because the pathogenesis of NAFLD and CAD is not fully elucidated, we attempted to identify mediators of these diseases and believe that adiponectin might be related to the development and progression of CAD in patients with NAFLD.
- Citation: Choi DH, Lee SJ, Kang CD, Park MO, Choi DW, Kim TS, Lee W, Cho BR, Kim YH, Lee BK, Ryu DR, Lee JW. Nonalcoholic fatty liver disease is associated with coronary artery disease in Koreans. World J Gastroenterol 2013; 19(38): 6453-6457
- URL: https://www.wjgnet.com/1007-9327/full/v19/i38/6453.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i38.6453
Nonalcoholic fatty liver disease (NAFLD) is a common disorder with an increasing prevalence of approximately 34% of the adult population in the United States[1]. Patients with NAFLD can progress to more aggressive forms of nonalcoholic steatohepatitis (NASH), which can progress to cirrhosis, end-stage liver disease, and eventually hepatocellular carcinoma[2]. Because NAFLD is related to metabolic syndrome and obesity, many patients with NAFLD have coronary artery disease (CAD). Several studies have reported that NAFLD is a strong independent risk factor for CAD[3,4]. However, these studies have some clinical application limitations because of the use of indirect modalities, such as coronary artery calcification or intima-media thickness despite coronary artery imaging. Authors of these studies suggested that the presence of CAD was indicated by coronary artery calcification or intima-media thickness despite conducting coronary artery imaging[5,6]. Many NAFLD studies conducted in Western populations have found a relationship between NAFLD and CAD in relatively obese patients, which has not been found in Asian populations[6,7]. Therefore, the relationship between NAFLD and CAD in relatively thin Asian people must be evaluated. This study was conducted to evaluate whether NAFLD independently affects angiographically proven CAD in Asians and, if so, which mediator is responsible for this association.
From January 2009 to June 27, 2011, 184 adult patients who underwent elective coronary angiography (CAG) at Kangwon National University Hospital were consecutively recruited. Indications for CAG included Canadian Cardiovascular Society class III or IV angina upon medical treatment, high-risk findings upon noninvasive testing, acute coronary syndrome, or a chest pain evaluation according to the American College of Cardiology/American Heart Association recommendations[8]. Standard selective CAG was performed by three experienced cardiologists and reviewed by another cardiologist. CAD was defined as the presence of at least a 50% stenosis in at least one major coronary artery. The patients were classified into four groups according to the number of major coronary arteries affected by CAD: A, insignificant coronary artery stenosis; B, one-vessel disease; C, two-vessel disease; and D, three-vessel disease.
We excluded patients with viral hepatitis (positive for hepatitis B surface antigen and anti-hepatitis C virus), history of alcohol ingestion (> 30 g/d for men and > 20 g/d for women), history of drug use reported to cause steatosis (steroids, estrogens, tamoxifen, amiodarone, valproic acid, diltiazem, or methotrexate), improved steatosis (metformin, statins, or glitazones) within 3 mo of enrollment, or other history of chronic liver disease. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg or using antihypertensive medications. Diabetes was defined as fasting blood sugar ≥ 126 mg/dL or using glucose-lowering medications (oral agents or insulin). Of the 184 patients, we excluded 50 with at least one potential cause for chronic liver disease. Altogether, 134 patients were enrolled and underwent abdominal ultrasonography within 2 d after CAG by a single experienced physician to determine the presence of four fatty liver grades: 0, no evidence of fatty liver; 1, mild; 2, moderate; and 3, severe degree. The presence of a fatty liver was identified by characteristic echo patterns such as a diffuse increase in the echogenicity of the liver compared with that of the kidney according to conventional criteria[9]. We measured not only known risk factors (i.e., age, male gender, high low-density lipoprotein, low high-density lipoprotein, triglyceride, body mass index, diabetes, and hypertension) for CAD but also serum insulin, HOMA index, adiponectin, interleukin (IL)-6, tumor necrosis factor (TNF)-α and high-sensitivity C-reactive protein (hs-CRP) levels. This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Kangwon National University Hospital.
Clinical and biochemical variables were compared between the two groups (A vs B-D). Continuous variables were assessed with the unpaired Student’s t-test, and nominal variables were compared with the chi- square test. Variables that were significantly different between the two groups were extracted and included as covariates in a binary logistic regression with CAD as the dependent and NAFLD as the independent variable. Correlations between CAD severity and NAFLD degree were analyzed using Pearson’s correlation analysis. A P-value < 0.05 was considered significant. All analyses were conducted using the SPSS for Windows 12.0.1 statistical software (SPSS, Inc., Chicago, IL, United States).
A total of 134 (37 males and 97 females) patients met the inclusion criteria for the study. Table 1 demonstrates the demographic, clinical and laboratory data of the subjects without CAD (A) and those with CAD (B-D). The two groups were significantly different in terms of age, total cholesterol, triglycerides, low-density lipoprotein levels and presence of NAFLD. In addition, there tended to be more clinical features associated with metabolic syndrome in the CAD group, but the difference was not significant. In each group, women were predominant, and all subjects were post-menopausal except for one person in the CAD group.
| Group A | Group B-D | P value | |
| Insignificant stenosis (n = 88) | Significant stenosis (n = 46) | ||
| Age (yr) | 62.5 ± 10.8 | 65.2 ± 9.2 | 0.010 |
| Sex (male) | 20 (22.7) | 17 (37.0) | 0.104 |
| DM | 11 (12.5) | 10 (21.7) | 0.211 |
| HTN | 49 (55.7) | 33 (71.7) | 0.093 |
| Height (cm) | 155.0 ± 7.4 | 156.4 ± 8.4 | 0.333 |
| Weight (kg) | 61.9 ± 8.2 | 62.5 ± 10.0 | 0.734 |
| BMI (kg/m2) | 25.8 ± 3.3 | 25.6 ± 3.4 | 0.697 |
| Waist circumference (cm) | 86.8 ± 13.4 | 89.7 ± 6.9 | 0.169 |
| Hip circumference (cm) | 97.6 ± 13.8 | 98.7 ± 8.3 | 0.607 |
| WHR | 0.89 ± 0.9 | 0.91 ± 0.8 | 0.238 |
| Total cholesterol (mg/dL) | 177.1 ± 30.8 | 195.6 ± 39.1 | 0.009 |
| HDL cholesterol (mg/dL) | 41.2 ± 12.2 | 38.4 ± 12.1 | 0.227 |
| Triglyceride (mg/dL) | 134.9 ± 72.4 | 177.4 ± 94.4 | 0.012 |
| Measured-LDL cholesterol (mg/dL) | 102.3 ± 26.1 | 115.5 ± 33.3 | 0.015 |
| Calculated-LDL cholesterol (mg/dL) | 108.6 ± 28.3 | 121.7 ± 33.7 | 0.033 |
| Creatinine (mg/dL) | 0.8 ± 0.3 | 1.1 ± 0.4 | 0.068 |
| Uric acid (mg/dL) | 4.6 ± 1.4 | 4.9 ± 1.6 | 0.399 |
| Hemoglobin (g/dL) | 13.4 ± 1.8 | 13.0 ± 1.5 | 0.356 |
| HbA1c (%) | 5.7 ± 0.7 | 6.3 ± 1.2 | 0.072 |
| Systolic BP (mmHg) | 123.3 ± 16.6 | 125.6 ± 15.6 | 0.409 |
| Diastolic BP (mmHg) | 73.8 ± 10.7 | 75.9 ± 9.6 | 0.250 |
| FBS (mg/dL) | 104.2 ± 21.2 | 115.3 ± 37.3 | 0.082 |
| Total bilirubin (mg/dL) | 1.0 ± 0.5 | 1.1 ± 0.6 | 0.432 |
| Albumin (g/dL) | 3.9 ± 0.3 | 3.9 ± 0.4 | 0.465 |
| AST (U/L) | 34.1 ± 55.0 | 27.1 ± 11.0 | 0.394 |
| ALT (U/L) | 30.7 ± 53.4 | 22.8 ± 9.7 | 0.321 |
| PT INR | 0.9 ± 0.3 | 0.8 ± 0.1 | 0.182 |
| HOMA-index | 6.29 ± 9.16 | 5.99 ± 5.39 | 0.838 |
| NAFLD | 44 (51.2) | 36 (78.3) | 0.002 |
An analysis of the relationship between NAFLD and the presence of CAD is shown in Table 2. In addition to the significantly different variables between the two groups in Table 1, well-known established risk factors for CAD, such as age, gender, glucose, HbA1c and body mass index, were considered as covariates in conducting the multivariate analysis. In those models, as shown in Table 2, NAFLD was the significant independent predictor for CAD (P = 0.03, OR = 1.685; 95%CI: 1.051-2.702).
| OR (95%CI) | P value | |
| NAFLD | 1.685 (1.051-2.702) | 0.030 |
| Age | 1.056 (1.010-1.104) | 0.057 |
| Total cholesterol (mg/dL) | 1.012 (0.982-1.043) | 0.427 |
| TG (mg/dL) | 1.004 (0.998-1.010) | 0.873 |
| Measured-LDL cholesterol (mg/dL) | 1.003 (0.970-1.036) | 0.225 |
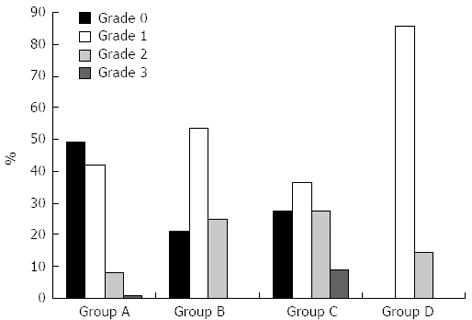
Next, we evaluated the correlation between the NAFLD degree and CAD severity. The proportion of patients with NAFLD increased from 51.1% in group A to 100% in group D. In group A, most of the fatty livers were grade 1. However, in the higher grade CAD group, the proportion of patients with more severe fatty livers was increased. No subject in group D (three-vessel disease) had a normal liver. Figure 1 shows that angiographically proven coronary artery stenosis was strongly associated with NAFLD in a grade-dependent manner by Pearson’s correlation analysis (P = 0.002).
In addition, we measured the serum level of candidate mediators of metabolic syndrome, such as insulin, the HOMA index, IL-6, TNF-α, and hs-CRP (Table 3). In our results, none of the factors assessed were found to be related to CAD. However, serum adiponectin level demonstrated a trend toward lowering based on CAD progression (P = 0.071).
| Group A | Group B-D | P value | |
| Insignificant stenosis (n = 88) | Significant stenosis (n = 46) | ||
| Adiponectin (μg/mL) | 8.40 ± 5.97 | 6.95 ± 5.85 | 0.071 |
| IL-6 (pg/mL) | 4.55 ± 7.75 | 4.71 ± 7.41 | 0.894 |
| TNF-α (ng/mL) | 4.00 ± 3.95 | 4.85 ± 4.73 | 0.273 |
| hs-CRP(mg/dL) | 0.45 ± 1.70 | 0.74 ± 1.18 | 0.366 |
Our findings demonstrate that NAFLD is strongly associated with coronary artery stenosis in a grade-dependent manner. Our results also demonstrate that NAFLD is a significant predictor of CAD independent of traditional risk factors in Asians. Furthermore, we suggest that adiponectin might have a potential pathogenic role in the development and progression of CAD in patients with NAFLD.
Because NAFLD is a hepatic manifestation of metabolic syndrome, many studies have suggested that NAFLD results in increased cardiovascular risk and mortality[7,10]. The risk for developing cardiovascular morbidity and mortality is thought to be higher than the risk for developing hepatic disease because of its slow progression. Therefore, many studies have investigated the association between NAFLD and cardiovascular diseases. As a result, a number of studies have demonstrated that NAFLD is an independent risk factor for CAD[4,5,11-13]. However, most studies used coronary artery calcification score, carotid artery intima-media thickness, carotid artery plaque measurements, or circulatory endothelial dysfunction as surrogate markers for CAD[5,6,14]. Despite the fact that the coronary calcification score is a well-known marker for an increased risk of coronary events, the direct relationship between the presence of NAFLD and clinical CAD must be evaluated for use in the clinical setting[3]. Recently, Wong et al[15] evaluated the interaction between fatty liver and cardiovascular outcomes using coronary angiograms in a prospective cohort study and demonstrated that fatty liver is associated with CAD independently of other metabolic factors, which is consistent with our results. In contrast, our study was different from that study because we demonstrated that angiographically proven coronary artery stenosis was strongly associated with fatty liver in a grade-dependent manner.
Although the pathogenesis of NAFLD and CAD has not been fully elucidated, several explanations are present for the relationship between NAFLD and CAD. One widely accepted hypothesis implicates low-grade inflammatory conditions as key factors leading to hepatic steatosis and atherosclerosis[16,17]. Moreover, oxidative stress is presumed to play a role in NASH pathogenesis. Many investigators have studied additional mechanisms that might be associated with NAFLD, which are supported by the levels of various biomarkers, such as reactive oxygen species, adipocytokines (leptin and adiponectin), CRP, and caspase-generated cytokeratin-18[18-21]. In this study, we also tried to find candidate mediators of the mechanism of this relationship. We investigated several mediators, including adiponectin, IL-6, TNF-α, and hs-CRP. Among these candidate mediators, adiponectin may have been related to the development and progression of CAD in patients with NAFLD in our study. Adiponectin is the most abundant adipose-specific adipokine, and decreases hepatic insulin resistance and attenuates liver inflammation[22]. Low levels of serum adiponectin might play an important role in the pathogenesis of clinical CAD and NAFLD. In contrast, NAFLD is also characterized by increased insulin resistance[23]. We measured fasting serum insulin levels and calculated the HOMA index to confirm this relationship in our study. Because we included obese Asians, which in contrast with previous Asian-Pacific NAFLD studies that included non-obese subjects, our study subjects had relatively high insulin resistance[24]. However, fasting serum insulin levels and HOMA-IR were not different between our two groups (with/-without CAD and with/-without a fatty liver).
Some limitations of our study merit comment. First, our results were not based on a biopsy-proven NAFLD. There is no histology or staging of fibrosis by use of elastography to determine the liver fibrosis. We diagnosed NAFLD using hepatic ultrasonography. This technique does not identify fatty infiltration < 30% although it is a safe and confirmed reliable noninvasive method[25]. This technique also has additional weak points, which are intra- and interobserver differences when making a diagnosis. However, to overcome these limitations, ultrasonography was performed by a single experienced physician to determine the presence of the four fatty liver grades. In addition, standard selective CAG was performed to diagnose and measure CAD severity by three experienced cardiologists in our study. To reduce interobserver variability for CAG, another cardiologist also reviewed all of the data. Second, this study was conducted at a single center in a rural area, which increased the chance for selection bias. Women were predominant in the included subject. A possible explanation for this gender imbalance is that men in this area had a high prevalence of alcohol intake and were excluded based on a history of alcohol ingestion.
Because NAFLD is considered a hepatic manifestation of metabolic syndrome, many studies have investigated the association between NAFLD and cardiovascular diseases. As a result, our study demonstrates that NAFLD is an independent risk factor for angiographically proven CAD in a grade-dependent manner. Because the pathogenesis of NAFLD and CAD are not fully elucidated, we also attempted to identify mediators and believe that adiponectin might be related to the development and progression of CAD in patients with NAFLD. Therefore, future large-scale studies are needed to elucidate the precise mechanism of this relationship.
Although recent many studies used coronary artery calcification score, carotid artery intima-media thickness, or carotid artery plaque measurements as surrogate markers for coronary artery disease (CAD), this study evaluated the interaction between fatty liver and cardiovascular outcomes using coronary angiograms in a prospective case-control study of Asians.
The relationship between nonalcoholic fatty liver disease (NAFLD) and CAD in relatively thin Asian people must be evaluated. Moreover, because the pathogenesis of NAFLD and CAD are not fully elucidated, the authors attempted to identify candidate mediators.
This article show that angiographically proven coronary artery stenosis was strongly associated with NAFLD in a grade-dependent manner. In addition, the authors attempted to identify mediators and believe that adiponectin might be related to the development and progression of CAD in patients with NAFLD.
By understanding the association between NAFLD and CAD, patients with a severe degree of fatty liver disease have to be concerned about CAD to improve their prognosis.
This is a prospective single center study, which investigate the relationship between NAFLD and CAD and seeks candidate mediators. Future large-scale studies are needed to elucidate the precise mechanism of this relationship.
P- Reviewers Baffy G, Enjoji M, Oliveira C, Scheimann A, Vijayvergiya R S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2695] [Article Influence: 128.3] [Reference Citation Analysis (3)] |
| 2. | Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 330] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 3. | Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, Kim YJ, Yoon JH, Jeong SH, Lee DH. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 4. | Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51:1947-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 5. | Choi SY, Kim D, Kang JH, Park MJ, Kim YS, Lim SH, Kim CH, Lee HS. [Nonalcoholic fatty liver disease as a risk factor of cardiovascular disease: relation of non-alcoholic fatty liver disease to carotid atherosclerosis]. Korean J Hepatol. 2008;14:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G, Arcaro G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29:1325-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Edens MA, Kuipers F, Stolk RP. Non-alcoholic fatty liver disease is associated with cardiovascular disease risk markers. Obes Rev. 2009;10:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148-e304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 732] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 9. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3718] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 10. | Watanabe S, Yaginuma R, Ikejima K, Miyazaki A. Liver diseases and metabolic syndrome. J Gastroenterol. 2008;43:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Choi SY, Kim D, Kim HJ, Kang JH, Chung SJ, Park MJ, Kim YS, Kim CH, Choi SH, Kim W. The relation between non-alcoholic fatty liver disease and the risk of coronary heart disease in Koreans. Am J Gastroenterol. 2009;104:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Mirbagheri SA, Rashidi A, Abdi S, Saedi D, Abouzari M. Liver: an alarm for the heart? Liver Int. 2007;27:891-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Arslan U, Türkoğlu S, Balcioğlu S, Tavil Y, Karakan T, Cengel A. Association between nonalcoholic fatty liver disease and coronary artery disease. Coron Artery Dis. 2007;18:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27-III32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 946] [Cited by in RCA: 1357] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 15. | Wong VW, Wong GL, Yip GW, Lo AO, Limquiaco J, Chu WC, Chim AM, Yu CM, Yu J, Chan FK. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60:1721-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 16. | Aller R, de Luis DA, Fernandez L, Calle F, Velayos B, Olcoz JL, Izaola O, Sagrado MG, Conde R, Gonzalez JM. Influence of insulin resistance and adipokines in the grade of steatosis of nonalcoholic fatty liver disease. Dig Dis Sci. 2008;53:1088-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541-3546. [PubMed] |
| 18. | Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 19. | Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 20. | Yilmaz Y, Dolar E, Ulukaya E, Akgoz S, Keskin M, Kiyici M, Aker S, Yilmaztepe A, Gurel S, Gulten M. Soluble forms of extracellular cytokeratin 18 may differentiate simple steatosis from nonalcoholic steatohepatitis. World J Gastroenterol. 2007;13:837-844. [PubMed] |
| 21. | Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1298] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 22. | Finelli C, Tarantino G. What is the role of adiponectin in obesity related non-alcoholic fatty liver disease? World J Gastroenterol. 2013;19:802-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 144] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (3)] |
| 23. | Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res. 2012;2012:145754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Liu CJ. Prevalence and risk factors for non-alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol. 2012;27:1555-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 25. | Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705-1725. [PubMed] |